Abstract
An improved enzyme-linked immunosorbent (ELISA) assay using one-step antibody immobilization has been developed for the detection of human fetuin A (HFA), a specific biomarker for atherosclerosis and hepatocellular carcinoma. The anti-HFA formed a stable complex with 3-aminopropyltriethoxysilane (APTES) by ionic and hydrophobic interactions. The complex adsorbed on microtiter plates exhibited a detection range of 4.9 pg mL−1 to 20 ng mL−1 HFA, with a limit of detection of 7 pg mL−1. Furthermore, an analytical sensitivity of 10 pg mL−1 was achieved, representing a 51-fold increase in sensitivity over the commercial sandwich ELISA kit. The results obtained for HFA spiked in diluted human whole blood and plasma showed the same precision as the commercial kit. When stored at 4°C in 0.1 M phosphate-buffered saline (PBS, pH 7.4), the anti-HFA bound microtiter plates displayed no significant decrease in their functional activity after two months. The new ELISA procedure was extended for the detection of C-reactive protein, human albumin and human lipocalin-2 with excellent analytical performance.
ELISA is the gold standard of in vitro diagnostics (IVD) during the last five decades for analysis of biomarkers and important analytes in healthcare and diversified analytical settings. With over 300,000 peer-reviewed articles to date, ELISA-based technologies have open up a lucrative, commercial market. Despite ongoing developments in immunosensors, labs-on-chips, and microfluidic and point-of-care technologies, ELISA with high throughput and omnipotent nature has been unmatched in reliability for the monitoring and management of disease markers. It is still the most widely used immunoassay format by pharmaceutical industries for routine monitoring of drugs and drug impurities (e.g. Chinese hamster ovary protein and monocyte chemotactic protein). Competing immunoassay technology must be compared to ELISA for precision and other analytical parameters.
Defined plasma biomarkers are of unique diagnostic relevance for early preventive intervention in chronic inflammatory diseases, highly prevalent in the Western world. One of those biomarkers is HFA where a highly sensitive and rapid assay is of value when combined with sensitive measurements of C-reactive protein1. HFA is a product of the liver and its concentration decreases during the acute phase reaction. Due to its anti-inflammatory properties by counteracting proinflammatory cytokine production, quantification in body fluids is highly relevant in guiding diagnostics and therapy of infection-independent diseases of liver, heart and vasculature. In vivo, HFA functions as an inhibitor of soft tissue calcification and is a specific biomarker for hepatocellular carcinoma2 and atherosclerosis3, and associated with arthritis4, cardiovascular diseases5,6,7, malaria8, diabetes9, and metabolic syndrome10, as well as neurological diseases such as multiple sclerosis11.
The last two decades have witnessed considerable advances in the development of improved immunoassay procedures for IVD including improved antibody (Ab) immobilization chemistries, signal enhancement strategies using micro-/nanomaterials or polymers, novel lab-on-a-chip technologies, biosensors and novel immunoassay formats. In all cases, the development of an appropriate Ab immobilization strategy is a critical requirement that significantly affects the analytical performance of an immunoassay12,13,14. Examples range from adsorption, oriented binding using fragment crystallizable (Fc) proteins, covalent binding using cross-linkers, site-directed immobilization, and non-covalent binding15,16,17,18,19,20,21. However, most of these procedures employ a complex multi-step procedure involving costly crosslinking agents.
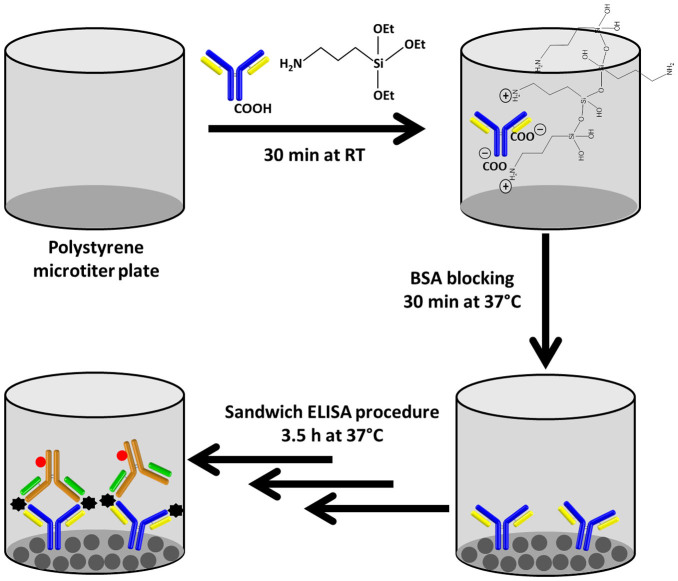
This article describes a simple immobilization-based sandwich ELISA procedure for the development of rapid, low-cost and highly-sensitive IVD kits. The new immobilization format (NIF) only involves the dilution of the antibody in APTES to form a stable complex. APTES-polymer/Ab complexes sorbed on the microtiter plate (MTP) will be evaluated with respect to detection limit, analytical sensitivity, storage stability and its applicability for detecting HFA in human whole blood and plasma.
