Abstract
Nicotine dependence and cocaine abuse are major public health problems, and most cocaine abusers also smoke cigarettes. An ideal treatment medication would reduce both cigarette smoking and cocaine abuse. Varenicline is a clinically available, partial agonist at α4β2* and α6β2* nicotinic acetylcholine receptors (nAChRs) and a full agonist at α7 nAChRs. Varenicline facilitates smoking cessation in clinical studies and reduced nicotine self-administration, and substituted for the nicotine-discriminative stimulus in preclinical studies. The present study examined the effects of chronic varenicline treatment on self-administration of IV nicotine, IV cocaine, IV nicotine+cocaine combinations, and concurrent food-maintained responding by five cocaine- and nicotine-experienced adult rhesus monkeys (Macaca mulatta). Varenicline (0.004–0.04 mg/kg/h) was administered intravenously every 20 min for 23 h each day for 7–10 consecutive days. Each varenicline treatment was followed by saline-control treatment until food- and drug-maintained responding returned to baseline. During control treatment, nicotine+cocaine combinations maintained significantly higher levels of drug self-administration than nicotine or cocaine alone (P<0.05–0.001). Varenicline dose-dependently reduced responding maintained by nicotine alone (0.0032 mg/kg/inj) (P<0.05), and in combination with cocaine (0.0032 mg/kg/inj) (P<0.05) with no significant effects on food-maintained responding. However, varenicline did not significantly decrease self-administration of a low dose of nicotine (0.001 mg/kg), cocaine alone (0.0032 and 0.01 mg/kg/inj), or 0.01 mg/kg cocaine combined with the same doses of nicotine. We conclude that varenicline selectively attenuates the reinforcing effects of nicotine alone but not cocaine alone, and its effects on nicotine+cocaine combinations are dependent on the dose of cocaine.
Keywords: cocaine, nicotine, nicotine+cocaine, nicotine addiction, polydrug abuse model, varenicline
INTRODUCTION
Both cigarette smoking and cocaine addiction are major public health problems (Benowitz, 2009; CDC, 2002, 2004, 2005; DAWN, 2010; SAMSHA, 2012), and there is a continuing search for more effective treatment medications (Henningfield et al, 2009; Mello and Mendelson, 2013; Pollock et al, 2009). Most cocaine abusers also smoke cigarettes (Budney et al, 1993) and smoking increases during cocaine use (Roll et al, 1996; Roll et al, 1997). Nicotine enhances the reinforcing effects of cocaine in rhesus monkeys (Mello and Newman, 2011), and similar findings were reported in behavioral studies of nicotine-cocaine interactions in rodents (Bechtholt and Mark, 2002; Horger et al, 1992; Levine et al, 2011) and rhesus monkeys (Freeman and Woolverton, 2009; Mello et al, 2013b). Nicotine's enhancement of cocaine's effects may reflect an increase in dopamine levels because equally potent doses of nicotine and cocaine have additive effects on dopamine release from the nucleus accumbens (Gerasimov et al, 2000; Sziraki et al, 1999; Zernig et al, 1997). Given that nicotine and cocaine alone each increase dopamine levels (DiChiara, 2000; DiChiara and Imperato, 1988a; Pettit and Justice, 1991), although through different mechanisms (Koob and LeMoal, 2006; Kuhar et al, 1991), identifying medications that may reduce abuse of nicotine+cocaine in combination is an intriguing challenge.
Varenicline (Chantix/Champix) was approved for the treatment of nicotine dependence by the FDA in 2006 and has been effective in facilitating smoking cessation (Cinciripini et al, 2013; Hawk et al, 2012; Hays et al, 2008; Jorenby et al, 2006; Niaura et al, 2006; Tonstad, 2006). Varenicline was designed as a partial agonist at α4β2* nicotinic acetylcholine receptors (nAChRs) (Faessel et al, 2010; Rollema et al, 2007a) and a full agonist at α7 nicotinic receptors (Coe et al, 2005; Mihalak et al, 2006). Activation of the α4β2* nAChR receptors on mesolimbic dopamine (DA) neurons leads to dopamine release that modulates the reinforcing effects of nicotine (Koob and LeMoal, 2006). Reduction of the abuse-related effects of nicotine by varenicline is usually attributed to its effects at α4β2* receptors. Nicotine produced higher levels of dopamine turnover and release than varenicline, and when varenicline was combined with nicotine, it reduced nicotine's effects on dopamine levels to those of varenicline alone (Coe et al, 2005; Rollema et al, 2007b). Recent evidence suggests that varenicline also acts as a partial agonist at α6β2* nAChRs in both rat and monkey striatal synaptosomes (Bordia et al, 2012). The importance of α6β2* nAChRs in modulating dopamine release is increasingly recognized (Exley et al, 2013; Wickham et al, 2013) For review, see (Quik and Wonnacott, 2011). Interestingly, the binding affinity of varenicline for α6β2* nAChRs was about 20-fold higher than that of nicotine (Bordia et al, 2012).
There has been relatively little research on varenicline's effects on the abuse-related effects of cocaine and results have been inconsistent. Clinical studies have reported significant decreases in cocaine use during chronic varenicline treatment (0.5–2 mg/day) (Plebani et al, 2012), and no effect of varenicline (2 mg/day ) on cocaine use (Poling et al, 2010). In rhesus monkeys, low doses of varenicline (0.03 and 0.1 mg/kg, PO (salt)) had no effect on cocaine self-administration, and higher doses (0.3–0.56 mg/kg, PO) potentiated the reinforcing effects of cocaine as well as cocaine's discriminative stimulus effects (0.1–0.3 mg/kg, IV) (Gould et al, 2011). However, varenicline did not substitute for the reinforcing or discriminative stimulus effects of cocaine (Gould et al, 2011). In rats, varenicline (2.0 mg/kg, SC) decreased cocaine self-administration, and in reinstatement studies, a high dose of varenicline increased and a low dose decreased cocaine-seeking behavior (Guillem and Peoples, 2010).
In contrast, preclinical studies of the effects of varenicline on the abuse-related effects of nicotine have consistently reported that varenicline reduced nicotine self-administration and substituted for the nicotine discriminative stimulus. For example, in rats, varenicline reliably reduced nicotine self-administration (George et al, 2011; Le Foll et al, 2011; O'Connor et al, 2010; Rollema et al, 2007b; Wouda et al, 2011). Most studies of nicotine discrimination agree that varenicline substituted for nicotine in rats, at levels that varied between 60 and 100% (Jutkiewicz et al, 2011; Le Foll et al, 2011; Lesage et al, 2009; Paterson et al, 2010; Reichel et al, 2010; Rollema et al, 2007b; Smith et al, 2007). Consistent with varenicline's classification as a partial agonist at nicotinic receptors (Faessel et al, 2010; Rollema et al, 2007b), pretreatment with varenicline (1.0 and 3.0 mg/kg, SC) significantly antagonized nicotine discrimination (Lesage et al, 2009). Mecamylamine, a nonselective nicotine antagonist, also blocked varenicline substitution for the nicotine discriminative stimulus (Rollema et al, 2007a).
