Abstract
Background
Pregabalin has a similar pharmacologic profile to that of its developmental predecessor gabapentin but has shown greater analgesic activity in rodent models of neuropathic pain.
Objective
The objective of the study was to compare the effects of 2 different doses of pregabalin and placebo on postoperative pain and morphine consumption.
Methods
Ninety patients who underwent abdominal hysterectomy were included in the study and randomly divided into 3 groups in a doubled-blinded manner. They were given 150 mg of pregabalin (group P300, n = 30), 300 mg of pregabalin (group P600, n = 30), or placebo capsules (group C, n = 30) 4 hours before the induction of anesthesia; they received a second dose of the drug 12 hours postoperatively. Morphine consumption, nausea, and vomiting, visual analogue scale-pain intensity (VAS-PI), sedation scores, and dissatisfaction scores were recorded in the postanesthesia care unit (PACU) and at 2, 4, 6, and 24 hours after operation.
Results
Morphine consumption at 24 hours was 40.80 (3.42) mg, 33.79 (5.77) mg, and 46.97 (6.67) mg in groups P300, P600, and C, respectively (P < 0.001). VAS-PI scores at movement and at rest in the PACU and at 2, 4, and 6 hours decreased in group P600 (P < 0.01). In the PACU and at 2, 4, and 6 hours, the sedation scores were increased in group P600 compared with the scores in group C (P < 0.001, P < 0.001, P = 0.01, P = 0.006, respectively). Patient satisfaction was higher in group P600 than in group C for all time points (P < 0.001, P < 0.001, P < 0.001, P = 0.001, P < 0.001, respectively). There were no statistically significant differences between the groups for side effects such as nausea, vomiting, and dizziness (P = 0.58).
Conclusions
Pregabalin at a total dose of 600 mg, administered before operation and at 12 hours postoperatively after abdominal hysterectomy, reduced morphine consumption and pain intensity and increased patient satisfaction. No significant differences in side effects were observed between the study groups.
Key words: morphine consumption, pain, postoperative, pregabalin
Introduction
Pain relief after surgical procedures continues to be a major medical challenge. Alleviation of pain has been given a high priority by medical professionals and health authorities. Improvement in perioperative analgesia is not only desirable for humanitarian reasons but also essential for its potential to reduce postoperative morbidity and mortality.1,2 Unsatisfactory analgesia increases discomfort of the patient and prolongs hospital stay.3 This means an increase in the incidence of complications and treatment costs.3 Moreover, it may lead to development of chronic pain as an adverse effect, one of the most devastating problems related to this issue if acute pain cannot be treated as required.4
Postoperative pain is not purely nociceptive in nature and may consist of inflammatory, neurogenic, and visceral components.5 Sensitization of the dorsal horn neurons has been demonstrated in acute pain models and may also play a role in the development of chronic pain after surgery.6 By reducing the hyperexcitability of dorsal horn neurons induced by tissue damage, gabapentin and pregabalin may have roles in the treatment of postoperative pain.7,8
Pregabalin is the pharmacologically active S-enantiomer of 3-aminomethyl-5-methyl-hexanoic acid.9 It is a structural analogue of γ-aminobutyric acid, one of the key inhibitory neurotransmitters in the brain. Its mode of action is believed to be mediated by the α-2-Δ-1 subunit protein of voltage-gated calcium channels, resulting in its anxiolytic, anticonvulsant, and antinociceptive effects.10 Pregabalin is rapidly absorbed with peak blood concentrations occurring within 1 hour. The average bioavailability exceeds 90% and is independent of dose, which may produce a more predictable patient response. The elimination half-life of pregabalin ranges between 5.5 and 6.7 hours and is independent of dose.9,11 Due to these specific properties of pregabalin, preoperative single dose administration is effective in postoperative pain therapy with no need for long-term treatment.12
Although opioids continue to have an important role in postoperative pain management, they have side effects.13,14 For this reason, multimodal analgesia was suggested to improve postoperative analgesia and to reduce opioid-related side effects.15 An antineuropathic pain drug like pregabalin, as a part of multimodal analgesia, can be useful for optimization of postoperative analgesia.7,8,12,16 Early preclinical studies suggesting analgesic efficacy after tissue injury led to the development of gabapentin and pregabalin as treatments for postoperative pain.17,18 Preoperative administration of 600 mg pregabalin, but not 300 mg, significantly reduced opioid usage in patients after laparoscopic hysterectomy.7 In addition, a study reported that 75 and 150 mg doses of pregabalin did not reduce postoperative analgesic requirements,8 and another study showed that a single preoperative 150 mg dose was useful to reduce morphine consumption after sleeve gastrectomy.12 In contrast, 300 mg pregabalin reduced postoperative opioid usage after abdominal hysterectomy.16
The aim of the present study was to determine the effect of 2 different doses of oral pregabalin (150 or 300 mg) with the same dose repeated after 12 hours on acute postoperative pain control and morphine consumption in a randomized and double-blinded manner.
