Abstract
Background
Fluorouracil (5-FU) is a pyrimidine analogue used as a cancer treatment. Its toxic side effects, including mucositis, are reported to occur in 40% of the treated patients. Because of the inflammatory component of mucositis, we explored the possibility of modulating this condition with an immunomodulatory agent and a tumor necrosis factor-α inhibitor.
Objective
The aim of this study was to evaluate the effect of 2 immunosuppressive agents, etanercept and cyclosporine, in a murine model of 5-FU–induced mucositis.
Methods
To study the short-term effects of 5-FU on mucositis, cyclosporine and etanercept were administered to mice after an injection of 5-FU. The animals (n = 8) were euthanized at 6 hours post-challenge. Hematoxylin and eosin–stained histologic sections of the small intestine were examined for signs of apoptosis. To further examine the potential of cyclosporine in the treatment of 5-FU–induced mucositis in a longer duration, the animals (N = 15) were given 2 challenges of 5-FU within 6 hours. All mice were dosed daily until day 9 with either cyclosporine (100 mg/kg) or phosphate-buffered saline (PBS).
Results
Six hours after 5-FU challenge, 25 mg/kg etanercept and 50 mg/kg cyclosporine had no effect on 5-FU–induced apoptosis (P > 0.05). However, 100 mg/kg cyclosporine significantly reduced the cumulative level of apoptosis >41.6% of the intestinal crypt surface (P < 0.05). During long-term observation, all mice began to lose weight at a rate of approximately 0.8 g/day after 5-FU exposure. The rates of weight loss and survival were not affected by cyclosporine treatment. The diarrhea onset began on day 4 with 46.7% of the PBS-treated mice showing signs of diarrhea compared with 53.3% in the cyclosporine group. The diarrhea score for both groups plateaued on day 7, with a cumulative score of 41 for the PBS group and 50 for the cyclosporine group. Cyclosporine treatment did not affect the diarrhea onset day or severity compared with the PBS-treated group (P > 0.05).
Conclusions
Our data indicated that etanercept is not a suitable treatment for 5-FU–induced mucositis. Despite decreased apoptosis in the gut, cyclosporine did not affect the severity of the diarrhea or survival. Therefore, we concluded that cyclosporine treatment was only effective in mediating the short-term apoptotic events in the intestines but has no long-term effect on the animals' survival and diarrhea.
Key Words: cyclosporine, etanercept, fluorouracil, mucositis
Introduction
Fluorouracil (5-FU) is a pyrimidine analogue that has been used as cancer therapy since the 1960s.1 It is an irreversible inhibitor of thymidylate synthase that is involved in DNA replication and repair. It inhibits the activity of the exosome complex and can incorporate its toxic metabolites into DNA and RNA, leading to cell cycle arrest and apoptosis.2 This mechanism of action also affects rapidly dividing healthy cells in the patients and causes a variety of side effects including mucositis. This compound was used successfully to induce gastrointestinal mucositis in both relevant mouse and rat models.3–5 In these models, the decrease in diarrhea correlated well with the reduction of intestinal inflammation and crypt cell survival.
During the pathogenesis of mucositis, epithelial damage caused by the initial insult is followed by local cytokine production, which leads to inflammation followed by ulceration.6 There are multiple lines of evidence that point to an inflammatory component in mucositis. Nuclear factor κB (NF-κB), cyclooxygenase-2 (COX-2), and proinflammatory cytokines such as interleukin 1 beta (IL-1β), IL-6, and tumor necrosis factor α (TNF-α) have been linked to the pathogenesis of mucositis.6–8 The increase in TNF-α production is well documented.9,10 It can activate NF-κB and cause the release of other proinflammatory cytokines, thus amplifying the signal.8,9 Because of this reason, the damaging effects caused by TNF-α release extend beyond the initial insult. In addition to its proinflammatory effects, TNF-α is also an apoptotic cytokine. The role of TNF-α in irradiation-induced toxicity had been demonstrated in both TNFR1 knockout mice and WT mice treated with antisense against TNFR1.11 Targeting the TNF-α signaling cascade led to attenuation of downstream apoptosis. This makes TNF-α inhibitors, such as etanercept, attractive agents for mediating mucositis.