Most medications to treat drug abuse are derived from advances in medicinal chemistry and neurobiology and initially are not available for testing on humans. As a consequence, preclinical models of drug addiction are essential for guiding decisions about which classes of medications are likely to be clinically useful. Retrospective validation of these models, using medications that have clinical efficacy, is one important component of the translational evaluation process (Mello, 2005). Comparison of clinical and preclinical studies of varenicline provides a valuable opportunity for retrospective evaluation of treatment medication effects on nicotine and cocaine in a nonhuman primate model of drug self-administration. In addition, there are many similarities between rhesus monkeys and humans in neuroanatomy, physiology, and neurochemistry of brain transmitter systems (Weerts et al, 2007). Moreover, nicotinic receptor distribution in cortex, thalamus, basal ganglia, and cerebellum is very similar in human and monkey brain, and there is considerable overlap between AChRs and mesolimbic dopamine neurons (Gotti and Clementi, 2004; Gotti et al, 2007; Han et al, 2000; Han et al, 2003). This is the first report comparing the effects of chronic varenicline treatment (0.004 and 0.04 mg/kg/h) on self-administration of nicotine alone (0.001–0.0032 mg/kg/inj, base), cocaine alone (0.0032–0.01 mg/kg/inj), and combinations of nicotine (0.001–0.0032 mg/kg/inj)+cocaine (0.0032–0.01 mg/kg/inj) in a nonhuman primate polydrug model (Mello and Newman, 2011).
MATERIALS AND METHODS
Subjects
Four male and two female rhesus monkeys (Macaca mulatta) that weighed between 6 and 10 kg were studied. All monkeys had a history of cocaine and nicotine self-administration. Although females were gonadally intact, cocaine and nicotine self-administration disrupted menstrual cycle regularity. Amenorrhea and abnormally short menstrual cycles were observed, similar to disruptions previously reported during chronic cocaine self-administration (Mello et al, 1997). Each day, monkeys received multiple vitamins, fresh fruit and vegetables and Lab Diet Jumbo Monkey Biscuits (PMI Feeds, St. Louis, MO) to supplement a response-contingent banana-flavored pellet diet, fortified with vitamin C (Formula 4TUR banana flavor, grain-based pellet, Purina Mills Test Diet, Richmond, IN). Food supplements were given twice a day between 0900 and 0930, and between 1700 and 1730. Water was continuously available from an automatic watering system. A 12-h light–dark cycle was in effect (lights on 0700–1900), and the experimental chamber was dark during food and drug self-administration sessions.
Animal maintenance and research were conducted in accordance with the guidelines provided by the NIH Office of Laboratory Animal Welfare (OLAW), The Committee on the Care and Use of Laboratory Animals, and the US Department of Agriculture (USDA). The facility is licensed by the USDA, and protocols were approved by the Institutional Animal Care and Use Committee (IACUC). Monkeys were observed at least twice every day, and any changes in general activity were noted. The health of the monkeys was periodically monitored by consultant veterinarians trained in primate medicine. Operant food and drug acquisition procedures provided an opportunity for enrichment and for monkeys to manipulate their environment (Line, 1987). Monkeys had many other enrichment devices and visual, auditory, and olfactory contact with other monkeys throughout the study.
IV Catheter Implantation
Nicotine, cocaine, and nicotine+cocaine solutions, and varenicline or saline were administered through surgically implanted venous catheters. Double lumen Silicone rubber catheters (I.D. 0.028 in, O.D. 0.088 in; Saint Gobain Performance Plastics, Beaverton, MI) were surgically implanted in an internal or external jugular or femoral vein. All surgical procedures were performed under aseptic conditions. Monkeys were initially sedated with ketamine (5–10 mg/kg, IM). Atropine (0.05 mg/kg) SC or IM was administered to reduce salivation. Following insertion of an endotracheal tube, anesthesia was maintained with isofluorane (1–2% mixed with oxygen). After surgery, monkeys were given procaine penicillin G at 20 000 units/kg, IM twice daily for 5 days, or cephalexin 20 mg/kg, PO twice daily for 5 days. An analgesic dose of buprenorphine (0.032 mg/kg, IM) and metacam (meloxicam) (0.1 mg/kg, SC) was administered twice daily for 3 days.
The intravenous catheter exited in the mid-scapular region and was protected by a tether system consisting of a custom-fitted nylon vest connected to a flexible stainless-steel cable and fluid swivel (Lomir Biomedical, Malone, NY). This flexible tether system permits monkeys to move freely. Catheter patency was evaluated periodically by administration of a short-acting barbiturate, methohexital sodium (4 mg/kg) through the catheter lumen. If muscle tone decreased within 10 s after drug administration, the catheter was considered patent.
Apparatus and Operant Procedures
Monkeys lived in stainless steel chambers (64 × 64 × 79 cm) equipped with a custom-designed operant response panel (28 × 28 cm), a pellet dispenser (Gerbrands Model G5210, Arlington, MA), and two syringe pumps (Model 981210, Harvard Apparatus, South Natick, MA), one for each lumen of the double-lumen catheter. During food self-administration sessions, the response key (6.4 × 6.4 cm) on the operant panel was illuminated with a red light. Completion of the response requirement under a fixed ratio 2, variable ratio 16 (FR 2, [VR 16:S]) schedule resulted in presentation of a 1-s red light beneath the response key. Completion of a second VR16 resulted in delivery of a 1 g banana-flavored pellet. During drug self-administration sessions, the response key was illuminated with a green light, and completion of the response requirement under an FR 2, [VR 16:S] schedule resulted in delivery of 0.1 ml of saline or a drug solution over 1 s through one lumen of the double-lumen catheter. A 10-s time-out followed delivery of each drug or saline injection or food pellet, during which stimulus lights remained off and responding had no scheduled consequences. If 25 food pellets or 20 injections were delivered before the end of the 1-hr session, then all stimulus lights were turned off, and responding had no scheduled consequences for the remainder of that session. Thus, a monkey could earn a maximum of 100 food pellets/day and 80 drug or saline injections/day in four daily food and drug self-administration sessions. The daily food self-administration sessions began at 1100, 1500, 1900, and 0600 the next morning, and the daily drug self-administration sessions began at 1200, 1600, 2000, and 0700 the next morning. Room lights were off during all experimental sessions. Schedules of reinforcement were programmed with custom-designed software and IBM-compatible computers and interface systems (Med Associates, St Albans, VT). Additional details of this apparatus have been described previously (Mello et al, 1995). Drug concentrations were varied by computer-controlled changes in pump infusion duration (Fivel, 2011).