Results
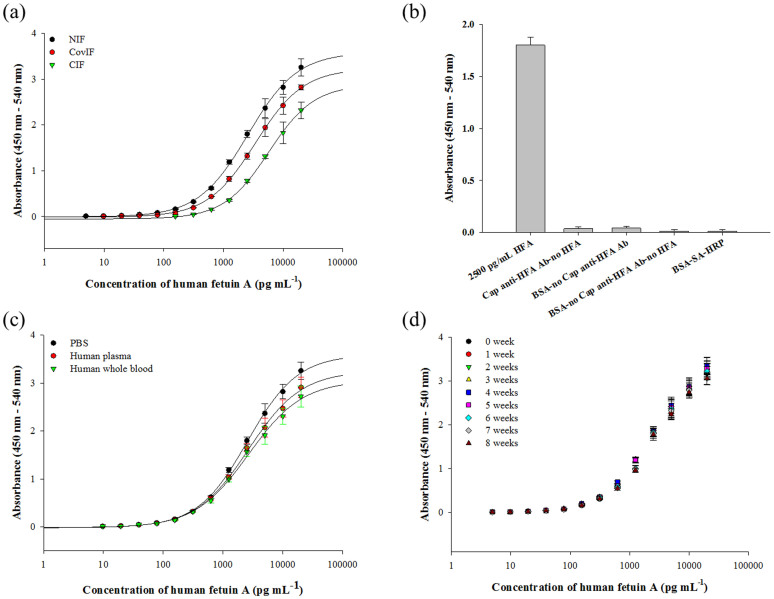
In this procedure, the capture Ab was admixed with 1% (v/v) APTES (1:1 v/v mixture), dispensed into the MTP wells and incubated for 30 min (Figure 1). Under the optimized condition (Supplementary Figures S1a–b), HFA from 4.9 to 20,000 pg mL−1 was detected with linearity of 156 to 20,000 pg mL−1 (Figure 2a). The estimated limit of detection (LOD) and analytical sensitivity were 7 pg mL−1 and 10 pg mL−1, respectively. The intraday variability of five assay repeats (in triplicate) in a single day ranged from 1.2 to 8.5, while the interday variability of five assay repeats (in triplicate) on five consecutive days was between 2.1 to 10.2. With a maximal half-effective concentration (EC50) of 2.6 ng mL−1, the NIF was highly specific to HFA without any interference from immunological reagents in different process controls (Figure 2b).
Figure 1. One-step antibody immobilization-based sandwich ELISA procedure for the detection of human fetuin A (HFA).
Figure 2. One-step antibody (Ab) immobilization-based sandwich ELISA.
(a) Detection of HFA by the NIF, covalent immobilization format (CovIF)-based5,9 and conventional immobilization format (CIF)-based (passive adsorption-based) sandwich ELISA procedures. (b) Specific HFA detection with respect to various experimental process controls. (c) Detection of HFA spiked in PBS (0.1 M, pH 7.4), diluted human whole blood and diluted human plasma. (d) Detection of HFA by the anti-HFA Ab-bound MTPs stored in 0.1 M PBS, pH 7.4 at 4°C for 8 weeks. All experiments were done in triplicate with the error bars representing the standard deviation.
The NIF was compared with a commercial ELISA kit and a typical sandwich ELISA procedure with covalently cross-linked Ab on APTES-functionalized surfaces17,22. All immunoassays were performed under the same conditions with the same assay components to minimize experimental variability. The NIF outperformed conventional immobilization format (CIF)-based sandwich ELISA with 28-fold faster Ab immobilization and 51-fold more sensitivity. Compared to the covalent immobilization format (CovIF) that involves the covalent crosslinking of Ab to the APTES-functionalized MTP, it is still 5-fold faster in terms of Ab immobilization and 3-fold more sensitive (Table 1, Supplementary Table S1). The Ab immobilization density of the NIF estimated by bicinchoninic acid protein assay was compared favorably with the results obtained by the CIF, CovIF and a new covalent immobilization format (NCIF) (which involves covalent antibody immobilization involving 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC)-activated anti-HFA Ab diluted in APTES (for covalent binding of Ab to APTES into KOH-pretreated MTP wells) (Supplementary Figure S2). Hence, the NIF is the most sensitive with minimal reagents and analysis time for the detection of HFA compared to various ELISA formats.