Cyclosporin A (CsA) is an 11 amino acid long fungus-derived peptide.12 It is an immunosuppressive drug with low myelotoxicity. Its effects were originally thought to affect mostly T and B lymphocytes,13–15 but have been found to extend to macrophages as well. CsA decreases human macrophage IL-6 synthesis at the post-transcriptional level16 and can decrease reactive oxygen species production in murine macrophages stimulated by phorbol 12-myristate 13-acetate.17 Other macrophage functions including antigen presentation,18–20 migration,12,21 and prostaglandin E production22 can also be down-regulated by CsA. Because macrophages are a major source of TNF-α production, it reasons that targeting macrophage function is potentially beneficial in modulating mucositis. Therefore, in this current set of studies, the possibility of using cyclosporine and etanercept, an antibody against TNF-α, to attenuate 5-FU–induced mucositis was examined.
Methods
Animals and Husbandry
Male C57B/6 mice, 10 to 12 weeks old, were used in all studies. All procedures were certified according to the UK Animals (Scientific Procedures) Act 1986. The animals were purchased from Harlan UK and allowed to acclimatize for 2 weeks before use. Both feed (Rat and Mouse Expanded diet, B&K Universal Ltd., North Humberside, United Kingdom) and water (from drinking bottles) were available ad libitum. Animal health was monitored daily, and cages were cleaned at regular intervals. All animals were kept in individually ventilated cages in a specific pathogen-free barrier unit. The room was maintained at 21°C (±2°C), with a mean (SD) relative humidity of 55% (10%), and 12-hour day/night cycles. The animals were identified by numbered cages and ear punches. For the acute observations, naive mice were randomized into groups of 8 for their respective treatments. For the long-term observations, 15 animals were randomly assigned to each group.
Experimental Procedures, Clinical Scoring, and Sample Collection
Etanercept (Lot 34648, Wyeth, Sandwich, United Kingdom) and cyclosporine (Lot 1361825, Fluka, Dorset, United Kingdom) were dissolved in phosphate-buffered saline (PBS) with brief vortexing. The high-dose cyclosporine required brief sonication to increase solubility. To study the short-term effect of 5-FU–induced mucositis, etanercept (25 mg/kg) and cyclosporine (50 or 100 mg/kg) were administered once by IP injection 15 minutes after an IP injection of clinical grade 5-FU (Medac, Hamburg, Germany) at 500 mg/kg. The doses of etanercept and cyclosporine were selected based on internal maximum tolerated dose studies. The dose at this level did not cause weight loss or clinical signs of adverse effects in the naive animals. The animals were euthanized 6 hours after administration of 5-FU by cervical dislocation. At the time of euthanasia, samples of the upper ileum and mid-colon were harvested for analysis.
To study the effects of long-term cyclosporine dosing, all mice were given a second IP injection of 500 mg/kg 5-FU 6 hours after the initial dose of 5-FU. This double-challenge regimen was necessary to generate sufficient crypt death and intestinal ulceration. The animals were then dosed daily until day 9 with either 100 mg/kg cyclosporine or PBS. Their weight and signs of diarrhea were noted daily until day 10. During the times of peak diarrhea incidence (days 4–10), mice were inspected twice daily for signs of diarrhea. Scores were recorded as 0, 1, 2, or 3, where 0 is normal stool consistency, 1 is loose stools, 2 is overt diarrhea with perianal soilage, and 3 is severe/bloody diarrhea with substantial tail soilage. The daily scores were then summed to give a cumulative score over the course of the study. The weight loss of the animals was also recorded as a surrogate marker for the general health of the animals. Mice were euthanized by cervical dislocation. Briefly, the animals were restrained on a flat surface, and the base of the tail was grasped firmly with 1 hand. The complete dislocation was achieved with a metal rod placed against the cervical area, and followed with a quick backward pull on the tail.