Training procedure
Monkeys were initially trained to self-administer banana-flavored food pellets and cocaine (0.1 mg/kg/inj). Once cocaine-maintained responding was stable, the unit dose was reduced to 0.01 or 0.032 mg/kg to limit the disruptive effects of each IV cocaine injection, and to facilitate higher levels of IV self-administration behavior throughout the session. Extinction training consisted of substituting saline for 0.032 mg/kg IV cocaine. Once saline extinction was reliable, drug dose–effect curves were determined over a dose range of 0.001–0.0032 mg/kg/inj IV nicotine. Combinations of doses on the ascending limb for nicotine with two doses of cocaine (0.0032 and 0.01 mg/kg/inj) were also tested. Saline and different doses of cocaine+nicotine were presented in an irregular order.
Drug dose-effect curve determinations
Training continued until monkeys met the following criteria for stable food and cocaine self-administration under the FR2 [VR16:S] schedule of reinforcement: (1) three consecutive days during which the number of drug injections per day varied by no more than 20% of the 3-day mean with no upward or downward trend and (2) the mean number of food pellets and injections delivered per day was equal to or greater than 60. Once responding was stable, self-administration of saline, nicotine (0.001–0.0032 mg/kg/inj), and combinations of cocaine (0.0032–0.01 mg/kg/inj)+nicotine (0.001–0.0032 mg/kg/inj) were studied. Each dose was substituted for a minimum of 7 days and until responding was stable according to the above criteria, or for a maximum of 10 days. Following each substitution test, monkeys were returned to the maintenance dose of cocaine, 0.01 or 0.032 mg/kg/inj, for at least three days and until responding was stable to ensure reliable baseline responding prior to the subsequent substitution test. Drug doses were presented in an irregular order that differed for each monkey.
Varenicline treatment procedures
Procedures for evaluating the effects of varenicline on the reinforcing effects of nicotine, cocaine, and combinations of cocaine+nicotine were similar to those used in our previous studies of the effects of chronic buspirone treatment on cocaine, nicotine, and cocaine+nicotine self-administration (Mello et al, 2013b). Evaluation of the chronic effects of candidate medications is important to determine if acute treatment effects are sustained through time (Mello, 2005; Mello and Negus, 1996). Varenicline or saline injections were delivered every 20 min for 23 h each day through one lumen of a double-lumen catheter. This procedure was designed to ensure that steady-state levels of the treatment medication and its metabolites were present during the four daily drug and food self-administration sessions each day (Mello et al, 2013a; Mello et al, 2013b; Negus and Mello, 2003; Newman et al, 2010). Varenicline doses in mg/kg/h and the equivalent doses in mg/kg/day were as follows: a varenicline dose of 0.004 mg/kg/h=0.1 mg/kg/day; a varenicline dose of 0.04 mg/kg/h=1.0 mg/kg/day. The total injection volume delivered was 6.9 ml in 69 injections.
The effects of varenicline (0.004 and 0.04 mg/kg/h) on the ascending limb of the nicotine or cocaine+nicotine dose effect curve were studied first to determine which doses of varenicline were effective, and to monitor possible side effects. Subsequently, the most effective doses of varenicline were tested on the dose-effect curve for nicotine alone, and cocaine+nicotine combinations. Each treatment dose was studied for 7–10 days until responding was stable according to the criteria described earlier. Successive varenicline doses were separated by an interval of saline treatment until drug-and food-maintained responding returned to baseline levels. The saline treatment interval was necessary to prevent any carryover effects from the preceding treatment condition.
Data analysis
The primary dependent variables were the total number of drug or saline injections and food pellets earned per day. The number of injections self-administered on the last three days of each treatment condition were averaged for statistical analysis. Repeated measures one-way analysis of variance (ANOVA) was used to determine varenicline's effect on drug self-administration, and food-maintained responding. A significant ANOVA (P<0.05) was followed by Dunnett's post hoc tests. To determine whether the number of injections earned with cocaine+nicotine combinations were significantly higher than either cocaine or nicotine alone, one-way ANOVA with Fisher's LSD post hocs were used. All statistical procedures and figures were drawn using GraphPad Prism v. 6.0.
Drugs
Cocaine HCl was provided by the National Institute on Drug Abuse (Rockville, MD) and prepared in sterile saline (0.9%). (-)-Nicotine hydrogen tartrate was obtained commercially (Sigma-Aldrich, St. Louis, MO) and solubilized in sterile water buffered with NaOH to achieve a pH of 6–7. Varenicline was prepared in sterile water. All IV drugs were sterile-filtered with a 0.22-μm syringe-driven filter. The cocaine+nicotine solutions were combined in the same syringe. Cocaine doses are expressed as the salt form, nicotine doses are expressed as the base. Varenicline was provided by Dr F Ivy Carroll, Center for Organic and Medicinal Chemistry, Research Triangle Institute, Research Triangle Park, NC.
RESULTS
Varenicline's Effects on Self-administration of Cocaine and Nicotine Alone
Cocaine
Varenicline (0.004 and 0.04 mg/kg/h) had no effect on self-administration of a low dose of cocaine (0.0032 mg/kg/inj) in comparison to saline treatment, and there was considerable variability between animals (Figure 1, top left). Varenicline also had no effect on self-administration of a higher dose of cocaine (0.01 mg/kg/inj) (Figure 1, bottom left). Monkeys self-administered all the cocaine available during saline and varenicline treatment at a unit dose of 0.01 mg/kg/inj cocaine.
Figure 1.
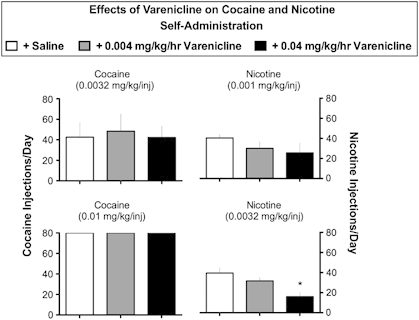
Effects of varenicline on cocaine and nicotine self-administration: each dose of cocaine or nicotine is shown above each set of bar graphs. Ordinate: the number of cocaine or nicotine injections per day for the last three days of the treatment period. Drug self-administration during saline treatment is shown as open bars. Drug self-administration during varenicline treatment is shown as gray bars (0.004 mg/kg/h) and black bars (0.04 mg/kg/h). The top row shows drug self-administration when low minimally reinforcing doses of cocaine and nicotine were available. The bottom row shows drug self-administration when higher reinforcing doses of cocaine and nicotine were available. The ANOVA found a significant effect of varenicline on self-administration of 0.0032 mg/kg/inj nicotine (F(2,6)=6.43; p=0.03) during treatment with 0.04 mg/kg/h varenicline (p=0.02). Varenicline did not significantly alter self-administration of 0.0032 or 0.01 mg/kg/inj cocaine and 0.001 mg/kg/inj nicotine (all Fs<1.73; ps=0.28–0.44). Each data point represents the mean±SEM of 3–4 monkeys. *p<0.05 vs baseline.