Methods
One hundred and five patients were assessed for eligibility; 15 patients were excluded, and the remaining 90 patients were included in this study. Institutional ethics committee approval of Inonu University Medical School, Malatya, Turkey, and written informed consent from each patient were obtained before the study. The patients (n = 90, American Society of Anesthesiologists physical status I and II; age range, 25–65 years) were scheduled for elective total abdominal hysterectomy under general anesthesia.
Exclusion criteria were known allergy to opioids or pregabalin, history of cardiovascular, renal or hepatic disease, psychiatric disorders, chronic pain syndromes, drugs or alcohol abuse, and refusal for participation. Patients receiving regular opioids or drugs (acetaminophen, NSAIDs, sedatives, or anticonvulsants) within 24 hours before surgery were also excluded. Patients with systolic arterial blood pressure levels >160 mm Hg or diastolic arterial blood pressure levels >90 mm Hg were also excluded. In this double-blind study, the patients were randomly allocated by a computer-generated block randomization procedure to receive pregabalin or placebo 4 hours before the induction of anesthesia and at 12 hours postoperatively. The researchers and participants were blinded to the drugs and procedures. The hypothesis that preoperative application of pregabalin would decrease postoperative opioid requirement with lower visual analogue score-pain intensity (VAS-PI) and dissatisfaction scores was analyzed. Patient controlled analgesia (PCA) technique and the verbal numerical rating scale (VAS-PI, 0 = no pain, 10 = worst pain imaginable) were explained to the patients during the preoperative anesthesia consultation.
No patients were premedicated. All the patients fasted from midnight before until the operation. All the patients were then randomly allocated using computerized randomization to receive 150 mg of pregabalin (group P300, n = 30) or 300 mg of pregabalin (group P600, n = 30) as the first study dose 4 hours before the induction of anesthesia, or as part of the control group (group C, n = 30). All the capsules, including placebo, were prepared by the hospital pharmacy as identical capsules to maintain blinding. The drugs were obtained by individually prepared opaque envelopes by an anesthesiologist with no further involvement in the study. To maintain blinding more properly, all data were obtained and recorded by an anesthesiologist who did not participate in this study.