Intestinal Histologic Analysis
Samples of the small (ileum) and large (mid-colon) intestine were removed and fixed in Carnoy's solution as described by Letari et al.3 Briefly, fixed tissue was dehydrated through a series of alcohols and xylene steps and then embedded in paraffin, using a Leica TP1020 tissue processor (Leica Microsystems, Buffalo Grove, Illinois) and an EG1140H workstation (Leica Microsystems). The fixed small intestines were specially positioned to obtain the ideal orientation of the crypts before paraffin. From each mouse, a series of intestine segments, approximately 1 cm long, were placed within a loop of surgical Micropore tape (3M, St. Paul, Minnesota) and the tape tightened to immobilize the lengths. This allowed the alignment of many pieces of fixed intestine alongside each other, so that every section contained 10 to 12 well-orientated cross sections. Sections 3 mm thick were cut using a Leica RM2125RTF microtome (Leica Microsystems), and air-dried on microscope slides overnight at 37°C. Paraffin blocks were sectioned to generate 2 nonserial sections per slide. Subsequently, slides were dewaxed in xylene and rehydrated through graded alcohols to PBS. All sections were then stained with hematoxylin and eosin and mounted. The number of apoptotic cells, as expressed as apoptotic indices, was analyzed for each cell position in the crypt and summarized as a cell position plot. This method divides the surface area of the intestinal crypt into distinct sectors, making it possible to estimate the percentage of surface of the crypt that is affected. The investigators were blinded to the treatment groups. Fifty half crypts per mouse were scored on a cell positional basis, generating 400 frequency scores per group of 8 animals, from which the means were generated.
Data Analysis
The statistical analysis of apoptosis indices was performed using the WinCrypts software that used an extension of the median test.3 At each cell position, the common median for all the animals was calculated by combining the groups. The individual values for each animal were classified as being below or above the median. These values were then cast on a 2 × 2 contingency table for each position and a χ2 test was applied. For survival analysis, Cox proportional hazard regression model was used. The proportional odds model was used to analyze the cumulative diarrhea score. P ≤ 0.05 was considered significant in all analysis. The group size was determined using power analysis according to the point biserial correlation model with a desired power of 0.95. The effect size |ρ| was calculated to be 0.707, based on a coefficient of determination value of 0.5.
Results
Effect of Etanercept and Cyclosporine Treatment on the Apoptotic Index
The effects of cyclosporine and etanercept treatment on intestinal crypt cells apoptosis at 6 hours after 5-FU challenge were examined. The data were summarized as cell positional plots (Figure 1). In normal tissue, the baseline level of spontaneous apoptosis was low, as reflected in a low apoptotic index throughout the crypt. 5-FU treatment induced significant apoptosis in the crypt epithelial cells, especially at cell positions 1 to 13. Cells in the vicinity of positions 3 to 7 were particularly sensitive. This region was rich with stem cells that were unable to repair DNA. These apoptotic cells generally take on a round appearance. The maximum apoptotic index observed was 25.33% in the PBS-treated animals. At 6 hours after 5-FU challenge, 25 mg/kg etanercept and 50 mg/kg cyclosporine had no effect on the level of apoptosis. However, 100 mg/kg cyclosporine decreased the level of apoptosis between cell positions 6 and 10. This represents 41.6% of the crypt surface showing beneficial effect.
Figure 1.
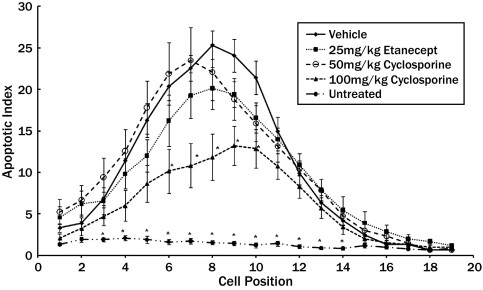
Cell positional frequency plots of the apoptotic index in the intestinal crypt 6 hours after systemic exposure to 5-fluorouracil (5-FU). The baseline apoptotic index of the naive (untreated) animals (•) is also shown. The frequency of apoptosis in all treatment groups was significantly increased compared with naïve animals in positions 3 to 14 (P ≤ 0.05). Apoptosis at cell positions 16 to 19 was generally not observed. Etanercept (■) and low-dose cyclosporine (50 mg/kg) (○) treatment had no effect on the frequency of crypt cell apoptosis after 5-FU exposure. High-dose cyclosporine (100 mg/kg) (▴) given before the 5-FU challenge significantly decreased the amount of apoptosis in positions 6 to 10 compared with the vehicle-treated animals.