Nicotine
When nicotine self-administration was maintained by a low, minimally reinforcing unit dose (0.001 mg/kg/inj), varenicline decreased intake slightly by about 35% (Figure 1, top right). At a nicotine unit dose on the ascending limb of the nicotine dose-effect curve (0.0032 mg/kg/inj), the lower dose of varenicline reduced nicotine self-administration non-significantly (Figure 1, bottom right). The higher varenicline dose reduced nicotine self-administration by 60 percent (P<0.05).
Varenicline's Effects on Self-administration of Cocaine+Nicotine Combinations
A combination of cocaine (0.0032 mg/kg/inj)+nicotine (0.001 mg/kg/inj) maintained high levels of responding during saline control treatment. A low dose of varenicline (0.004 mg/kg/h) did not decrease drug self-administration significantly. A higher dose of varenicline (0.04 mg/kg/h) decreased drug self-administration by 23% (Figure 2, top left).
Figure 2.
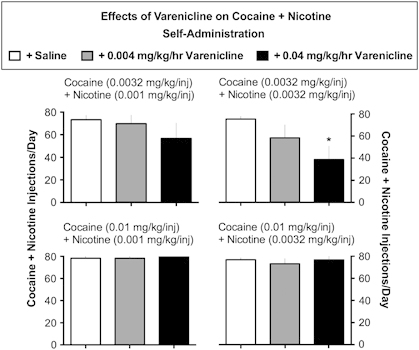
Effects of varenicline on cocaine+nicotine self-administration: each dose of cocaine+nicotine is shown above each set of bar graphs. Ordinate: the number of cocaine+nicotine injections per day for the last three days of the treatment period. Drug self-administration during saline treatment is shown as open bars. Drug self-administration during varenicline treatment is shown as gray bars (0.004 mg/kg/h) and black bars (0.04 mg/kg/h). The top row shows drug self-administration when low minimally reinforcing doses of cocaine+nicotine were available. The bottom row shows drug self-administration when higher reinforcing doses of cocaine+nicotine were available. The ANOVA found a significant effect of varenicline (0.04 mg/kg/h; p=0.025) on self-administration of 0.0032 mg/kg/inj cocaine+0.0032 mg/kg/inj nicotine (F(2,8)=4.936; p=0.04) but not on 0.0032 mg/kg/inj cocaine+0.001 mg/kg/inj nicotine, 0.01 mg/kg/inj cocaine+0.001 mg/kg/inj nicotine, or 0.01 mg/kg/inj cocaine+0.0032 mg/kg/inj nicotine (all Fs<1.08; ps=0.38–0.44). Each data point represents the mean±SEM of 4–5 monkeys. *p<0.05 vs baseline.
A combination of the same dose of cocaine (0.0032 mg/kg/inj) with a higher dose of nicotine (0.0032 mg/kg/inj) also maintained high levels of responding during saline control treatment (Figure 2, top right). Chronic varenicline treatment (0.004–0.04 mg/kg/h) dose-dependently and significantly decreased cocaine+nicotine self-administration (P<0.025) (Figure 2, top right).
A reinforcing dose of cocaine (0.01 mg/kg/inj) combined with the same doses of nicotine (0.0032 and 0.001 mg/kg/inj) maintained high levels of drug self-administration during saline control treatment (Figure 2, bottom row, left+right). Chronic treatment with varenicline had no appreciable effect on drug self-administration (Figure 2, bottom row, left+right).
Food-maintained responding
The effects of saline, cocaine, nicotine, and cocaine+nicotine combinations on food self-administration is summarized in Table 1. Under most conditions, food-maintained responding did not change from baseline levels of 92±8 to 100 pellets per day. Food-maintained responding remained stable at the lower dose of nicotine, but decreased at most by 20% during treatment with 0.4 mg/kg/h varenicline.
Table 1. Effects of Chronic Treatment with Varenicline on Food-maintained Responding.
| +Saline | +0.004 mg/kg/h varenicline | +0.04 mg/kg/h varenicline | ||
|---|---|---|---|---|
| 0.0032 mg/kg/inj | Cocaine | 100+0 | 96.33±3.67 | 78.92±19.77 |
| 0.01 mg/kg/inj | Cocaine | 100+0 | 95.89±4.11 | 93.78±6.22 |
| 0.001 mg/kg/inj | Nicotine | 100+0 | 96.89±3.11 | 77.22±22.78 |
| 0.0032 mg/kg/inj | Nicotine | 100+0 | 99.58±0.42 | 93.92±3.85 |
| 0.0032+0.001 mg/kg/inj | Cocaine+nicotine | 94.80+3.51 | 98.87±1.13 | 90.80±6.73 |
| 0.0032+0.0032 mg/kg/inj | Cocaine+nicotine | 100+0 | 99.07±0.93 | 91.20±6.97 |
| 0.01+0.001 mg/kg/inj | Cocaine+nicotine | 92.00±8.0 | 100±0 | 90.75±9.25 |
| 0.01+0.0032 mg/kg/inj | Cocaine+nicotine | 97.92±2.08 | 99.00±1.0 | 78.58±13.13 |
Mean±SEM number of food pellets earned during the last 3 days of control or varenicline treatment in rhesus monkeys responding for cocaine, nicotine, or cocaine+nicotine polydrug combinations. One-way ANOVAs found that chronic varenicline treatment had no significant effects on food-maintained responding (all Fs<2.77; ps=0.14–0.42).
Comparison of reinforcing effects of cocaine, nicotine, and cocaine+nicotine combinations
Figure 3 shows that the low dose of cocaine (0.0032 mg/kg/inj) alone produced about 50% of the 80 cocaine injections available (38.40±12.01 inj per day), whereas monkeys earned near maximal injections available at a higher unit dose of cocaine (0.01 mg/kg/inj; 79.8±0.20 inj per day). Nicotine alone produced about 40% of the maximal available reinforcers at the low dose (0.001 mg/kg/inj; 33.13±5.93 inj per day) and about 57% of the maximal injections at the higher dose of nicotine (0.0032 mg/kg/inj; 46.13±8.16 inj per day). Each combination of cocaine+nicotine maintained higher levels of drug self-administration (73.3±3.9 and 77.47±1.6 inj per day) than the low dose of cocaine (0.0032 mg/kg/inj) alone or nicotine (0.001 and 0.0032 mg/kg/inj) alone.
Figure 3.
