Summary
Pediatric patients who experience a bone marrow relapse of precursor-B acute lymphoblastic leukemia are cured < 50% of the time. This study was designed to determine if intensification of therapies with known activity in this disease would improve the cure rates for patients with relapsed acute lymphoblastic leukemia. Patients were treated with intensive asparaginase during induction followed by repeated cycles of ifosfamide/etoposide and cytarabine/idarubicin. Patients with well-matched related donors were encouraged to undergo hematopoietic stem cell transplant as consolidation. The results of this study demonstrate no significant difference in disease-free survival in patients who received chemotherapy alone (45%) or chemotherapy followed by allogeneic stem cell transplant (50%). Furthermore, results from this study show no significant difference in event-free survival (39.9% ± 6.2%) or overall survival (41.6% ± 6.1%) at 8 years when compared with previous studies using less intensive regimens. Our results suggest that alternative therapies are needed to improve cure rates for pediatric patients with relapsed leukemia.
Keywords: relapsed precursor-B, ALL, intensive chemotherapy, POG study 9411, SIMAL 9
Despite intensive chemotherapy that includes central nervous system (CNS) prophylaxis, about 20% of children with precursor-B acute lymphoblastic leukemia (ALL) will relapse. Using chemotherapy plus irradiation, 70% of those with a late isolated extramedullary recurrence and 40% to 50% with early extramedullary recurrence are cured.1,2 However, using chemotherapy, cure for patients with late bone marrow relapse is only 50% and for patients with early bone marrow relapse is only 10% to 20%.3,4 Stem cell transplantation (SCT) may improve the prognosis of children with early marrow relapse but is of less value to patients with later marrow relapse or isolated CNS relapse.5,6
One approach to improving cure rates after relapse would be intensification of chemotherapy. This includes using higher doses of agents to which patients were previously exposed or the use of agents to which patients had not been previously exposed. Weekly PEG asparaginase was superior to biweekly PEG for induction therapy (95% vs. 81% CR rates) for patients with relapsed ALL in POG 9310.7 Ifosfamide/etoposide induced remission in 6/16 children with multiple relapsed ALL in POG 8870.8 High-dose cytosine arabinoside plus an anthracycline had activity in adults and children with advanced ALL.9 Idarubicin has theoretical advantages versus other anthracyclines: longer half life, better CNS penetration, and activity against multiple drug-resistant cells.10 Thioguanine was more effective than mercaptopurine in some studies.11 Divided dose of oral methotrexate with leucovorin rescue has given promising results in newly diagnosed children with ALL.12
POG 9411 was designed to test whether intensifying the use of known active agents in precursor-B ALL (more intensive asparaginase, ifosfamide-etoposide, and high-dose cytosine arabinoside-idarubicin) would result in an improved outcome in children with recurrent ALL. A companion study, POG 9410 piloted an alternative intensification with purged autologous stem cell transplant as consolidation. These results have been previously reported.13 The data reported in this paper show no improvement in outcome when compared with other reported regimens.
Patients and Methods
Between October 1, 1995 and April 15, 1997, 99 eligible patients were enrolled in the study. The protocol was approved by local institutional review boards. Informed consent was obtained from the patient or their guardians as appropriate.
Eligibility requirements included: younger than 21 years at relapse, non-T non-B ALL (precursor-B ALL), first marrow relapse with > 25% blasts (with or without extramedullary disease), or first extramedullary relapse with 5% to 25% marrow blasts. Patients with prior induction failure on an asparaginase-containing regimen were not eligible. Pregnant or lactating females were not eligible.
The chemotherapy regimen is summarized in Table 1. Patients were randomized to Escherichia coli or PEG asparaginase during induction. However, 8 patients with a history of ≥ grade 2 allergic reactions to E. coli asparaginase were nonrandomly assigned to receive PEG. Patients who entered remission proceeded to intensification followed by continuation. Each cycle of chemotherapy began after the specified interval provided that the ANC was > 500/μL and the platelets > 75,000/μL. Patients with a suitable donor underwent SCT after the second intensification treatment.