Table 1. Analytical comparison of the NIF with the conventional immobilization format (CIF) and covalent immobilization format (CovIF)-based5,9 sandwich ELISAs for the detection of HFA.
| NIF1 | CovIF2 | CIF3 | |
|---|---|---|---|
| Time required for antibody immobilization (h) | 0.5 | 2.5 | 14 |
| Assay duration (h) | ~4 | ~6 | ~20 |
| Detection range (pg mL−1) | 4.9–20,000 | 9.8–20,000 | 151–20,000 |
| LOD (pg mL−1) | 7 | 12 | 226 |
| Analytical sensitivity (pg mL−1) | 10 | 30 | 510 |
| EC50 (ng mL−1) | 2.6 | 3.4 | 5.8 |
| % CV | |||
| Intra-day (n = 5) | 1.2–8.5 | 2.4–10.4 | 4.7–17.4 |
| Inter-day (n = 5) | 2.1–10.2 | 1.7–17.6 | 3.6–20.0 |
| Assays on various substrates | Yes | Yes | No |
| Requirement for crosslinkers | No | Yes | No |
1NIF: new immobilization format by diluting antibody in APTES followed by physical adsorption on the microtiter plate (MTP).
2CovIF: covalent immobilization format by covalent crosslinking of antibody on an APTES modified MTP surface with EDC.
3CIF: conventional immobilization format by physical adsorption of antibody on the MTP.
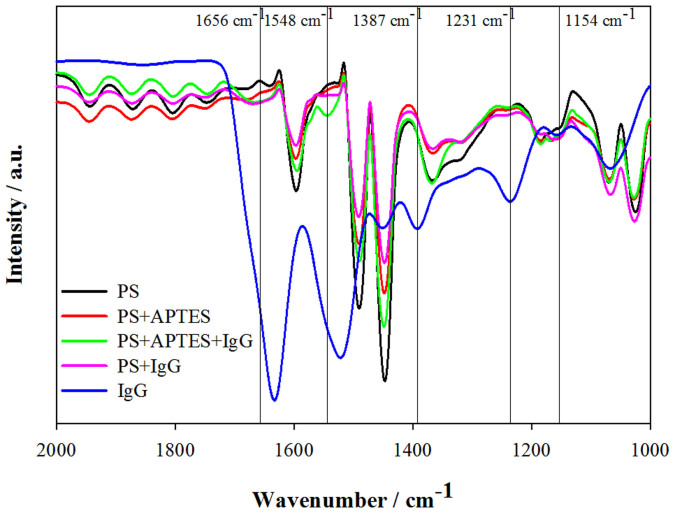
Central to the NIF is the sorption of the APTES-polymer/Ab complex to the MTP surface. To elucidate the binding of the complex to a polystyrene (PS) MTP in the absence of a crosslinker, immunoglobulin G (IgG) bovine was used as the model. PS beads were immersed in a solution of APTES/IgG in PBS buffer (0.2 mg/mL IgG and 0.5% APTES) for 30 min. The IgG/APTES solution was decanted and the resulting PS beads were washed five times with PBS buffer, followed by three times with deionized water to remove residual Na2HPO4 and KH2PO4 salts that will interfere with Fourier transform infrared spectroscopy (FTIR) and energy-dispersive X-ray (EDX) analyses. The IgG concentration was increased to provide greater peak intensity for IgG bands in the FTIR spectra for facilitating bonding elucidation. In aqueous solution, APTES reacts with the free hydroxyls of an oxidized substrate by SN2 exchange with loss of ethanol. The next step is condensation that leads to polymerization when an APTES molecule forms a siloxane with its neighboring APTES (Figure 1).
Figure 3 shows five FTIR spectra: PS, PS+APTES, PS+APTES+IgG, PS+IgG and IgG. PS exhibits the usual FTIR peaks consistent for all PS derivatives. Upon addition of APTES, the spectrum shows the Si-O-Si character at 1154 cm−1, with 1671 cm−1 and a broad 3400 cm−1 peaks for the primary NH, confirming the presence of APTES and its polymerization17,23. For derivatives with IgG, additional bands for the amides at 1656, 1548 cm−1 with broad OH, NH bands centered at 3305 cm−1 that match with the IgG spectrum, although the two peaks for IgG at 1387 and 1231 cm−1 have diminished in those PS derivatives. The bands associated with the PO4 buffer salts are not present in the spectra to obscure the identity of the bands of interest. EDX analysis of the materials shows that the Si wt.% content is 0.07% for the PS derivatives with APTES with the absence of Na, K and P from the PBS buffer salts, confirming the validity of the water-wash procedure prior to FTIR acquisition. The amount of APTES bound to PS is low, but consistent with the fact that PS lacks the polar, hydrogen bond accepting groups that promote the initial adsorption chain reaction postulated for the binding of APTES to different polymers with available surface hydroxyl groups24.