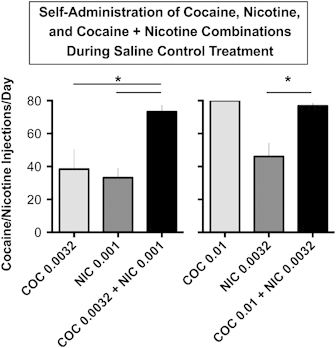
Comparison of the number of injections of cocaine and nicotine alone with cocaine+nicotine combinations during saline control treatment: Abscissa: doses of cocaine (0.0032 or 0.01 mg/kg/inj) shown as light gray rectangles; doses of nicotine (0.001 or 0.0032 mg/kg/inj) shown as dark gray rectangles; and the same doses of cocaine and nicotine in combination are shown as black rectangles. Ordinate: number of drug injections per day during saline treatment. Each data point is the average (±SEM) of 5 monkeys during the last three days of each drug condition. One-way repeated measures analysis of variance (ANOVA) with Fisher's LSD post hoc tests were used to determine whether self-administration of the cocaine plus nicotine combinations was significantly higher than the same doses of nicotine or cocaine alone as measured by the number of injections per day. The ANOVAs found a significant main effect of reinforcer when 0.0032 mg/kg cocaine, 0.001 mg/kg/inj nicotine, and 0.0032 mg/kg/inj cocaine+0.001 mg/kg/inj nicotine were compared (F(2,8)=5.495; p=0.03) and when 0.01 mg/kg/inj cocaine, 0.0032 mg/kg/inj nicotine, and 0.01 mg/kg/inj cocaine+0.0032 mg/kg/inj nicotine were compared (F(2,8)=16.97; p=0.001). Post hoc tests found that self-administration of the 0.0032 mg/kg/inj cocaine+0.001 mg/kg/inj nicotine combination was significantly greater than either 0.0032 mg/kg/inj cocaine or 0.001 mg/kg/inj nicotine alone (ps=0.029 and 0.016, respectively). Further, self-administration of 0.01 mg/kg/inj cocaine+0.0032 mg/kg/inj nicotine was significantly greater than 0.0032 mg/kg/inj nicotine alone (p=0.0025) but not 0.01 mg/kg/inj cocaine alone (p−0.725) *=p<0.05.
DISCUSSION
This is the first report of the effects of chronic varenicline treatment on the reinforcing effects of nicotine alone, cocaine alone, and a combination of nicotine+cocaine in rhesus monkeys. Our major findings were that chronic varenicline treatment dose-dependently and selectively reduced self-administration of nicotine alone and combinations of nicotine+a low dose of cocaine. However, varenicline did not reduce self-administration of cocaine alone or the same doses of nicotine in combination with a high dose of cocaine. Another major finding was that combining low, minimally reinforcing doses of nicotine and cocaine increased drug self-administration significantly above levels maintained by the same dose of each drug alone. These data confirm and extend our previous reports of the behavioral effects of low dose nicotine+cocaine combinations in rhesus monkeys (Mello et al, 2013a; Mello and Newman, 2011). Taken together with our earlier report of the effects of buspirone on nicotine+cocaine combinations (Mello et al, 2013a), these findings indicate that this polydrug model is useful for the evaluation of treatment medications that may attenuate dual addiction to nicotine and cocaine. The relation of these findings to some previous studies of varenicline, and possible mechanisms accounting for varenicline's divergent effects on nicotine and cocaine self-administration are discussed below.
Varenicline and Cocaine Interactions
Clinical and preclinical studies of varenicline's effects on the abuse-related effects of cocaine have inconsistent results. Our finding that varenicline did not reduce cocaine self-administration is consistent with one clinical study (Poling et al, 2010), and one study in rhesus monkeys (Gould et al, 2011). Methadone-maintained cocaine users and cigarette smokers reported that varenicline (2 mg or 0.028 mg/kg in a 70 kg man) had no effect on cocaine use, but decreased cigarette smoking by 52.8%, whereas placebo treatment decreased cigarette smoking by 8% (Poling et al, 2010). In rhesus monkeys, varenicline at doses of 0.03 and 0.1 mg/kg, PO (salt) had no effect on cocaine self-administration, and higher doses (0.3–0.56 mg/kg, PO), potentiated cocaine's reinforcing effects. When varenicline was administered intravenously (0.1–0.3 mg/kg), it also potentiated cocaine's discriminative stimulus effects, but did not substitute for cocaine in drug discrimination or drug self-administration studies (Gould et al, 2011). Comparisons between these studies in rhesus monkeys are limited by differences in schedules of reinforcement, drug doses, route and frequency of varenicline administration, and frequency of access to cocaine.
In contrast, treatment-seeking cocaine-dependent volunteers significantly reduced cocaine use (as assessed by three urine samples per week) during 8 weeks of varenicline treatment (0.5–2 mg/kg) in comparison to placebo treatment (Plebani et al, 2012). In rats, varenicline (2.0 mg/kg SC) also decreased cocaine self-administration (0.75 mg/kg, inj) maintained on a fixed ratio 1 (FR1) schedule of reinforcement, but other doses (0.3 and 1.0 mg/kg, SC) had no effect (Guillem and Peoples, 2010). Varenicline had dose-related, but opposite effects on cue and drug-induced reinstatement. A high dose of varenicline increased whereas low doses decreased cocaine-seeking behavior in a reinstatement paradigm (Guillem and Peoples, 2010).
Varenicline and Nicotine Interactions
Varenicline significantly and selectively reduced self-administration of nicotine and nicotine+low dose cocaine combinations, with no significant changes in concurrent food-maintained responding. The selective and sustained decreases in nicotine and nicotine+cocaine self-administration were due to varenicline treatment and not to sedation or a general disruption of operant responding. All monkeys resumed drug self-administration at baseline levels after varenicline treatment was discontinued. This indicates that IV catheters were patent and catheter malfunction did not account for the observed decreases in drug-maintained responding. The same monkeys were studied as their own control across successive saline and varenicline treatment conditions. Importantly, this was the first study of nicotine and nicotine+cocaine self-administration in which varenicline was administered every 20 min for 23 h each day to insure that treatment doses were present during each of the 4 daily drug and food self-administration sessions. These data are consistent with our previous reports that chronic buspirone treatment significantly and selectively reduced nicotine and cocaine+nicotine self-administration by rhesus monkeys (Mello et al, 2013a).
Varenicline has activity at several receptor subtypes that comprise nAChRs. The α4β2 receptor is one of the most abundant, and appears to be an important modulator of the reinforcing effects of nicotine (Benowitz, 2009; Picciotto et al, 1998; Rose, 2007; Watkins et al, 2000). Nicotine did not stimulate dopamine release and did not maintain nicotine self-administration in β2 knockout mice in contrast to wild-type mice (Picciotto et al, 1998). Varenicline also acts as a partial agonist at α6β2* nAChRs, and its binding affinity was very similar to that of α4β2* nAChRs (Bordia et al, 2012; Grady et al, 2010). In competition binding studies in monkey striatum, varenicline was ∼6 times more potent than nicotine at α6β2* and α4β2* nAChRs (Bordia et al, 2012). The similarities between varenicline's effects at α6β2* and α4β2* nAChRs suggests that both receptor subtypes are important for its anti-smoking effects (Bordia et al, 2012). Consistent with this interpretation, several recent reports show that α6β2* nAChRs are an important modulator of dopamine neurotransmission (Exley et al, 2013; Wickham et al, 2013) For review, see (Quik and Wonnacott, 2011).