Table 1. Chemotherapy Regimens.
| Induction | |||||
| Prednisone | 40 mg/m2 PO in 3 divided doses for 28 d | ||||
| Vincristine | 1.5 mg/m2 IV days 1, 8, 15, and 22 (maximum dose 2 mg) | ||||
| Doxorubicin | 30 mg/m2 IV on days 1, 2 | ||||
| Asparaginase (randomized) | |||||
| PEG | 2500 U/m2 IM days 1, 8, 15, and 22 or | ||||
| Escherichia coli | 10,000 U/m2 IM days 1, 3, 5, 8, 10, 12, 15, 17, 19, 22, 24, and 26 | ||||
| Intrathecal therapy (age adjusted): days 1, 8, and 22 (also days 15 and 29 if CNS disease) | |||||
| Age (y) | < 1 | 1 | 2 | 3-8 | ≥ 9 |
| MTX | 7.5 | 8 | 10 | 12 | 15 |
| HDC | 7.5 | 8 | 10 | 12 | 15 |
| Ara-C | 15 | 16 | 20 | 24 | 30 |
| Consolidation 1 | |||||
| Ifosfamide | 2.8 g/m2 IV days 1-5 with MESNA 350 mg/m2 IV q 3h × 8, days 1-5 | ||||
| Etoposide | 100 mg/m2 IV days 1-5 | ||||
| G-CSF | 5 μg/kg SC daily until ANC ≥ 5000 or > 1500 on 2 occasions (after the ANC nadir) | ||||
| Consolidation 2 | |||||
| Ara-C | 1 g/m2 IV over 6 h days 1-4 | ||||
| Idarubicin | 5 mg/m2 IV days 1-4 | ||||
| G-CSF | as per consolidation 1 | ||||
| If cumulative lifetime dose of anthracycline (doxorubicin equivalents) is 450 mg/m2, substitute PEG asparaginase (2500 U/m2) weekly × 2 for idarubicin | |||||
| Patients with a closely matched (6/6 or 5/6) related donor may be removed from study for a stem cell transplant after Consolidation 2 | |||||
| Continuation (5 cycles) | |||||
| Week 1 | |||||
| Ifosfamide | 3.4 g/m2 IV days 1-3 with MESNA 425 mg/m2 IV q 3h × 8, days 1-3 | ||||
| Etoposide | 100 mg/m2 IV days 1-3 | ||||
| Week 4 | |||||
| 6-thioguanine | 75 mg/m2 PO days 1-14 | ||||
| Methotrexate | 25 mg/m2 PO q 6 h × 4, days 1 and 8 | ||||
| Leucovorin | 5 mg/m2 PO/IV q 12 h × 3 doses at days 3 and 10 | ||||
| Triple IT | day 1; doses as per induction | ||||
| Week 6 | |||||
| Ara-C | 1 gm/m2 IV over 6 h days 1-3 | ||||
| Idarubicin | 5 mg/m2 IV days 1-3; discontinue if cumulative lifetime dose (doxorubicin equivalents) approaches 450 mg/m2 | ||||
| Week 9 | |||||
| Dexamethasone | 8 mg/m2 PO in 3 divided doses days 1-14 | ||||
| Vincristine | 2 mg/m2 IV days 1 and 8 (maximum dose 2 mg) | ||||
| PEG Asparaginase | 2500 U/m2 IM days 1 and 8 | ||||
Patients with combined CNS and systemic relapse will have cranial (1800 cGy) and spinal (1200 cGy) radiation delayed until 2 cycles of continuation therapy have been delivered (approximately 6 mo into therapy).
CNS leukemia was deemed present if the CSF cell count was ≥ 5 with morphologic blasts. Patients with a lower CSF cell count were considered CNS negative. Complete remission was defined as a marrow with < 5% blasts, adequate hematopoiesis (ANC > 500/μL and platelets > 75,000/μL), and no evidence of extramedullary leukemia. Patients with 5% to 25% marrow blasts at the end of induction were considered induction failures. Marrow relapse was defined as > 25% blasts. CNS relapse required the CSF cell count to be ≥ 5 with definite blasts by morphology or a lower CSF cell count with definite blasts on at least 2 occasions at least a week apart. Other extramedullary disease required biopsy proof of leukemia. Toxicities were graded according to standard NCI toxicity criteria.