Figure 3. FTIR spectra pertaining to the APTES-functionalization and the immobilization of antibody on polystyrene (PS) surface.
IgG may physically adsorb to the PS surface by interaction with the hydrophobic groups of antibody molecules without the need for APTES25. When PS was immersed in a PBS solution of IgG, IgG bands were present in the purified PS+IgG FTIR spectrum (Figure 3, purple band). Since no coupling agents were employed to initiate the amide coupling between APTES and IgG, the binding of IgG to PS must be one of physical adsorption. The presence of water has two effects in the binding scheme of PS with APTES and IgG. Water can render the amine groups of APTES to be positively charged, allowing the APTES to bind to the anionic carboxylate groups of IgG through electrostatic interaction26. Water can also catalyze the polymerization of APTES as it hydrolyzes the ethoxy groups to give reactive silanol groups23, which would lead to the formation of Si-O-Si bonds detected in the PS+APTES+IgG FTIR spectrum.
PS was hydroxylated by KOH and followed by EDC coupling of IgGs as a covalent immobilization control in NCIF. The reactivity of PS with KOH is negligible as evident by very little change in the FTIR spectrum (Supplementary Figure S3). The amide bond formation between EDC-activated IgG and APTES displays two new bands at 1552 cm−1 and 1642 cm−1 (Supplementary Figure S3, inset), which are mutually exclusive from the amide bands associated with the bound IgG. The peak at 1154 cm−1 reflects the polymerization of APTES (Si-O-Si bond) as described previously. Using anti-HFA Ab, the NCIF control offered no better analytical performance compared to the NIF (Supplementary Figure S4). It was reasoned that a lower Ab immobilization density was due to a decrease in hydrophobic character for the covalently coupled APTES-Ab entity.
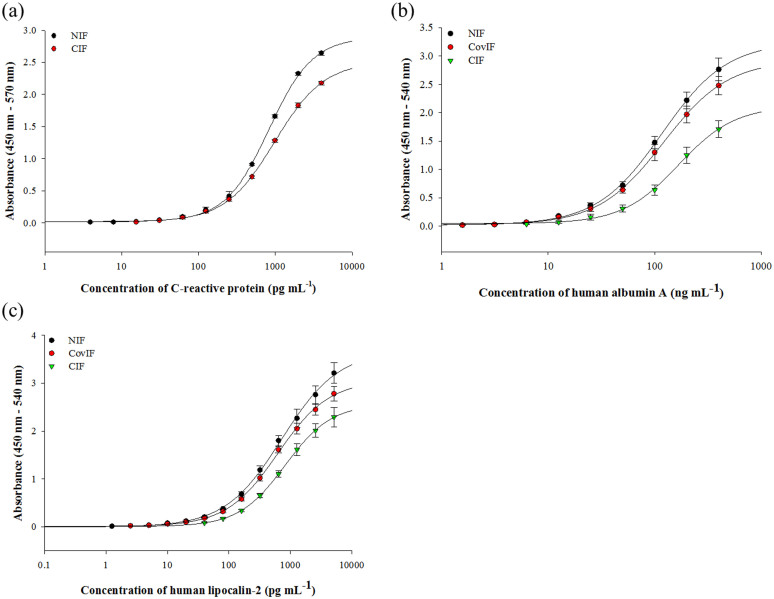
The NIF is multisubstrate-compatible and adaptable to various commercial substrates including the modified MTP format (Supplementary Figure S5)27. The detection of HFA spiked in diluted human whole blood and plasma (Figure 2c) using the NIF exhibited similar precision as the commercial kit, showing its applicability for the determination of HFA in clinical and bioanalytical settings (Table 2). The NIF-based sandwich ELISA is the most sensitive immunoassay format in comparison to the commercial and previously developed sandwich ELISA formats for HFA (Table 3). The stability of Ab immobilized on MTPs was assessed over a two months period at 4°C in 0.1 M PBS (pH 7.4). No significant decrease in the Ab functional activity or FTIR signature was observed after eight weeks (Figure 2d, Supplementary Figure S6), attesting the leach-proof Ab immobilization. Lastly, the NIF was applicable for detecting C-reactive protein (CRP), human albumin and human lipocalin-2 (Figure 4) with superior performance over the commercial kit.