Although varenicline is a full agonist at α7 nicotinic receptors (Coe et al, 2005; Mihalak et al, 2006), the contribution of α7 receptors to varenicline's effects on nicotine self-administration is unclear (Shama and Vijayaraghavan, 2008). A series of novel compounds with high affinity for α4β2 nicotinic receptors showed dose-dependent and complete substitution for the nicotine discriminative stimulus in rats (Smith et al, 2007). Other novel compounds that were selective for α7 and β4 nicotinic receptors did not substitute for nicotine. These data suggested that α4β2 receptors are critical for the discriminative stimulus properties of nicotine, whereas β4 and α7 receptors are not involved (Smith et al, 2007). In mice given oral access to nicotine over the course of 5 months, the α7-knockout mice decreased nicotine consumption in comparison to wild-type controls, whereas β2 receptor-knockout mice increased nicotine consumption (Levin et al, 2009). These data were interpreted to suggest that α7 receptor antagonists might be useful to treat nicotine addiction (Levin et al, 2009). It is likely that varenicline's actions as an α4β2 partial agonist are more important than its α7 agonist activity in reducing nicotine self-administration.
Nicotine-Cocaine Interactions
It is generally agreed that cocaine maintains higher levels of IV self-administration than nicotine in nonhuman primates (Le Foll et al, 2007; Mello et al, 2013a; Mello et al, 2013b; Mello and Newman, 2011). For example, a nicotine dose at the peak of the dose-effect curve (0.0032 mg/kg/inj) maintained 46 injections per day, whereas a cocaine dose at the peak of the dose-effect curve (0.032 mg/kg/inj) maintained 76 injections per day in the same rhesus monkeys (Mello and Newman, 2011). When marginally reinforcing doses of cocaine (0.0032 mg/kg/inj), and nicotine (0.001 mg/kg/inj) were combined, the number of drug injections per day was significantly higher than for either drug alone (Mello et al, 2013a; Mello and Newman, 2011). Similarly, in the present study, a combination of the same doses of cocaine and nicotine maintained higher levels of self-administration than either drug alone (Figure 3).
The extent to which simultaneous activation of dopamine release by nicotine and blockade of dopamine reuptake by cocaine may account for enhancement of the reinforcing effects of low doses of nicotine+cocaine in combination is unclear. However, evidence from microdialysis studies indicates that combinations of equi-potent doses of cocaine+nicotine produce additive effects on dopamine release (Gerasimov et al, 2000; Sziraki et al, 1999; Zernig et al, 1997). In addition, overlapping patterns of fos-related protein expression in rat brains after nicotine (0.03 mg/kg/inj) and cocaine (0.25 mg/kg/inj) self-administration, but not after saline control self-administration, were interpreted to suggest that there is a common anatomical substrate for cocaine and nicotine addiction (Pich et al, 1997). In mice, exposure to nicotine increased cocaine-induced locomotor activity and conditioned place preference in comparison to placebo, but exposure to cocaine did not enhance nicotine-induced behaviors (Levine et al, 2011). These findings were interpreted as evidence that nicotine alters the brain to increase its susceptibility to cocaine by increasing synaptic plasticity and increasing FosB responses to cocaine, secondary to higher histone acetylation levels in the striatum (Levine et al, 2011). Importantly, the priming influence of nicotine on cocaine's effects only occurred when multiple doses of nicotine were given concurrently with cocaine, but not after a 14-day nicotine-free interval (Levine et al, 2011). It remains to be determined if this molecular mechanism accounts for the enhanced reinforcing effects of nicotine+cocaine combinations in drug-experienced rhesus monkeys.
The Role of Dopamine in Nicotine-Cocaine Interactions
Interpretation of the differences in varenicline's effects on nicotine and cocaine is complicated by the fact that all three drugs activate the mesolimbic dopamine system and increase extracellular dopamine levels by different mechanisms. Nicotine and varenicline induce dopamine release by stimulating nAChRs on the cell bodies of mesolimbic dopamine neurons (Coe et al, 2005; Corrigall et al, 1994; DiChiara, 2000; DiChiara and Imperato, 1988; Nisell et al, 1994; Stolerman and Shoaib, 1991; Watkins et al, 2000), whereas cocaine blocks dopamine reuptake by the dopamine transporter (Kuhar et al, 1991; Ritz et al, 1987, 1988). When varenicline was combined with nicotine, dopamine release was reduced to the level measured after varenicline alone, with a corresponding decrease in nicotine self-administration (Coe et al, 2005; Rollema et al, 2007a). A parsimonious explanation of varenicline's lack of effects on cocaine self-administration could be that because cocaine-induced increases in extracellular dopamine levels occur independently of nAChR stimulation of mesolimbic dopamine neurons, the resulting dopamine levels were sufficient to maintain drug self-administration.
The importance of dopamine in the reinforcing effects of nicotine was initially suggested by the fact that dopamine D1-like and D2-like receptor antagonists, as well as nicotinic receptor antagonists, reduced nicotine self-administration in preclinical studies (Pierce and Kumaresan, 2006; Watkins et al, 2000). There has been increasing interest in the possible role of dopamine D3 and D4 receptor antagonists and partial agonists for the treatment of both cocaine and nicotine addiction (Newman et al, 2012). There is evidence that D3 antagonists can modify some of the abuse-related properties of both nicotine (Andreoli et al, 2003; Khaled et al, 2010; Pak et al, 2006; Ross et al, 2007; Spiller et al, 2008) and cocaine (see for review (Heidbreder et al, 2005; Heidbreder and Newman, 2010; Le Foll et al, 2005; Newman et al, 2012; Newman et al, 2009; Newman et al, 2005). It has been suggested that dopamine D3 receptor antagonists may be valuable for the treatment of relapse to nicotine seeking, but are less likely to substitute for nicotine or to attenuate withdrawal signs and symptoms (Le Foll et al, 2007). The contrast between the effects of buspirone and varenicline on cocaine, nicotine, and nicotine+cocaine further suggests the possible importance of the dopamine D3 and D4 antagonist component of buspirone (Mello et al, 2013a; Mello et al, 2013b).
Translational Implications of Varenicline's Reduction of Nicotine and Nicotine+Cocaine Self-Administration
Varenicline is FDA approved for the treatment of cigarette smoking, and this will greatly facilitate examining its clinical effectiveness in persons with dual addiction to nicotine and cocaine. Cocaine abusers are often heavy smokers, and a medication that could safely reduce both cocaine abuse and cigarette smoking, with minimal side effects would be clinically useful. Clinical laboratory studies also have consistently shown interactions between nicotine and cocaine. In cocaine-dependent cigarette smokers, an acute dose of transdermal nicotine (44 mg) enhanced reports of cocaine craving induced by visual cues and paraphernalia related to crack-cocaine, whereas placebo patches had no effect (Reid et al, 1998). In a subsequent study of cue-induced cocaine craving, a nicotine antagonist, mecamylamine reduced reports of craving in comparison to placebo (Reid et al, 1999). As noted earlier, cocaine users report smoking more cigarettes during cocaine use (Roll et al, 1996; Roll et al, 1997) and smokers use more cocaine than non-smokers (Budney et al, 1993). The conflicting clinical data on varenicline's effects on cocaine use (Plebani et al, 2012; Poling et al, 2010) suggest that further studies are warranted. Although varenicline may not be equally effective in all smokers, these studies in rhesus monkeys suggest it may be useful for some smokers, especially those who use low doses of cocaine recreationally.