With standard hemodynamic monitors in place (electrocardiograph, heart rate, pulse oxymeter oxygen saturation, noninvasive blood pressure), anesthesia was induced using 2% lidocaine (1 mg/kg), fentanly (1 μg/kg), and thiopental (3–5 mg/kg) until the eyelash reflex disappeared. Vecuronium bromide (0.1–0.2 mg/kg) was administered to facilitate tracheal intubation and maintained using bolus injections at a dose of 0.01 mg/kg. Anesthesia was maintained using nitrous oxide 60% and sevoflurane 1.5% to 2.5% in oxygen. The patients were ventilated through controlled mechanical ventilation with an initial tidal volume of 6 to 8 mL/kg, and respiratory frequency of 10 breaths/min was adjusted to maintain the end-tidal carbon dioxide between 35 and 40 mm Hg. After skin closure at the end of surgery, the residual neuromuscular blockade was antagonized with intravenous neostigmine (50 μg/kg) and atropine (20 μg/kg). The anesthetic agents were then discontinued, and the patients were extubated. No further analgesic drugs were administered. All the patients received PCA with intravenous morphine and were followed for 24 hours, and pain and sedation scores were evaluated by study nurses who were blinded to the study protocol. After administration of 5 mg morphine over 30 minutes, starting 15 minutes before the estimated time of completion of surgery, the PCA device was set to deliver 2 mg of morphine with a lockout of 15 minutes and a 4 hour limit of 20 mg, and no continuous infusion. If analgesia was felt to be inadequate at any time during the study, the lockout time was shortened to 5 minutes. The primary goal with respect to the efficacy of 2 different doses of pregabalin was morphine consumption for 24 hours postoperatively. The secondary goal was to determine VAS-PI at rest and at movement, sedation, patient satisfaction with pain control, and number of patients who experienced side effects (such as respiratory depression, nausea and vomiting, headache, and pruritis). Sedation was assessed using the Ramsay sedation scale. Patient satisfaction with pain control was assessed on a 4-point scale as 1 = very satisfied, 2 = satisfied, 3 = neutral, and 4 = dissatisfied. Patients were evaluated for pain scores, morphine consumption, and sedation at 1, 2, 4, 6, and 24 hours postoperatively. PCA was discontinued when the respiratory rate was <12 breaths/min or oxygen saturation was <95%. If nausea and vomiting occurred, 10 mg of metoclopramid was administered intravenously.
Statistical Analysis
The primary outcome of this study was cumulative morphine consumption. Accordingly, in a 1-way ANOVA study, sample sizes of 30, 30, and 30 from the 3 groups achieved nearly 100% power to detect differences among the means versus the alternative of equal means using an F test with a 0.05 significance level. Power analyses were calculated by PASS software.19 Values are expressed as mean (SD) or numbers (percent) as appropriate. To evaluate normality, the Shapiro-Wilk test was performed. The comparisons of the 3 groups were made using 1-way ANOVA with the post hoc Tukey honestly significant different test for homogeneous variances or Tamhane's T2 test for nonhomogeneous variances in multiple comparisons. Categorical data and postoperative side effects were analyzed by Pearson's χ2 test. A P value < 0.05 was considered statistically significant. The statistical analyses were performed by an expert statistician.
Results
A total of 105 patients who fulfilled the inclusion criteria were considered for inclusion in the study (Figure). Fifteen patients were excluded from the study during the preoperative visit. Ninety patients were included in the study. Demographic characteristics, including age, body weight, height, the duration of anesthesia, the duration of surgical procedures, and ASA status of the patients in the 3 groups, were not different (Table I). Table II presents the comparison of the results of the cumulative morphine consumption, pain scores (VAS at rest, VAS at movement), the Ramsay sedation scores, and patient satisfaction scores with respect to the 3 groups.
Figure.
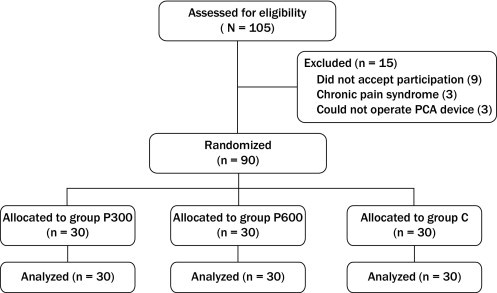
Flow diagram of patient distribution. Group C = patients given placebo; Group P300 = patients given 150 mg of pregabalin; Group P600 = patients given 300 mg of pregabalin; PCA = patient-controlled anesthesia.
Table I.