Effects of Etanercept and Cyclosporine Treatment on Weight Loss, Diarrhea Score, and Survival
During the long-term observation, weight loss was observed in both cyclosporine- and PBS-treated groups after 5-FU challenge (Figure 2). Seven days post-irradiation, the weight loss in both treatment groups began to plateau. Some animals in the study were moribund and were euthanized according to Institutional Animal Care and Use Committee recommendation. There was no difference in the rate of weight loss in all the treatment groups. There was no difference in the 8 lost between the cyclosporine-treated animals and the PBS-treated group.
Figure 2.
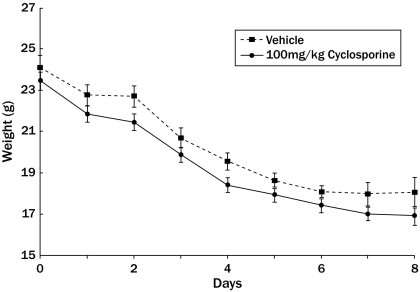
Effects of cyclosporine (•) treatment on weight loss after 5-fluorouracil challenge. The animals received daily dosing of either cyclosporine or vehicle. The weight loss in the cyclosporine-treated animals were not significantly different from that in the vehicle-treated animals throughout the study (*P ≤ 0.05).
The onset of diarrhea usually took place at the end of day 4 of the model (Figure 3). Treatment with cyclosporine did not change the diarrhea onset time. At the onset of the diarrhea, 46.7% of the PBS treated mice had demonstrable diarrhea compared with 53.3% of the cyclosporine group. There was no significant treatment effect on the diarrhea scores from both drugs compared with the PBS treated animals. The diarrhea score for both the PBS- and the cyclosporine-treated groups plateaued on day 7, with a cumulative score of 41 for the PBS group and 50 for the cyclosporine group. There was no statistical significance between the 2 groups (P > 0.05).
Figure 3.
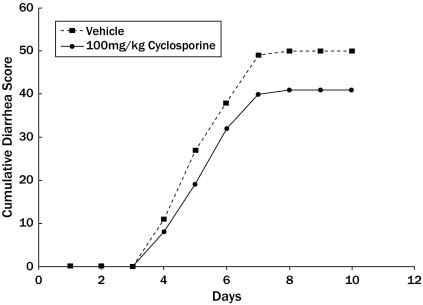
Effects of cyclosporine treatment on the diarrhea severity of the 5-fluorouracil (5-FU)–challenged animals. The cumulative diarrhea score increased exponentially 3 days after 5-FU challenge. The severity of diarrhea in the vehicle- and cyclosporine-treated animals plateaued after 7 days. Cyclosporine had no significant effect on the diarrhea severity.
The animals in both treatment groups began to die at 7 days after 5-FU challenge (Figure 4). The survival rate on day 7 was 53% for the PBS-treated animals, and 87% for the cyclosporine-treated group. Despite this initial higher survival rate in the cyclosporine group, 100% of the animals were dead in the PBS and cyclosporine groups by days 9 and 10, respectively. Statistical analysis showed that cyclosporine treatment offered no benefit on the survival rate compared with PBS control.
Figure 4.
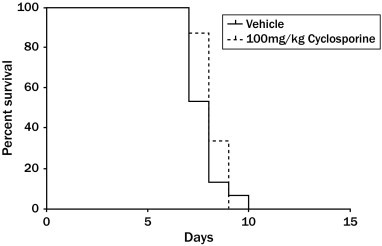
Effects of cyclosporine treatment on the survival of the 5-fluorouracil– challenged animals. Fifteen animals were assigned to each treatment group at the beginning of the study. Death of the animals was first observed at day 7 after challenge. Daily treatment with cyclosporine had no effect on the survival rate of the animals throughout the study.