FUNDING AND DISCLOSURE
The authors declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. The authors declare no conflict of interest.
Acknowledgments
We thank Sherilyn Pattel and Olga Smirnova for excellent technical assistance. Supported in part by grants R01-DA02519 and R01-DA026892 (NK Mello, PI); R01-DA12001 (FI Carroll, PI) from the National Institute on Drug Abuse, NIH.
References
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology (Berl) 2002;162:178–185. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Phys Chem. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Hrachova M, Chin M, McIntoch JM, Quik M. Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther. 2012;342:327–334. doi: 10.1124/jpet.112.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- CDC Cigarette smoking among adults - United States. 2000. MMWR Morb Mortal Weekly Report. 2002;51:642–645. [PubMed] [Google Scholar]
- CDC Adult cigarette smoking in the United States: current estimates. National Center for Chronic Disease Prevention and Health Promotion Tobacco Information and Prevention Source (TIPS) 2004;15:20–58. [Google Scholar]
- CDC Annual smoking-attributable mortality, years of potential life ost and productivity losses - United States, 1997-2001. MMWR Morb Mortal Weekly Report. 2005;54:625–628. [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on proloonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. 2013;70:522–533. doi: 10.1001/jamapsychiatry.2013.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- DAWN 2010Drug Abuse Warning Network, 2010: Area Profiles of Drug-Related Mortality Substance Abuse and Mental Health Services Administration: Rockville, MD; Vol Series D-36HHS Publication No. (SMA) 12-4699294 [Google Scholar]
- DiChiara G. Role of dopamine in the behavioural actions of nicotine related to addiction (In Process Citation) Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- DiChiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- DiChiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Franklin M, Bermudez I, et al. 2013Striatal dopamine transmission is reduced after chronic nicotine with a decrease in α6-nicotinic receptor control in nucleus accumbens Eur J Neurosc10 July 2013 doi: 10.1111/ejn.12298(e-pub ahead of print). [DOI] [PubMed]
- Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet. 2010;49:799–816. doi: 10.2165/11537850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Fivel PA. Computer-controlled drug doses for IV drug self-administration. Exp Clin Psychopharmacol. 2011;19:131–133. doi: 10.1037/a0023037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Woolverton WL. Self-administration of cocaine and nicotine mixtures by rhesus monkeys. Psychopharmacology. 2009;207:99–106. doi: 10.1007/s00213-009-1637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, Koob GF. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology. 2011;213:715–722. doi: 10.1007/s00213-010-2024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL. Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synapse. 2000;38:432–437. doi: 10.1002/1098-2396(20001215)38:4<432::AID-SYN8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharm. 2007;74:1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Gould RW, Czoty PW, Nader SH, Nader MA. Effects of varenicline on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2011;339:678–686. doi: 10.1124/jpet.111.185538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Federov NB, et al. Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58:1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Peoples LL. Varenicline effects on cocaine self administration and reinstatement behavior. Biochem Pharm. 2010;21:96–103. doi: 10.1097/FBP.0b013e328336e9c5. [DOI] [PubMed] [Google Scholar]
- Han ZY, Le Novere N, Zoli M, Hill JAJ, Champtiaux N, Changeux JP. Localization of nAChR subunit mRNAs in the brain of macaca mulatta. Eur J Neurosc. 2000;12:3664–3674. doi: 10.1046/j.1460-9568.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- Han ZY, Zoli M, Cardona A, Bourgeois JP, Changeux JP, Le Novere N. Localization of [3H]Nicotine, [3H]Cytisine, [3H]Epibatidine, and [125I]a-Bungarotoxin Binding Sites in the Brain of Macaca mulatta. J Comp Neurol. 2003;461:49–60. doi: 10.1002/cne.10659. [DOI] [PubMed] [Google Scholar]
- Hawk LWJ, Ashare RL, Lohnes SF, Schlienz nJ, Rhodes JD, Tiffany ST, et al. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clin Pharmacol Ther. 2012;91:172–180. doi: 10.1038/clpt.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JT, Ebbert JO, Sood A. Efficacy and safety of varenicline for smoking cessation. Am J Med. 2008;121:S32–S42. doi: 10.1016/j.amjmed.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi Z-X, Thanos PK, Mugnaini M, Hagan JJ, et al. The role of central dopamine D3 receptors in drug addiciton: a review of pharmacological evidence. Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann NY Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Shiffman S, Ferguson SG, Gritz ER. Tobacco dependence and withdrawal: science base, challenges and opportunities for pharmacotherapy. Pharmacol Ther. 2009;123:1–16. doi: 10.1016/j.pharmthera.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology (Berl) 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays LT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. J Am Med Assoc. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Brooks EA, Kynaston AD, Rice KC, Woods JH. Patterns of nicotinic receptor antagonism: Nicotine discriminative studies. J Pharmacol Exp Ther. 2011;380:194–203. doi: 10.1124/jpet.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled MATM, Araki KF, Li B, Coen KM, Marinelli PW, Varga J, et al. The selective dopamine D3 receptor antagonist SB-277011A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacol. 2010;13:181–190. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M.(eds) (2006Neurobiology of Addiction Academic Press: London; 490 [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, et al. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacology. 2011;15:1265–1274. doi: 10.1017/S1461145711001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. Dopamine D3 receptor ligands for the treatment of tobacco dependence. Expert Opin Investig. 2007;16:45–57. doi: 10.1517/13543784.16.1.45. [DOI] [PubMed] [Google Scholar]
- Lesage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav. 2009;91:461–467. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, et al. Nicotinic α7 or β2-containing receptor knockout: Effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Huang Y, Drisaldi B, Griffin EA, Pollack DD, Xu S, et al. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3:107–109. doi: 10.1126/scitranslmed.3003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line SW. Environmental enrichment for laboratory primates. J Am Vet Med Assoc. 1987;190:854–859. [PubMed] [Google Scholar]