Patient demographic characteristics and duration of anesthesia and surgery. Data are given as mean (SD) or number of patients.
| Variable | P300 (n = 30) | P600 (n = 30) | Control (n = 30) |
|---|---|---|---|
| Age, y | 46.4 (9.1) | 43.27 (7.40) | 42.47 (9.31) |
| Weight, kg | 68.80 (9.2) | 70.90 (10.23) | 70.66 (10.03) |
| Height, cm | 159.90 (5.08) | 162.40 (8.15) | 162.43 (6.66) |
| Duration of anesthesia, min | 108.43 (13.53) | 104.83 (16.05) | 104.67 (16.50) |
| Duration of surgical procedures, min | 89.33 (18.18) | 84.67 (12.92) | 86 (17.58) |
| ASA I/II | 23/7 | 23/7 | 20/10 |
ASA = American Society of Anesthesiologists; Control = patients given placebo; P300 = patients given 150 mg of pregabalin; P600 = patients given 300 mg of pregabalin.
Table II.
Comparison of the results of the variables with respect to groups. Data are given as mean (SD).
| Variable | P300 (n = 30) | P600 (n = 30) | Control (n = 30) | P‡ |
|---|---|---|---|---|
| Cumulative morphine consumption | ||||
| PACU | 8.03 (0.81)⁎† | 6.93 (0.83)† | 9.17 (0.38) | <0.001 |
| 2 h | 13.03 (1.30)⁎† | 11.37 (2.17)† | 14.77 (1.45) | <0.001 |
| 4 h | 20.63 (3.13)⁎† | 17.20 (3.12)† | 23.90 (3.20) | <0.001 |
| 6 h | 27.70 (3.01)⁎† | 22.67 (4.27)† | 32.60 (5.30) | <0.001 |
| 24 h | 40.80 (3.42)⁎† | 33.79 (5.77)† | 46.97 (6.67) | <0.001 |
| VAS rest | ||||
| PACU | 7.0 (0.7)⁎ | 5.50 (0.51)† | 7.20 (0.61) | <0.001 |
| 2 h | 6.03 (0.66)⁎ | 4.37 (0.49)† | 6.23 (0.56) | <0.001 |
| 4 h | 5.10 (0.75)⁎ | 3.57 (0.72)† | 5.30 (0.59) | <0.001 |
| 6 h | 3.20 (0.60)⁎ | 2.53 (0.7)† | 3.33 (1.0) | <0.001 |
| 24 h | 1.57 (0.6) | 1.47 (0.5) | 1.73 (0.9) | 0.39 |
| VAS movement | ||||
| PACU | 7.97 (0.5)⁎ | 6.9 (0.6)† | 8.27 (0.6) | <0.001 |
| 2 h | 6.80 (0.6)⁎ | 6.17 (0.6)† | 7 (0.8) | <0.001 |
| 4 h | 5.6 (0.7)⁎ | 4.97 (0.49)† | 5.83 (0.9) | <0.001 |
| 6 h | 4.23 (0.6)⁎ | 3.57 (0.6)† | 4.40 (1.0) | <0.001 |
| 24 h | 2.57 (0.6) | 2.43 (0.9) | 2.73 (0.9) | 0.40 |
| Ramsay sedation score | ||||
| PACU | 2.53 (0.51)⁎ | 2.87 (0.51)† | 2.33 (0.48) | <0.001 |
| 2 h | 2.40 (0.56)⁎† | 2.77 (0.43)† | 2.03 (0.32) | <0.001 |
| 4 h | 1.83 (0.53) | 1.93 (0.25)† | 1.60 (0.50) | 0.01 |
| 6 h | 1.50 (0.63) | 1.67 (0.48)† | 1.27 (0.45) | 0.01 |
| 24 h | 1.10 (0.31) | 1.23 (0.50) | 1.10 (0.31) | 0.30 |
| Patient satisfaction score | ||||
| PACU | 3.17 (0.37)⁎ | 2.73 (0.4)† | 3.30 (0.5) | <0.001 |
| 2 h | 2.87 (0.34)⁎ | 2.20 (0.4)† | 2.97 (0.18) | <0.001 |
| 4 h | 2.23 (0.4)⁎ | 1.87 (0.3)† | 2.40 (0.49) | <0.001 |
| 6 h | 1.63 (0.4) | 1.37 (0.4)† | 1.9 (0.60) | 0.001 |
| 24 h | 1.23 (0.43)⁎ | 0.93 (0.2)† | 1.43 (0.50) | <0.001 |
P300 = patients given 150 mg of pregabalin; P600 = patients given 300 mg of pregabalin; PACU = postanesthesia care unit; VAS = visual analogue scale.