Statistical Considerations
This was a pilot study, with the primary endpoint of event-free survival (EFS), computed as the time from enrollment to first event (induction failure, relapse, secondary malignancy, or death) or last contact (nonfailures). The secondary endpoint was disease-free survival (DFS) for the patients who had achieved complete response (CR) during induction therapy, which was computed as the time from the end of induction to first event (relapse, secondary malignancy, or death) or last contact (nonfailures). Univariate analyses were conducted to study the association of age at relapse, sex, ethnicity, and induction regimen with EFS. The Kaplan-Meier method was used to obtain estimates of EFS, and the method of Peto was used to compute the corresponding SEs of estimates. The Pearson χ2 test was used to compare response rates, and the log-rank test was used to compare survival curves. Results are given as estimate ± SE in this report.
Results
Data received as of January 2006 are included in this report. Patient characteristics (Table 2) are similar to those of relapsed patients in other reports. There were 8 patients with prior significant allergic reactions to E. coli asparaginase. These patients were nonrandomly assigned to the PEG regimen.
Table 2. Patient Characteristics.
| Sex | |
| Male | 63 |
| Female | 36 |
| Age (y) | |
| 1-10 | 80 |
| > 10 | 19 |
| WBC | |
| ≤ 5000 | 25 |
| 5000-50,000 | 35 |
| > 50,000 | 7 |
| Race | |
| White | 67 |
| Black | 9 |
| Hispanic | 12 |
| Native American | 3 |
| Oriental | 5 |
| Other | 3 |
| CR1 (mo) | |
| < 24 | 24 |
| ≥ 24 | 75 |
| CR1 (mo) | |
| < 36 | 40 |
| ≥ 36 | 59 |
| CNS status | |
| CNS 3 | 1 |
| Prior Escherichia coli hypersensitivity ≥ grade 2 | 8 |
Ninety-five patients were evaluable for response to induction therapy. Fifty-two patients received PEG and 43 E. coli asparaginase. Eighty patients achieved a CR, 2 achieved partial response (5% to 25% blasts), and 1 had no response to induction therapy. Twelve patients died during induction: 10 deaths were due to infections, 1 to tumor and drug, and 1 to fever and neutropenia with negative cultures. The CR rate for the PEG regimen was 86.5% and for patients treated with E. coli asparaginase it was 81.4% (P = 0.58).
EFS for the whole group was 39.9% ± 6.2% at 8 years (Fig. 1). There have been no failures beyond 7 years among the 31 patients who were still at risk at that time. Overall survival (OS) was 41.6% ± 6.1% at 8 years. EFS and OS were not related to patient age at relapse, initial WBC, sex, ethnicity, or induction regimen. EFS was significantly better in patients with a longer duration of CR1 regardless of whether the cut was made at 24 months (P = 0.0002) or 36 months (P = 0.0001) (Fig. 2).
Figure 1.
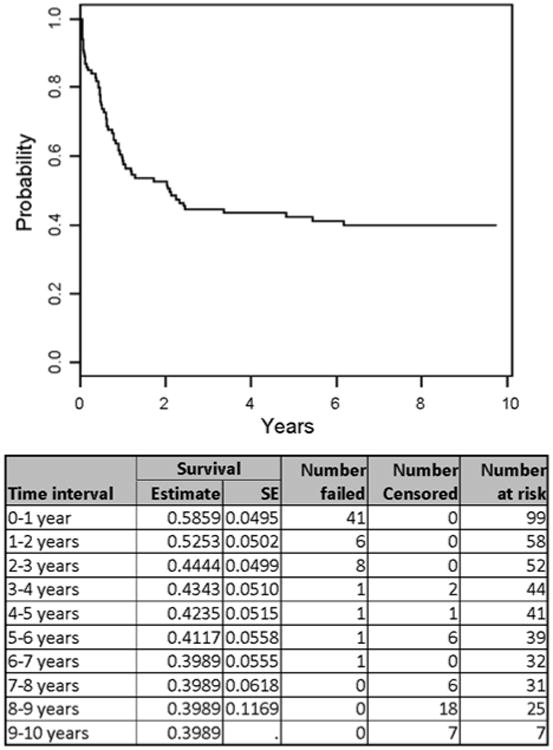
Event-free survival for all patients.