Table 2. Determination of spiked HFA concentrations in diluted human whole blood and plasma by NIF- and CIF-based sandwich ELISAs. The experiments were performed in triplicate, while the results are presented as mean ± S.D.
| Sample matrix | Added conc. (in ng/mL) | NIF-based sandwich ELISA | CIF-based sandwich ELISA |
|---|---|---|---|
| Diluted human whole blood | 20 | 20.1 ± 0.19 | 20.2 ± 0.18 |
| 10 | 10.2 ± 0.16 | 10.3 ± 0.24 | |
| 5 | 5.0 ± 0.17 | 5.1 ± 0.09 | |
| 2.5 | 2.4 ± 0.09 | 2.5 ± 0.05 | |
| 1.2 | 1.3 ± 0.06 | 1.4 ± 0.05 | |
| 0.6 | 0.5 ± 0.03 | 0.7 ± 0.03 | |
| 0.3 | 0.3 ± 0.01 | 0.4 ± 0.02 | |
| Diluted human plasma | 20 | 20.2 ± 0.15 | 20.1 ± 0.17 |
| 10 | 10.1 ± 0.18 | 9.8 ± 0.16 | |
| 5 | 4.9 ± 0.19 | 4.7 ± 0.18 | |
| 2.5 | 2.5 ± 0.11 | 2.7 ± 0.12 | |
| 1.2 | 1.2 ± 0.08 | 1.3 ± 0.09 | |
| 0.6 | 0.6 ± 0.04 | 0.5 ± 0.03 | |
| 0.3 | 0.3 ± 0.02 | 0.3 ± 0.01 |
Table 3. Comparison of NIF with various sandwich ELISA procedures and the commercial kits for the detection of HFA.
| Manufacturer/Immunoassay Procedures | Antibody binding | Sensitivity (ng mL−1) | References |
|---|---|---|---|
| New ELISA format (NIF) | One-step | 0.01 | This work |
| ELISA-covalent binding of antibody | Covalent | 0.03 | This work5 using the covalent immobilization of the antibody as described by Dixit et al.5 |
| Conventional ELISA | Passively adsorbed | 0.5 | This work |
| RnD systems | Passively adsorbed | 0.37 | http://www.rndsystems.com/pdf/DY1184.pdf |
| Biovendor | Passively adsorbed | 0.35 | http://www.biovendor.com/product/immunoassays/fetuin-a-ahsg-human-elisa |
| Alpco Diagnostics | Passively adsorbed | 5.00 | http://www.alpco.com/pdfs/43/43-NSEHU-E01.pdf |
| Immunology Consultants Laboratory, Inc. | Passively adsorbed | 6.25 | http://www.life-sciences.com.br/pdf/icllab.pdf |
| Genway Biotech, Inc. | Passively adsorbed | 6.25 | http://www.genwaybio.com/images/gw_tds/elisa_kits/40-374-130036.pdf |
| Assay Pro | Passively adsorbed | 6.25 | http://www.assaypro.com/datasheet/eg3501_1.pdf |
Figure 4. Immunoassays based the NIF for (a) C-reactive protein (CRP), (b) human albumin and (c) human lipocalin-2.
The NIF-based immunoassays were compared with the CIF and CovIF-based sandwich ELISAs. All experiments were done in triplicate with the error bars representing the standard deviation.
Discussion
The NIF was developed through physical adsorption of APTES-polymer/Ab complexes onto a MTP in just 30 min. The improved analytical performance, high simplicity and cost-effectiveness of the NIF was compared to commercial sandwich ELISA kits with high protein immobilization density, prolonged stability, high reproducibility and less biofouling and interferences28,29,30,31,32,33,34. The NIF, where APTES serves as a diluting and binding agent for anti-HFA capture Ab rather than a surface functionalization agent, demonstrates superior analytical performance and high stability.
With the NIF, only 0.5% APTES was used in the new procedure as compared to 2% APTES for the step-wise binding of Ab onto an APTES-functionalized PS surface17 in CovIF. The presence of the aqueous PBS buffer has two effects in the binding scheme of PS with APTES and Ab. Water can catalyze the polymerization of APTES as it hydrolyzes the ethoxy groups to reactive silanol groups (see Figure 1)23. Confirmation of polymerization of APTES (Si-O-Si bond) is given by the band at 1154 cm−1 in the FTIR spectra of APTES-possessing species. There is the potential for polymerization of the APTES to form single and multilayers of APTES. In horizontal polymerization with respect to the –(CH2)2NH2 plane, the binding is strong owing to ionic interactions of the two carboxylate groups of the Ab (heavy chain and/or light chain) with two adjacent amino groups of the APTES polymer network. Water can also render the amine groups of APTES to be positively charged, thereby allowing the APTES to bind to the anionic carboxylate groups of Ab via electrostatic interaction (Figure 1)26. In addition, the silanol group is also capable of displaying intra and inter ionic interactions with the amino groups of APTES and Ab (heavy chain and/or light chain). The silanol and amino groups of APTES also display extensive hydrogen bonding with the amino and carboxylic group of the Ab to form a stable APTES-Ab polymer network. One might anticipate a more favorable interaction between Ab and APTES in the liquid form compared to that of Ab with the APTES-functionalized surface. The orientation of bound Ab might also affect its bioanalytical performance.