Significantly different compared with P600 (P < 0.05).
Significantly different compared with control (P < 0.05).
1-way ANOVA test.
Morphine consumption at all the evaluation time points was significantly lower in group P300 compared with group C (P = 0.003, P = 0.01, P = 0.002, P < 0.001, P < 0.001, respectively). Similarly, at all the evaluation time points, morphine consumption was significantly lower in group P600 compared with group C (P < 0.001). Morphine consumption values in the postanesthesia care unit (PACU) and at 2, 4, 6, and 24 hours postoperatively in group P600 were significantly lower than those of group P300 (P = 0.003, P = 0.023, P = 0.002, P = 0.001, P < 0.001, respectively).
Pain scores at rest and at movement in the PACU and at 2, 4, and 6 hours postoperatively were significantly lower in group P600 than in groups P300 and C (P < 0.01), whereas the comparison of groups P300 and P600 revealed significantly higher VAS-PI at rest and at movement at 2, 4, and 6 hours postoperatively in group P300 than in group P600 (P < 0.001, P < 0.001, P = 0.007, respectively). There were no differences for all the evaluation time points in pain scores of groups P300 and C at rest and at movement (P = 0.19, P = 0.31, P = 0.35, P = 0.68, P = 0.80, respectively).
The sedation scores in the PACU and at 2 hours postoperatively in group P300 were significantly lower than those of group P600 (P = 0.03, P = 0.005), whereas the comparison of groups P300 and C revealed significantly higher sedation scores at 2 hours postoperatively in group P300 than those in group C (P = 0.004). The sedation scores in the PACU and at 2, 4, and 6 hours postoperatively in group P600 were significantly higher than those in group C (P < 0.001, P < 0.001, P = 0.01, P = 0.006, respectively).
The patient satisfaction scores in the PACU and at 2, 4, and 24 hours postoperatively in group P300 were higher than those in group P600 (P = 0.005, P < 0.001, P = 0.01, P = 0.01, respectively). At all the evaluation time points, the patient satisfaction scores in group P600 were lower than those in group C (P < 0.001, P < 0.001, P < 0.001, P = 0.001, P < 0.001, respectively).
There were no significant differences between the groups for postoperative side effects such as nausea, vomiting, and dizziness (Table III) (P = 0.58).
Table III.
Adverse events. Data are given as number of events.
| Adverse Event | P300 (n = 30) | P600 (n = 30) | Control (n = 30) | Total |
|---|---|---|---|---|
| Nausea and vomiting | 2 | 5 | 7 | 14 |
| Pruritus | 5 | 5 | 6 | 16 |
| Dizziness | 10 | 11 | 8 | 29 |
| Total | 17 | 21 | 21 | 59 |
Discussion
The results of this randomized clinical trial showed that perioperative administration of 2 different doses of oral pregabalin (150 or 300 mg twice, 1 hour before surgery and 12 hours after the initial dose) was effective in reducing postoperative pain and provided additional analgesia during the early postoperative period in patients who underwent total abdominal hysterectomy. There were no significant differences for side effects (postoperative nausea or vomiting and dizziness) among the 3 groups. The pregabalin groups also had significantly lower VAS-PI scores and higher patient satisfaction in the first postoperative 24 hours than the control group.