Figure 2.
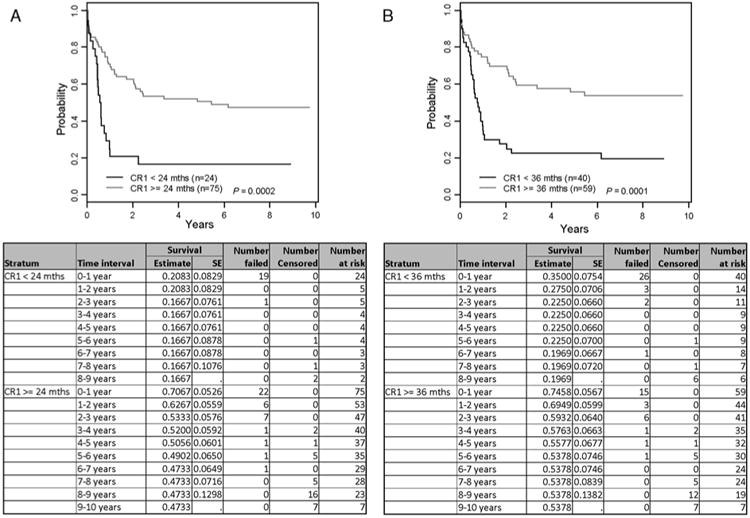
A, Event-free survival by duration of first complete remission (CR1) < 24 vs. ≥ 24 months. B, Event-free survival by duration of first complete remission (CR1) < 36 vs. ≥ 36 months.
Among the 80 patients who achieved a CR, 28 went on to receive chemotherapy alone, whereas the remaining 52 patients were withdrawn for SCT. There were no significant differences in DFS at 8 years for patients treated with chemotherapy alone (45.3% ± 11.2%) or chemotherapy followed by SCT (50% ± 9.1%) (P = 0.978). For the 28 patients who received chemotherapy alone, 14 patients relapsed and 1 patient died in remission of toxicity. Grade 3 and 4 toxicities related to chemotherapy are summarized in Table 3. There was significant myelosuppression in a majority of patients with serious bacterial infections documented in approximately half. Surprisingly there were few allergic reactions to either asparaginase preparation and toxicities did not differ between the asparaginase regimens. One patient developed a second malignancy (diffuse lymphoblastic non-Hodgkin lymphoma) 27 months after study entry. He had a short CR1 (< 24 mo) and was treated on the E. coli regimen. No patient developed secondary AML. For the 52 patients that received transplant, 26 died: 12 died of relapsed disease, 4 died of infections, and the remainder died of GVHD and/or organ dysfunction posttransplant. There was no significant difference in DFS between transplants from matched sibling donors versus other donors (P = 0.471). There was no effect of the induction randomization on DFS of the transplanted patients (P = 0.514).