Discussion
Mucositis is a common side effect of chemotherapy, which affects a large population of the oncology patients. The initial toxic symptoms of nausea and vomiting are usually followed by diarrhea. It occurs in approximately 40% of the standard-dose chemotherapy patients and 100% of the high-dose chemotherapy and stem cell or bone marrow transplantation patients.23,24 Many of these symptoms are thought to be associated with epithelial damage, which is preceded by microvasculature damage.25
There is a strong rationale for examining the potential therapeutic role of etanercept and cyclosporine in mucositis protection. Proinflammatory cytokines, including TNF-α, have been implicated in the pathogenesis of mucositis.26–29 In mouse oral mucosa, irradiation induced up-regulation of TNF-α and COX-2.30 Antisense treatment against TNFR1 has demonstrated radioprotective effects.31 Macrophages are one of the key cell types that mediate inflammatory responses. Cyclosporine has been shown to down-regulate proinflammatory cytokine production by both human and rodent macrophages.32–34 Thus, the potential therapeutic effects of etanercept and cyclosporine were studied in the current set of experiments.
In our studies, we quantified the level of apoptosis in the intestinal crypt caused by systemic 5-FU challenge. The apoptotic index increased toward the apical surface of the crypt. Cyclosporine (50 mg/kg) and etanercept (25 mg/kg) did not affect the level of apoptosis. However, 100 mg/kg cyclosporine was effective in significantly down-regulating apoptosis in the intestinal crypt. These doses were chosen based on safety data derived from internal maximum tolerated dose studies. Even though TNF-α has been considered a main driving cytokine in many inflammatory processes,29 its pivotal role in mucositis pathology had been controversial. In our current study, it is possible that TNF-α does not play a large role in the early apoptotic process in the intestinal crypt after 5-FU challenge, but the examination of the temporal role of TNF- is beyond the scope of this article. Our observations echo those of Haagen et al.30 who questioned the role of TNF-α in other models of mucositis. The authors reported that infliximab treatment did not affect irradiation-induced oral mucositis in the mouse.30 Even though it had been suggested that the lack of infliximab treatment effects might be attributed to species specificity,35 the doses used in the experiment had been shown to be effective in other murine models. It is possible that other parallel inflammatory pathways are influencing the response.
Based on the observation that 100 mg/kg cyclosporine affected the apoptosis of the intestinal epithelial cells, we examined the long-term effects of high-dose cyclosporine on various clinical parameters in a more severe disease state. In this paradigm, cyclosporine treatment did not improve the survival of the animals. The weight loss and cumulative diarrhea score were also not affected by cyclosporine treatment compared with the PBS-treated animals. Both parameters began to plateau at 7 days post-challenge. According to a priori power analysis using the point biserial correlation model, a sample size of 13 (α = 0.05) is adequate to provide a power of 0.958 to detected a significant difference of P < 0.05 between the treatment groups. Because 15 animals were assigned to each group, this study was powered adequately to support a meaningful analysis. One factor worthy of further investigation is a more frequent dosing regimen of etanercept and cyclosporine. Even though both compounds were dosed within the maximum tolerated dose, it is possible to dose the animals at a higher frequency and still remain within the established safety margin. Another parameter that can be explored is the effect of challenging the animals with a lower dose of 5-FU. It is possible that the severity of the intestinal damage caused by the current dose is too high for these immunomodulators to down-regulate.
In this study, we quantified 5-FU–induced local changes in the intestinal epithelium, which led to subsequent weight loss and diarrhea. We further demonstrated the lack of association between intestinal epithelial cell apoptosis and the survival of the animals. Even though some investigators reported up-regulation of TNF-α and activation of macrophages in mucositis, treatment with etanercept and cyclosporine did not affect many parameters. It is possible that the increase in TNF-α expression is incidental, and other pathways can compensate for the TNF-α inhibition. The interaction of different cytokines and inflammatory cell types is complex, and it is likely that targeting TNF-α alone is not sufficient to affect these pathologies. Further investigation would be required to establish the relationship between various proinflammatory cytokines and these disease parameters.
Conflicts of Interest
This study was designed and sponsored by Biomed-Valley Discoveries. The study execution and data analysis were performed by Epistem.