Various classes of analgesics have been used in reducing postoperative pain. Recent studies about postoperative pain care demonstrated that the combinations of various antihyperalgesics, such as gabapentin and analgesic drugs, might reduce pain, the need for opioid analgesics, and side effects.11,18 Therefore, multimodal analgesic techniques using a number of drugs acting on different analgesic mechanisms are becoming increasingly popular.5
The current concept of multimodal postoperative analgesia is mainly based on the combination of opioids, NSAIDs or paracetamol, low dose ketamine, anticonvulsants, and perioperative administration of local anesthetics. The goal of multimodal analgesia is not only the improved quality of analgesia but also reduction in opioid and other drug-related side effects.
Dahl et al20 reported that postoperative pain might be considered a transient or reversible type of neuropathic pain. Therefore, gabapentin and pregabalin, which are commonly used in chronic neuropathic pain, are effective in controlling postoperative pain. A randomized, double-blind, placebo-controlled, parallel-group trial was performed by Hill et al21 to compare pregabalin 50 and 300 mg with placebo and ibuprofen 400 mg using a dental pain model. They established that the analgesic effects of pregabalin 300 mg were as effective as that of ibuprofen 400 mg in a dental approach to reduce acute postoperative pain. Similar to this study, Jokela et al7 studied doses of 150 or 300 mg twice, at 1 hour before surgery and 12 hours after the initial dose. They observed that 300 mg of pregabalin was more influential than pregabalin 150 mg in terms of oxycodone consumption. They also indicated that the analgesic effect of 300 mg of pregabalin was marginal and that patients experienced significant side effects.
Mathiesen et al22 demonstrated that a single preoperative dose of pregabalin (300 mg) resulted in an almost 50% reduction in 24-hour postoperative morphine requirements in patients who underwent hip surgery. The decrease in 24 hour morphine consumption in this study was nearly 35% (600 mg) and 15% (300 mg). The main reason for this difference between the results of the study by Mathiesen et al22 and this study was related to spinal anesthesia. The duration of analgesic effects of spinal anesthesia significantly decreased morphine consumption in the early postoperative period, when the maximum analgesic requirement was unavoidable.
These studies showed that pregabalin had a significant analgesic effect on acute postoperative pain. These results showed that 150 and 300 mg of pregabalin were effective in pain relief, management of pain at movement and at rest, use of morphine, and patient satisfaction scores.
In contrast, Mathiesen et al23 applied 300 mg pregabalin and 8 mg dexamethasone in combination with 1000 mg paracetamol 1 hour before anesthesia for pain control in patients who underwent abdominal hysterectomy. They reported that a combination of paracetamol and pregabalin or paracetamol and pregabalin and dexamethasone did not lessen morphine consumption and pain score compared with only paracetamol. The authors concluded that the use of pregabalin with a major visceral ingredient was controversial.
In another study, Jokela et al8 evaluated 90 patients who underwent gynecologic laparoscopic surgery who were randomized to receive either oral pregabalin 75 or 150 mg compared with diazepam 5 mg 1 hour before surgery. Pregabalin 150 mg reduced pain scores at rest and during mobilization for the first 8 postoperative hours, but it reduced neither pain during cough nor the amount of postoperative analgesic. These findings were different from our findings, which might point to a rather limited effect of pregabalin.
The differences of surgical procedures with comparatively low grade of pain in the postoperative period might have limited the ability to detect significant effect of analgesics on postoperative pain and the need for opioid analgesic medication. In this study, abdominal hysterectomy patients were preferred as an increase of abdominal pressure during movement, coughing, and respiration might bring to light the effects of analgesia.
The conflicting results obtained in a study by White et al24 might be due to the difference in the surgical procedures applied in their study. Jokela et al5 observed significantly lower analgesic consumption in laparoscopic surgeries as the analgesic requirement was lower in such surgeries, and the analgesic sparing effects of perioperative administration of pregabalin on postoperative pain were determined only with the perioperative administration of pregabalin 600 mg, but not with 300 mg.