Table 3. Worst Toxicities of Chemotherapy in 95 Patients Who Were Evaluable for Response.
| Grade 3 | Grade 4 | |
|---|---|---|
| Absolute neutrophils (ANC) | 2 | 90 |
| Hemoglobin | 19 | 46 |
| Platelets | 7 | 75 |
| AST/ALT | 7 | 0 |
| Bilirubin | 0 | 1 |
| Stomatitis | 11 | 8 |
| Allergic reactions | 4 | 1 |
| Pancreatitis | 4 | 1 |
| Hyperglycemia | 6 | 3 |
| Bacteremia | 2 | 40 |
| Abscess | 8 | 0 |
| Other bacterial | 5 | 1 |
| Viral | 7 | 2 |
| Pneumocystis | 1 | 1 |
Discussion
POG 9411 was designed to assess the effects of therapy intensification on outcome in a group of pediatric patients with first relapse of precursor-B ALL. The results show that the strategies used in this study, including addition of active agents and hematopoietic SCT did not significantly change the EFS or OS for this population when compared with previous less intensive regimens.4 Possible reasons for these disappointing results include: (a) the “new” combinations shared resistance mechanisms with previous treatments; (b) the incremental dosage increases were not sufficient to overcome resistance; (c) the patient population was biased toward higher risk individuals; (d) no maintenance phase (only 1 y total Rx); and (e) toxicity of treatment limited dose intensification.
The chemotherapy utilized in POG 9411 was rotating polychemotherapy cycles lasting approximately 1 year. Death due to recurrent disease was a major contributor of failure in patients who received chemotherapy alone, suggesting that further intensification by higher dose therapies or longer duration of treatment could be beneficial. However, a significant percentage of study patients receiving chemotherapy experienced grade 4 hematologic and nonhematologic toxicities including life-threatening infections. More recent trials utilizing different blocks of intensive polychemotherapy with addition of conventional maintenance therapy have not resulted in appreciable increases in EFS or OS3,14 for this patient population. In contrast, Parker and colleagues demonstrated that patients with first relapse ALL treated with a mitoxantrone-containing regimen experienced significantly better disease control and fewer toxicities than those receiving idarubicin-based chemotherapy. These results were true in patients who were treated with chemotherapy alone or chemotherapy followed by allogeneic SCT, suggesting that the choice of chemotherapy agents is more important than intensity of treatment for survival of patients with relapsed precursor-B ALL.15
Allogeneic SCT was used in our study as an intensified consolidation therapy. SCT resulted in better disease control than chemotherapy alone. However, the 30% treatment-related mortality observed in this study likely mitigated any survival gains due to leukemia control in patients receiving this therapy. Recent advances in SCT have reduced treatment-related mortality16; however, studies have failed to show a consistent survival benefit of SCT over chemotherapy alone in relapsed ALL populations.3,15,17 Improved survival after allogeneic SCT when compared with chemotherapy alone has been demonstrated for patients with high risk but not intermediate or low risk, relapsed disease.3,17,18 Despite improved outcomes in patients with HR disease features after allogeneic SCT, 30% to 60% continue to die of recurrent disease and/or transplant-related complications; numbers that are not significantly different from those reported here for POG 9411.
The major favorable prognostic factor in this study was a longer length of the initial remission (CR1). This has been noted in previous publications.19 Patients with a longer initial remission (≥ 24 mo) should be offered the options of chemotherapy or SCT. How individuals weigh the risks and benefits of each approach varies greatly. This study was not designed to test the relative merits of chemotherapy versus SCT, and provides limited additional information for that choice.
In evaluating results of different therapeutic options long-term follow-up is essential. Although most second relapses occur within a shorter time than the first relapse, late second failures do occur. In addition, OS depends not only upon the initial success rate but upon the salvage rate of treatment failures. Follow-up of our patients has shown limited success in the salvage of patients whose leukemia recurs for a second time. New approaches are required for this patient population.
Acknowledgments
The authors thank Drs Lynda Mandell and Angela Ogden for their contribution to this manuscript.
Supported by grants: CA30969, CA29139, CA98543, CA98413 from NIH.
Footnotes
The authors declare no conflict of interest.