Typically without KOH/plasma treatment, the number of APTES molecules bound to PS is low due to the lack of the polar, hydrogen bond accepting groups that promote an initial adsorption chain reaction postulated for the binding of APTES to different polymers24. However, Ab may physically adsorb to the surface of PS by interaction with the hydrophobic groups of Ab molecules without the need for APTES25. Since the new procedure does not use crosslinking agents to initiate any amide coupling between APTES and Ab, physical adsorption could be attributed to the binding of Ab to the surface. Considering slight hydrophobicity of the aliphatic chain of APTES35, a higher Ab immobilization density could be achieved to promote greater physical adsorption of the pre-formed APTES polymer-Ab complex onto the PS surface. Based on the extreme simplicity and time saving granted by the NIF, the end-users can prepare the anti-HFA capture Ab-bound plates just before their intended use. This obviates the need for storing Ab-prebound MTPs, which can lead to tremendous cost-savings and improved analytical performance due to the skipping of storage effects. The NIF is generic and applicable for detecting three selected biomarkers; therefore, this assay format will be of immense utility in the field of sandwich ELISA-based IVD kits.
Methods
Ab immobilization and HFA ELISA
The anti-HFA (8 μg/mL in PBS) was mixed with 1% APTES in the ratio of 1:1 (v/v). Thereafter, the anti-HFA solution, with a final concentration of 4 μg/mL in 0.5% APTES, was added to the MTP wells and incubated for 30 min at room temperature. After washing with PBS, the anti-HFA-bound MTP wells were blocked with 1% (v/v) bovine serum albumin (BSA, diluted in 0.1 M PBS, pH 7.4) for 30 min and washed with PBS. The anti-HFA-bound MTP wells were then incubated with various HFA concentrations (4.9 pg mL−1 to 20 ng mL−1) for 1 h and washed with PBS. Thereafter, biotinylated anti-HFA (200 ng mL−1) was provided and incubated for 1 h followed by PBS washings. Subsequently, HRP-conjugated streptavidin, at a dilution of 1:200, was added to the MTP wells and incubated for 20 min followed by PBS washings. Such steps were performed at 37°C. The 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was then added as per the manufacturer's guidelines, and the enzyme-substrate reaction was stopped after 20 min by adding 50 μL of 2 N H2SO4. The absorbance was measured at 450 nm, taking 540 nm as the reference wavelength as per the manufacturer's guidelines. All experiments were carried out in triplicate and the absorbance of the blank (0 ng mL−1 HFA in 0.1 M PBS, pH 7.4) was subtracted from all assay values.
The conventional sandwich ELISA was performed as per the manufacturer's guidelines provided in the product information sheet without any modification. Various experimental process controls were employed to determine the efficiency of the BSA blocking, non-specific interactions of BSA with HFA, biotinylated anti-HFA and streptavidin-conjugated horseradish peroxidase (SA-HRP), and non-specific interaction of capture anti-HFA with biotinylated anti-HFA. The capture antibody being used in the commercial HFA ELISA kit is polyclonal mouse anti-HFA, while the detection antibody is monoclonal biotinylated goat anti-HFA. The commercial kit also states that the sandwich ELISA exhibits no cross-reactivity or interference with several recombinant human analytes (bone morphogenetic protein-2 (BMP-2), BMP-4, BMP-6, cathepsin V, matrix metalloproteinase-2 (MMP-2), MMP-9, transforming growth factor-β1 (TGF-β1), TGF-β2) and recombinant mouse fetuin A.
All datasets were subjected to standard curve analysis using SigmaPlot software, version 11.2. The EC50, R2 and Hill slope values were determined from the report generated by the software during standard curve analysis based on a four-parameter logistic function. The analytical sensitivity and LOD are calculated by the standard formulae, as mentioned below and further specified in the literature17,22,36,37.
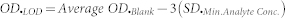 |
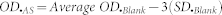 |
where OD.LOD and OD.AS are the optical densities corresponding to LOD and analytical sensitivity, respectively; OD.Blank is the optical density of the blank; and SD.Min Analyte Conc. and SD.Blank are the standard deviations of the minimum analyte concentration and the blank, respectively.