The main aim of multimodal analgesia was in obtaining effective analgesia and minimizing possible adverse effects. The prevalence of nausea and vomiting in this study with pregabalin use occurred in 4 of 16 patients with 300 mg and 12 of 18 patients with 600 mg and was higher than the rates found in the study by Jokela et al7, where dexamethasone was administered to prevent nausea and vomiting, and oxycodone, ibuprofen, or acetaminophen plus codeine combination was used postoperatively to decrease opioid requirement. Adverse events were reported by Hill et al21 more often in the pregabalin 300 group. Of the 50 patients who received 300 mg of pregabalin, 24 (48%) experienced side effects, most commonly dizziness, somnolence, and vomiting.
Jokela et al7 reported that the degree of drowsiness was similar after perioperative administration of diazepam 10 mg and pregabalin 300 or 600 mg. They also indicated that the incidence of dizziness was higher after perioperative administration of pregabalin 600 mg during the study. They concluded that the high incidence of side effects in their study might reflect the female gender of the study population, as women were more susceptible to the multiple side effects of drugs than men.
The limitations of this study were initiation of treatment with low doses of pregabalin and gradual increase of the dose to the maximum suggested daily dose of 600 mg. A 600 mg dose of pregabalin, given to a patient unaccustomed to this medication, might cause adverse effects. However, 600 mg of pregabalin did not increase the adverse effects. Also, the present study had a small sample size and did not involve a priori power analysis.
Conclusions
Preoperative administration of 300 mg of pregabalin with a second dose administered 12 hours after surgery resulted in significant reduction in morphine requirement, VAS scores at rest and at movement and at cough without increasing the rate of adverse effects in the postoperative 24 hours in patients who underwent abdominal hysterectomy. Pregabalin also provided significant patient satisfaction scores.
Acknowledgments
The authors received no financial support. The authors have indicated that they have no conflicts of interest regarding the content of this article.
Dr. Yücel was responsible for the literature search and study design. Dr. Özturk was responsible for study design and data analysis. Dr. Aydoğan was responsible for figure creation and data collection. Dr. Durmus was responsible for the study design and data interpretation. Dr. Çolak was responsible for data interpretation and writing of the manuscript. Dr. Ersoy was responsible for the data collection and literature search. All authors contributed to the writing of the manuscript.
References
- 1.Werner M.U., Søholm L., Rotbøll-Nielsen P., Kehlet H. Does an acute pain service improve postoperative outcome? Anesth Analg. 2002;95:1361–1372. doi: 10.1097/00000539-200211000-00049. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H., Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth. 2001;87:62–72. doi: 10.1093/bja/87.1.62. [DOI] [PubMed] [Google Scholar]
- 3.Tang R., Evans H., Chaput A., Kim C. Multimodal analgesia for hip arthroplasty. Orthop Clin North Am. 2009;40:377–387. doi: 10.1016/j.ocl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Durkin B., Page C., Glass P. Pregabalin for the treatment of postsurgical pain. Expert Opin Pharmacother. 2010;11:2751–2758. doi: 10.1517/14656566.2010.526106. [DOI] [PubMed] [Google Scholar]
- 5.Kong V.K.F., Irwin M.G. Gabapentin: a multimodal perioperative drug? Br J Anaesth. 2007;99:775–786. doi: 10.1093/bja/aem316. [DOI] [PubMed] [Google Scholar]