References
- 1.Barredo JC, Devidas M, Lauer SJ, et al. Isolated CNS relapse of acute lymphoblastic leukemia treated with intensive systemic chemotherapy and delayed CNS radiation: a pediatric oncology group study. J Clin Oncol. 2006;24:3142–3149. doi: 10.1200/JCO.2005.03.3373. [DOI] [PubMed] [Google Scholar]
- 2.Uderzo C, Grazia Zurlo M, Adamoli L, et al. Treatment of isolated testicular relapse in childhood acute lymphoblastic leukemia: an Italian multicenter study. Associazione Italiana Ematologia ed Oncologia Pediatrica. J Clin Oncol. 1990;8:672–677. doi: 10.1200/JCO.1990.8.4.672. [DOI] [PubMed] [Google Scholar]
- 3.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after timepoint and site-of-relapse stratification and intensified shortcourse multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder H, Garwicz S, Kristinsson J, et al. Outcome after first relapse in children with acute lymphoblastic leukemia: a population-based study of 315 patients from the Nordic Society of Pediatric Hematology and Oncology (NOPHO) Med Pediatr Oncol. 1995;25:372–378. doi: 10.1002/mpo.2950250503. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Raetz E, Zhang MJ, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107:4961–4967. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eapen M, Zhang MJ, Devidas M, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with acute lymphoblastic leukemia in a second remission after an isolated central nervous system relapse: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Leukemia. 2008;22:281–286. doi: 10.1038/sj.leu.2405037. [DOI] [PubMed] [Google Scholar]
- 7.Abshire TC, Pollock BH, Billett AL, et al. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Blood. 2000;96:1709–1715. [PubMed] [Google Scholar]
- 8.Bernstein ML, Whitehead VM, Devine S, et al. Ifosfamide with mesna uroprotection and etoposide in recurrent, refractory acute leukemia in childhood. A Pediatric Oncology Group Study. Cancer. 1993;72:1790–1794. doi: 10.1002/1097-0142(19930901)72:5<1790::aid-cncr2820720545>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian HM, Walters RL, Keating MJ, et al. Mitoxantrone and high-dose cytosine arabinoside for the treatment of refractory acute lymphocytic leukemia. Cancer. 1990;65:5–8. doi: 10.1002/1097-0142(19900101)65:1<5::aid-cncr2820650104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Berman E. A review of idarubicin in acute leukemia. Oncology (Williston Park) 1993;7:91–98. 104. discussion 104-107. [PubMed] [Google Scholar]
- 11.Stork LC, Matloub Y, Broxson E, et al. Oral 6-mercaptopurine versus oral 6-thioguanine and veno-occlusive disease in children with standard-risk acute lymphoblastic leukemia: report of the Children's Oncology Group CCG-1952 clinical trial. Blood. 2010;115:2740–2748. doi: 10.1182/blood-2009-07-230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winick N, Shuster JJ, Bowman WP, et al. Intensive oral methotrexate protects against lymphoid marrow relapse in childhood B-precursor acute lymphoblastic leukemia. J Clin Oncol. 1996;14:2803–2811. doi: 10.1200/JCO.1996.14.10.2803. [DOI] [PubMed] [Google Scholar]
- 13.Sandler ES, Homans A, Mandell L, et al. Hematopoietic stem cell transplantation after first marrow relapse of non-T, non-B acute lymphoblastic leukemia: a pediatric oncology group pilot feasibility study. J Pediatr Hematol Oncol. 2006;28:210–215. doi: 10.1097/01.mph.0000212902.84146.81. [DOI] [PubMed] [Google Scholar]
- 14.Einsiedel HG, von Stackelberg A, Hartmann R, et al. Longterm outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemiarelapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005;23:7942–7950. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 15.Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376:2009–2017. doi: 10.1016/S0140-6736(10)62002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacMillan ML, Davies SM, Nelson GO, et al. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:16–22. doi: 10.1016/j.bbmt.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Borgmann A, von Stackelberg A, Hartmann R, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003;101:3835–3839. doi: 10.1182/blood.V101.10.3835. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler K, Richards S, Bailey C, et al. Comparison of bone marrow transplant and chemotherapy for relapsed childhood acute lymphoblastic leukaemia: the MRC UKALL X experience. Medical Research Council Working Party on Childhood Leukaemia. Br J Haematol. 1998;101:94–103. doi: 10.1046/j.1365-2141.1998.00676.x. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]