Buffers and solutions were prepared in Milli-Q deionized water. The dilution of all HFA assay components and BSA was made in 0.1 M PBS, whereas KOH and APTES were diluted in deionized water. The HFA-spiked samples were prepared by admixing various concentrations of HFA in diluted human plasma and whole blood. The HFA dilution was made in BSA-preblocked glass vials, prepared by incubation with 1% (w/v) BSA for 30 min to minimize analyte loss due to non-specific adsorption on sample tube surfaces and/or altered immunogenicity38. Deionized water and PBS washings were done five times with 300 μL of the respective solutions, while 100 μL was taken for other solutions, i.e. 1% KOH, anti-HFA solution (where anti-HFA was mixed with 1% APTES in the ratio of 1:1 (v/v)), HFA, biotinylated anti-HFA, SA-HRP and TMB substrate. Unless otherwise indicated, the assay temperature and other protocols were maintained at 37°C using a thermostat while the absorbance was measured by a Tecan Infinite M200 Pro microplate reader. The details of the materials used and the characterization experiments performed are provided in the supplementary information.
Author Contributions
S.K.V. proposed the developed sandwich ELISA procedure and one-step antibody immobilization strategy, and performed the immunoassay experiments. E.L. and S.H. conducted the characterization experiments, while E.M.S. and J.H.T.L. contributed in the design of experiments and research supervision. All the authors contributed to the drafting of this manuscript.
Supplementary Material
Supplementary Infomrtaion
References
- Lim P. et al. Usefulness of fetuin-A and C-reactive protein concentrations for prediction of outcome in acute coronary syndromes (from the French registry of acute ST-elevation non-ST-elevation myocardial infarction [FAST-MI]). Am. J. Cardiol. 111, 31–37 (2013). [DOI] [PubMed] [Google Scholar]
- Drake R. R. et al. Lectin capture strategies combined with mass spectrometry for the discovery of serum glycoprotein biomarkers. Mol. Cell. Proteomics 5, 1957–1967 (2006). [DOI] [PubMed] [Google Scholar]
- Lim P. et al. Fetuin-A is an independent predictor of death after ST-elevation myocardial infarction. Clin. Chem. 53, 1835–1840 (2007). [DOI] [PubMed] [Google Scholar]
- Sato H. et al. Decreased levels of circulating α2-Heremans-Schmid glycoprotein/fetuin-A (AHSG) in patients with rheumatoid arthritis. Intern. Med. 46, 1685–1692 (2007). [DOI] [PubMed] [Google Scholar]
- Weikert C. et al. Plasma fetuin-A levels and the risk of myocardial infarction and ischemic stroke. Circulation 118, 2555–2562 (2008). [DOI] [PubMed] [Google Scholar]
- Honda H. et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin A as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am. J. Kidney. Dis. 47, 139–148 (2006). [DOI] [PubMed] [Google Scholar]
- Ketteler M. et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet 361, 827–833 (2003). [DOI] [PubMed] [Google Scholar]
- Jethwaney D. et al. Fetuin-A, a hepatocyte-specific protein that binds Plasmodium berghei thrombospondin-related adhesive protein: a potential role in infectivity. Infect. Immun. 73, 5883–5891 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas P. et al. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol. Endocrinol. 7, 1445–1455 (1993). [DOI] [PubMed] [Google Scholar]
- Ix J. H. et al. Association between human fetuin-A and the metabolic syndrome data from the heart and soul study. Circulation 113, 1760–1767 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris V. K. et al. Cerebrospinal fluid fetuin-A is a biomarker of active multiple sclerosis. Mult. Scler. J. 19, 1462–1472 (2013). [DOI] [PubMed] [Google Scholar]
- Vashist S. K., Dixit C. K., MacCraith B. D. & O'Kennedy R. Effect of antibody immobilization strategies on the analytical performance of a surface plasmon resonance-based immunoassay. Analyst 136, 4431–4436 (2011). [DOI] [PubMed] [Google Scholar]
- Kausaite-Minkstimiene A., Ramanaviciene A., Kirlyte J. & Ramanavicius A. Comparative study of random and oriented antibody immobilization techniques on the binding capacity of immunosensor. Anal. Chem. 82, 6401–6408 (2010). [DOI] [PubMed] [Google Scholar]
- Vashist S. K., Saraswat M. & Holthöfer H. Development of a rapid sandwich enzyme linked immunoassay procedure for the highly sensitive detection of human lipocalin-2/NGAL. Procedia Chem. 6, 141–148 (2012). [Google Scholar]
- Wong L. S., Khan F. & Micklefield J. Selective covalent protein immobilization: strategies and applications. Chem. Rev. 109, 4025–4053 (2009). [DOI] [PubMed] [Google Scholar]
- Jung Y. et al. Photoactivable antibody binding protein: site-selective and covalent coupling of antibody. Anal. Chem. 81, 936–942 (2009). [DOI] [PubMed] [Google Scholar]
- Dixit C. K., Vashist S. K., MacCraith B. D. & O'Kennedy R. Multisubstrate-compatible ELISA procedures for rapid and high-sensitivity immunoassays. Nat. Protoc. 6, 439–445 (2011). [DOI] [PubMed] [Google Scholar]
- Lu B., Smyth M. R. & O'Kennedy R. Tutorial review. Oriented immobilization of antibodies and its applications in immunoassays and immunosensors. Analyst 121, 29R–32R (1996). [DOI] [PubMed] [Google Scholar]
- Shriver-Lake L. C. et al. Antibody immobilization using heterobifunctional crosslinkers. Biosens. Bioelectron. 12, 1101–1106 (1997). [DOI] [PubMed] [Google Scholar]
- Vashist S. K., Saraswat M. & Holthofer H. Comparative study of the developed chemiluminescent, ELISA and SPR immunoassay formats for the highly sensitive detection of human albumin. Procedia Chem. 6, 184–193 (2012). [Google Scholar]
- Vashist S. et al. A multi-well plate for biological assays. WO 2010044083 A2, (2010).