Acknowledgments
All authors contributed equally to the conduct of the study and creation of the manuscript.
References
- 1.Heidelberger C., Ansfield F.J. Experimental and clinical use of fluorinated pyrimidines in cancer chemotherapy. Cancer Res. 1963;23:1226–1243. [PubMed] [Google Scholar]
- 2.Ardalan B., Glazer R. An update on the biochemistry of 5-fluorouracil. Cancer Treat Rev. 1981;8:157–167. doi: 10.1016/s0305-7372(81)80014-x. [DOI] [PubMed] [Google Scholar]
- 3.Letari O., Booth C., Bonazzi A. Efficacy of CR3294, a new benzamidine derivative, in the prevention of 5-fluorouracil-induced gastrointestinal mucositis and diarrhea in mice. Cancer Chemother Pharmacol. 2010;66:819–827. doi: 10.1007/s00280-009-1224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith C.L., Geier M.S., Yazbeck R. Lactobacillus fermentum BR11 and fructo-oligosaccharide partially reduce jejunal inflammation in a model of intestinal mucositis in rats. Nutr Cancer. 2008;60:757–767. doi: 10.1080/01635580802192841. [DOI] [PubMed] [Google Scholar]
- 5.Whitford E.J., Cummins A.G., Butler R.N. Effects of Streptococcus thermophilus TH-4 on intestinal mucositis induced by the chemotherapeutic agent, 5-Fluorouracil (5-FU) Cancer Biol Ther. 2009;8:505–511. [PubMed] [Google Scholar]
- 6.Sonis S.T. Pathobiology of mucositis. Semin Oncol Nurs. 2004;20:11–15. doi: 10.1053/j.soncn.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Logan R.M., Gibson R.J., Bowen J.M. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol. 2008;62:33–41. doi: 10.1007/s00280-007-0570-0. [DOI] [PubMed] [Google Scholar]
- 8.Sonis S.T. Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol. 2007;5:3–11. [PubMed] [Google Scholar]
- 9.Franko A.J., Sharplin J., Ghahary A., Barcellos-Hoff M.H. Immunohistochemical localization of transforming growth factor beta and tumor necrosis factor alpha in the lungs of fibrosis-prone and “non-fibrosing” mice during the latent period and early phase after irradiation. Radiat Res. 1997;147:245–256. [PubMed] [Google Scholar]
- 10.Johnston C.J., Piedboeuf B., Rubin P. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996;145:762–767. [PubMed] [Google Scholar]
- 11.Zhang M., Qian J., Xing X. Inhibition of the tumor necrosis factor-alpha pathway is radioprotective for the lung. Clin Cancer Res. 2008;14:1868–1876. doi: 10.1158/1078-0432.CCR-07-1894. [DOI] [PubMed] [Google Scholar]
- 12.Matsushima Y., Baba T. The in vivo effect of cyclosporine A on macrophages. J Exp Pathol. 1990;5:39–48. [PubMed] [Google Scholar]
- 13.Hess A.D., Esa A.H., Colombani P.M. Mechanisms of action of cyclosporine: effect on cells of the immune system and on subcellular events in T cell activation. Transplant Proc. 1988;20:29–40. [PubMed] [Google Scholar]
- 14.Colombani P.M., Hess A.D. T-lymphocyte inhibition by cyclosporine: Potential mechanisms of action. Biochem Pharmacol. 1987;36:3789–3793. doi: 10.1016/0006-2952(87)90438-2. [DOI] [PubMed] [Google Scholar]
- 15.Bunjes D., Hardt C., Rollinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981;11:657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- 16.Garcia J.E., Lopez A.M., de Cabo M.R. Cyclosporin A decreases human macrophage interleukin-6 synthesis at post-transcriptional level. Mediators Inflamm. 1999;8:253–259. doi: 10.1080/09629359990423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiara M.D., Bedoya F., Sobrino F. Cyclosporin A inhibits phorbol ester-induced activation of superoxide production in resident mouse peritoneal macrophages. Biochem J. 1989;264:21–26. doi: 10.1042/bj2640021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palay D.A., Cluff C.W., Wentworth P.A., Ziegler H.K. Cyclosporine inhibits macrophage-mediated antigen presentation. J Immunol. 1986;136:4348–4353. [PubMed] [Google Scholar]