- Mello NK.2005Marian W. Fischman Memorial Lecture (2004). Evaluation of drug abuse treatment medications: Concordance between clinical and preclinical studiesIn: Dewey WL, (ed)Problems of Drug Dependence 2004: Proceedings of the 66th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc U.S. Department of Health and Human Services, National Institutes of Health: Bethesda, MD; 82–104. [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Fivel PA, Kohut SJ. Effects of chronic buspirone treatment on nicotine and concurrent nicotine+cocaine self-administration. Neuropsychopharmacology. 2013;38:1264–1275. doi: 10.1038/npp.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Fivel PA, Kohut SJ, Bergman J. Effects of chronic buspirone treatment on cocaine self-administration. Neuropsychopharmacology. 2013;38:455–467. doi: 10.1038/npp.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH.2013Cocaine and other commonly abused drugsIn: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, (eds)Harrison's Principles of Internal Medicine19th EditionMcGraw-Hill Co.: New York; (in press. [Google Scholar]
- Mello NK, Mendelson JH, Kelly M, Diaz-Migoyo N, Sholar JW. The effects of chronic cocaine self-administration on the menstrual cycle in rhesus monkeys. J Pharmacol Exp Ther. 1997;281:70–83. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opiate abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, Lukas SE, Mendelson JH, Sholar JW, Drieze JM. A primate model of polydrug abuse: cocaine and heroin combinations. J Pharmacol Exp Ther. 1995;274:1325–1337. [PubMed] [Google Scholar]
- Mello NK, Newman JL. Discriminative and reinforcing stimulus effects of nicotine, cocaine, and cocaine+nicotine combinations in rhesus monkeys. Exp Clin Psychopharmacol. 2011;19:203–214. doi: 10.1037/a0023373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alc Depend. 2003;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P. Medication discovery for addiction: Translating the dopamine D3 receptor hypothesis. Biochem Pharm. 2012;84:992–890. doi: 10.1016/j.bcp.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Cyriac G, Deschamps JR, Taylor M, Kumar R, et al. N-(4-(4-(2,3-Dichloro- or 2-methoxyphenyl)piperazin-1-yl)butyl)heterobiarylcarboxamides with functionalized linking chains as high affinity and enantioselective D3 receptor antagonists. J Med Chem. 2009;52:2559–2570. doi: 10.1021/jm900095y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- Newman JL, Negus SS, Lozama A, Prisinzano TE, Mello NK. Behavioral evaluation of modafinil and the abuse-related effects of cocaine in rhesus monkeys. Exp Clin Psychopharmacol. 2010;18:395–408. doi: 10.1037/a0021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Jones C, Kirkpatrick P. Varenicline. Nature Rev. 2006;5:537–538. doi: 10.1038/nrd2088. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- O'Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology. 2010;208:365–376. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- Pak AC, Ashby CR, Heidbreder CA, Pilla M, Gilbert JG, Xi X-Z, et al. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:1–18. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Min W, Hackett A, Lowe D, Hanania T, Caldarone BJ, et al. The high-affinity nAChR agonists varenicline and sazetidine-A exhibit reinforcing properties in rats. Prog Neuropsychopharmacol & Biol Psych. 2010;34:1455–1464. doi: 10.1016/j.pnpbp.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB. Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res. 1991;539:94–102. doi: 10.1016/0006-8993(91)90690-w. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Lynch KG, Yu Q, Pettinati HM, O'Brien CP, Kampman KM. Results of an initial clinical trial of varenicline for the treatment of cocaine dependence. Drug Alc Depend. 2012;121:163–166. doi: 10.1016/j.drugalcdep.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. Am J Addict. 2010;19:401–408. doi: 10.1111/j.1521-0391.2010.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JD, Koustova E, Hoffman A, Shurtleff D, Volkow ND. Treatments for nicotine addiction should be a top priority. Lancet. 2009;374:513–514. doi: 10.1016/S0140-6736(09)60352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Wonnacott S. α6β2* and α4β2* nicotinic acetylcholine receptors as drug targets for Parkinson's Disease. Pharmacol Rev. 2011;63:938–966. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Barr JD, Bevins RA. Extinction with varenicline and nornicotine, but not ABT-418, weakens conditioned responding evoked by the interoceptive stimulus effects of nicotine. Neuropharmacology. 2010;58:1237–1245. doi: 10.1016/j.neuropharm.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alc Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine self-administration appears to be mediated by dopamine uptake inhibition. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:233–239. doi: 10.1016/0278-5846(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Depend. 1996;40:195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Tidey J. Cocaine use can increase cigarette smoking: evidence from laboratory and naturalistic settings. Exp Clin Psychopharmacol. 1997;5:263–268. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rose JE. Multiple brain pathways and receptors underlying tobacco addiction. Biochem Pharmacol. 2007;74:1263–1270. doi: 10.1016/j.bcp.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Ross JT, Corrigall WA, Heidbreder CA, LeSage MG. Effects of the selective dopamine D3 receptor antagonist SB-277011A on the reinforcing effects of nicotine as measured by a progressive-ratio schedule in rats. Eur J Clin Pharmacol. 2007;559:173–179. doi: 10.1016/j.ejphar.2007.01.004. [DOI] [PubMed] [Google Scholar]
- SAMSHA (ed) (2012Substance Abuse and Mental Health Services Administration, Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-44, HHS Publication No. (SMA) 12-4713: Rockville, MD.
- Shama G, Vijayaraghavan S. Nicotinic receptors containing the a7 subunit: a model for rational drug design. Curr Med Chem. 2008;15:2921–2932. doi: 10.2174/092986708786848703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, et al. Ligands selective for α4β2 but not α3β4 or α7 nicotinic receptors generalise to the nicotine discriminative stimulus in rats. Psychopharmacology. 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi XZ, Peng XQ, Newman AH, Ashby CRJ, Heidbreder C, et al. The selective dopamine D3 receptor antagonists SB-277011A and NGB 2904 and the putative partial D3 receptor agonist BP-897 attenuate methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology. 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends Pharmacol Sci. 1991;12:467–473. doi: 10.1016/0165-6147(91)90638-9. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Sershen H, Benuck M, Hashim A, Laitha A. Differences in receptor system participation between nicotine- and cocaine-induced dopamine overflow in nucleus accumbens. NY Acad Sci. 1999;877:800–802. doi: 10.1111/j.1749-6632.1999.tb09326.x. [DOI] [PubMed] [Google Scholar]
- Tonstad S. Smoking cessation efficacy and safety of varenicline, an alpha4beta2 nicotinic receptor partial agonist. J Cardiovasc Nurs. 2006;21:433–436. doi: 10.1097/00005082-200611000-00004. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol. 2007;15:309–327. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- Wickham R, Solecki W, Rathbun L, McIntosh JM, Addy NA. Ventral tegmental area α6β2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core. Psychopharmacology. 2013;229:73–82. doi: 10.1007/s00213-013-3082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegerman M, van Mourik Y, Schetters D, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology. 2011;216:267–277. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, O'Laughlin IA, Fibiger HC. Nicotine and heroin augment cocaine-induced dopamine overflow in nucleus accumbens. Eur J Pharmacol. 1997;337:1–10. doi: 10.1016/s0014-2999(97)01184-9. [DOI] [PubMed] [Google Scholar]