- Dixit C. K. et al. Development of a high sensitivity rapid sandwich ELISA procedure and its comparison with the conventional approach. Anal. Chem. 82, 7049–7052 (2010). [DOI] [PubMed] [Google Scholar]
- Vandenberg E. T. et al. Structure of 3-aminopropyl triethoxy silane on silicon oxide. J. Colloid Interface Sci. 147, 103–118 (1991). [Google Scholar]
- Howarter J. A. & Youngblood J. P. Surface modification of polymers with 3-aminopropyltriethoxysilane as a general pretreatment for controlled wettability. Macromolecules 40, 1128–1132 (2007). [Google Scholar]
- Liu X. et al. BSA-templated MnO2 nanoparticles as both peroxidase and oxidase mimics. Analyst 137, 4552–4558 (2012). [DOI] [PubMed] [Google Scholar]
- Wang Z.-H. & Jin G. Silicon surface modification with a mixed silanes layer to immobilize proteins for biosensor with imaging ellipsometry. Colloids Surf. B 34, 173–177 (2004). [DOI] [PubMed] [Google Scholar]
- Vashist S. K. Comparison of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide based strategies to crosslink antibodies on amine-functionalized platforms for immunodiagnostic applications. Diagnostics 2, 23–33 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R. et al. inventors; Medtronic Minimed, Inc., assignee. Long term analyte sensor array. US 7,577,470. 2009 Aug 18.
- Shah R., Reghabi B., Gottlieb R., Hoss U. & Mastrototaro J. inventors; Medtronic Minimed, Inc, assignee. Analyte sensors and methods for making and using them. US20050272989 A1. 2005 Dec 8.
- Shah R. et al. inventors; Medtronic Minimed, Inc., assignee. Biosensors and methods for making and using them. US 7,813,780 B2. 2010 Oct 12.
- Zheng D. et al. Effect of 3-aminopropyltriethoxysilane on the electrocatalysis of carbon nanotubes for reagentless glucose biosensing. J. Nanopharmaceutics Drug Del. 1, 64–73 (2013). [Google Scholar]
- Zheng D., Vashist S. K., Al-Rubeaan K., Luong J. H. T. & Sheu F. S. Mediatorless amperometric glucose biosensing using 3-aminopropyltriethoxysilane-functionalized graphene. Talanta 99, 22–28 (2012). [DOI] [PubMed] [Google Scholar]
- Zheng D., Vashist S. K., Al-Rubeaan K., Luong J. H. T. & Sheu F. S. Rapid and simple preparation of a reagentless glucose electrochemical biosensor. Analyst 137, 3800–3805 (2012). [DOI] [PubMed] [Google Scholar]
- Zheng D. et al. Graphene versus multi-walled carbon nanotubes for electrochemical glucose biosensing. Materials 6, 1011–1027 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe D., Smart C., Alexander C. & Vulfson E. Bacterial adhesion at synthetic surfaces. Appl. Environ. Microbiol. 65, 4995–5002 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist S. K. A sub-picogram sensitive rapid chemiluminescent immunoassay for the detection of human fetuin A. Biosens. Bioelectron. 40, 297–302 (2013). [DOI] [PubMed] [Google Scholar]
- Vashist S. K. Graphene-based immunoassay for human lipocalin-2. Anal. Biochem. 446, 96–101 (2014). [DOI] [PubMed] [Google Scholar]
- Dixit C. K., Vashist S. K., MacCraith B. D. & O'Kennedy R. Evaluation of apparent non-specific protein loss due to adsorption on sample tube surfaces and/or altered immunogenicity. Analyst 136, 1406–1411 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Infomrtaion