- 6.Perkins F.M., Kehlet H. Chronic postoperative pain as an outcome of surgery: A review of predictive factors. Anesthesiology. 2000;93:1123–1133. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 7.Jokela R., Ahonen J., Tallgren M. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain. 2008;134:106–112. doi: 10.1016/j.pain.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Jokela R., Ahonen J., Tallgren M. Premedication with pregabalin 75 or 150 mg with ibuprofen to control pain after day-case gynaecological laparoscopic surgery. Br J Anaesth. 2008;100:834–840. doi: 10.1093/bja/aen098. [DOI] [PubMed] [Google Scholar]
- 9.Frampton J.E., Scott L.J. Pregabalin in the treatment of painful diabetic peripheral neuropathy. Drugs. 2004;64:2813–2820. doi: 10.2165/00003495-200464240-00006. [DOI] [PubMed] [Google Scholar]
- 10.Owen R.T. Pregabalin: its efficacy, safety and tolerability profile in generalized anxiety. Drug Today (Barcelona) 2007;43:601–610. doi: 10.1358/dot.2007.43.9.1133188. [DOI] [PubMed] [Google Scholar]
- 11.Gajraj N.M. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007;105:1805–1815. doi: 10.1213/01.ane.0000287643.13410.5e. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera Schulmeyer M.C., De la Maza J., Ovalle C. Analgesic effects of a single preoperative dose of pregabalin after laparoscopic sleeve gastrectomy. Obes Surg. 2010;20:1678–1681. doi: 10.1007/s11695-009-9944-1. [DOI] [PubMed] [Google Scholar]
- 13.Kehlet H. Postoperative opioid sparing to hasten recovery: what are the issues? Anesthesiology. 2005;102:1083–1085. doi: 10.1097/00000542-200506000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Vadivelu N., Mitra S., Narayan D. Recent advances in postoperative pain management. Yale J Biol Med. 2010;83:11–25. [PMC free article] [PubMed] [Google Scholar]
- 15.Kehlet H., Dahl J.B. The value of ‘multi-modal' or ‘balanced analgesia’ in postoperative pain treatment. Anesth Analg. 1993;77:1048–1056. doi: 10.1213/00000539-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 16.Ittichaikulthol W., Virankabutra T., Kunopart M. Effects of pregabalin on post operative morphine consumption and pain after abdominal hysterectomy with/without salphingo-oophorectomy: a randomized, double-blind trial. J Med Assoc Thailand. 2009;92:1318–1323. [PubMed] [Google Scholar]
- 17.Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence, future directions. Curr Opin Anaesthesiol. 2007;20:456–472. doi: 10.1097/ACO.0b013e3282effaa7. [DOI] [PubMed] [Google Scholar]
- 18.Mathiesen O., Moiniche S., Dahl J.B. Gabapentin and postoperative pain: a qualitative and quantitative systematic review, with focus on procedure. BMC Anesthesiol. 2007;7 doi: 10.1186/1471-2253-7-6. 7–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hintze J. NCSS, LLC; Kaysville, Utah: 2008. Power analysis and sample size.www.ncss.com Accessed May 1, 2011. [Google Scholar]
- 20.Dahl J.B., Mathiesen O., Moiniche S. ‘Protective premedication’: an option with gabapentin and related drugs?: A review of gabapentin and pregabalin in the treatment of post-operative pain. Acta Anaesthesiol Scand. 2004;48:1130–1136. doi: 10.1111/j.1399-6576.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 21.Hill C.M., Balkenohl M., Thomas D.W. Pregabalin in patients with postoperative dental pain. Eur J Pain. 2001;5:119–124. doi: 10.1053/eujp.2001.0235. [DOI] [PubMed] [Google Scholar]
- 22.Mathiesen O., Jacobsen L.S., Holm H.E. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth. 2008;101:535–541. doi: 10.1093/bja/aen215. [DOI] [PubMed] [Google Scholar]
- 23.Mathiesen O., Rasmussen M.L., Dierking G. Pregabalin and dexamethasone in combination with paracetamol for postoperative pain control after abdominal hysterectomy: A randomized clinical trial. Acta Anaesthesiol Scand. 2009;53:227–235. doi: 10.1111/j.1399-6576.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 24.White P.F., Tufanogullari B., Taylor J., Klein K. The effect of pregabalin on preoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg. 2009;108:1058–1061. doi: 10.1213/ane.0b013e31818d40ce. [DOI] [PubMed] [Google Scholar]