- 19.Manca F., Fenoglio D., Kunkl A. Effect of cyclosporine on the antigen-presenting function of human and murine accessory cells. Transplantation. 1988;46:40S–43S. doi: 10.1097/00007890-198808001-00008. [DOI] [PubMed] [Google Scholar]
- 20.Manca F., Kunkl A., Celada F. Inhibition of the accessory function of murine macrophages in vitro by cyclosporine. Transplantation. 1985;39:644–649. doi: 10.1097/00007890-198506000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Drath D.B., Kahan B.D. Alterations in rat pulmonary macrophage function by the immunosuppressive agents cyclosporine, azathioprine, and prednisolone. Transplantation. 1983;35:588–592. doi: 10.1097/00007890-198306000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Whisler R.L., Lindsey J.A., Proctor K.V. Characteristics of cyclosporine induction of increased prostaglandin levels from human peripheral blood monocytes. Transplantation. 1984;38:377–381. doi: 10.1097/00007890-198410000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Keefe D.M., Cummins A.G., Dale B.M. Effect of high-dose chemotherapy on intestinal permeability in humans. Clin Sci (Lond) 1997;92:385–389. doi: 10.1042/cs0920385. [DOI] [PubMed] [Google Scholar]
- 24.Keefe D.M., Brealey J., Goland G.J., Cummins A.G. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000;47:632–637. doi: 10.1136/gut.47.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maj J.G., Paris F., Haimovitz-Friedman A. Microvascular function regulates intestinal crypt response to radiation. Cancer Res. 2003;63:4338–4341. [PubMed] [Google Scholar]
- 26.Hong J.H., Chiang C.S., Tsao C.Y. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75:1421–1427. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 27.Xun C.Q., Thompson J.S., Jennings C.D. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. [PubMed] [Google Scholar]
- 28.Ong Z.Y., Gibson R.J., Bowen J.M. Pro-inflammatory cytokines play a key role in the development of radiotherapy-induced gastrointestinal mucositis. Radiat Oncol. 2010;5:22. doi: 10.1186/1748-717X-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logan R.M., Stringer A.M., Bowen J.M. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev. 2007;33:448–460. doi: 10.1016/j.ctrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Haagen J., Krohn H., Rollig S. Effect of selective inhibitors of inflammation on oral mucositis: preclinical studies. Radiother Oncol. 2009;92:472–476. doi: 10.1016/j.radonc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Huang X.W., Yang J., Dragovic A.F. Antisense oligonucleotide inhibition of tumor necrosis factor receptor 1 protects the liver from radiation-induced apoptosis. Clin Cancer Res. 2006;12:2849–2855. doi: 10.1158/1078-0432.CCR-06-0360. [DOI] [PubMed] [Google Scholar]
- 32.Losa Garcia J.E., Rodriguez F.M., Martin de Cabo M.R. Evaluation of inflammatory cytokine secretion by human alveolar macrophages. Mediators Inflamm. 1999;8:43–51. doi: 10.1080/09629359990711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tipton D.A., Pabst M.J., Dabbous M.K. Interleukin-1 beta- and tumor necrosis factor-alpha-independent monocyte stimulation of fibroblast collagenase activity. J Cell Biochem. 1990;44:253–264. doi: 10.1002/jcb.240440407. [DOI] [PubMed] [Google Scholar]
- 34.Rofe A.M., Philcox J.C., Haynes D.R. Changes in plasma zinc, copper, iron, and hepatic metallothionein in adjuvant-induced arthritis treated with cyclosporin. Biol Trace Elem Res. 1992;34:237–248. doi: 10.1007/BF02783679. [DOI] [PubMed] [Google Scholar]
- 35.Fox B.S., Sonis S. TNF and oral mucositis: response to effect of selective inhibitors of inflammation on oral mucositis: preclinical studies. Radiother Oncol. 2009;92:472–476. doi: 10.1016/j.radonc.2009.06.006. [DOI] [PubMed] [Google Scholar]


