Abstract
Cathepsin K is consistently and diffusely expressed in alveolar soft part sarcoma (ASPS) and a subset of translocation renal cell carcinomas (RCCs). However, cathepsin K expression in human neoplasms has not been systematically analyzed. We constructed tissue microarrays (TMA) from a wide variety of human neoplasms, and performed cathepsin K immunohistochemistry (IHC). Only 2.7% of 1,140 carcinomas from various sites exhibited cathepsin K labeling, thus suggesting that among carcinomas, cathepsin K labeling is highly specific for translocation RCC. In contrast to carcinomas, cathepsin K labeling was relatively common (54.6%) in the 414 mesenchymal lesions studied, including granular cell tumor, melanoma, and histiocytic lesions, but not paraganglioma, all of which are in the morphologic differential diagnosis of ASPS. Cathepsin K IHC can be helpful in distinguishing ASPS and translocation RCC from some but not all of the lesions in their differential diagnosis.
Keywords: Cathepsin K, TFE3, TFEB, Immunohistochemistry
Cathepsin K is a lysosomal papain–like cystine proteinase with strong collagenolytic and elastolytic activity, which is highly expressed in osteoclasts involved in bone homeostasis.1 Its expression has also been demonstrated in skin and lung fibroblasts, suggesting a physiologic role in maintaining homeostasis of the extracellular matrix outside the bone.2,3 Germline mutations in the cathepsin K gene underlie the sclerosing bone disorder pycnodysostosis.4 Cathepsin K knockout mice show profound osteosclerosis.5 Cathepsin K is also expressed in reactive activated macrophages, but not in resident macrophages; along these lines, consistent expression of cathepsin K has been demonstrated in granulomatous disorders including hypersensitivity pneumonitis, sarcoidosis, Wegener granulomatosis, berylliosis, and tuberculosis.6,7 In macrophages, the expression of cathepsin K is regulated by microphthalmia transcription factor (MiTF),4 which is also expressed in the melanocytic lineage where it activates expression of genes associated with melanin pigmentation. Not surprisingly, other MiTF-expressing neoplasms, such as melanoma,8 giant cell tumor,9,10 and perivascular epithelioid cell tumor,11–13 have also been shown to be immunoreactive for cathepsin K.
MiTF belongs to a family of closely related basic helix-loop-helix zipper transcription factors, designated the MiTF-TFE family, which also includes TFE3, TFEB, and TFEC.14 All of these proteins bind the same specific target DNA sequences, and thus have overlapping transcriptional targets.14 Among them, TFE3 and TFEB are implicated in gene fusions resulting from chromosome translocations in a subset of renal cell carcinoma (RCC), including the Xp11 translocation RCC15 and the t(6;11)(p21;q12) RCC.16 We hypothesized that overexpression of TFE3 fusion proteins and native TFEB in these translocation RCCs activates targets that are normally activated by MiTF in other cell types, thus explaining the frequent expression of melanocytic markers in the translocation RCC. Supporting this hypothesis, we recently showed that cathepsin K is frequently expressed in translocation RCCs, but not other RCCs.17 The expression of cathepsin K in other carcinomas has not been systematically analyzed.
Alveolar soft part sarcoma (ASPS), a rare sarcoma of unknown histogenesis, consistently harbors a der(17) t(X:17) (p11; p25), and shares the same ASPL-TFE3 gene fusion with a subset of the Xp11 translocation RCC (the ASPL-TFE3 RCC). The characteristic microscopic features of ASPS include uniform discohesive nests of eosinophilic polygonal tumor cells with distinct cell borders and abundant eosinophilic cytoplasm.18 ASPS cells are consistently negative for epithelial markers, neuroendocrine markers, and melanocytic markers.18 The differential diagnosis of ASPS is broad, and includes carcinomas such as RCCs, adrenal cortical carcinomas, and hepatocellular carcinomas, along with melanoma, paraganglioma, clear cell sarcoma, rhabdomyosarcoma, granular cell tumor, and histiocytic lesions. This differential diagnosis can be particularly challenging in limited material such as core biopsy specimens. Recently, we showed that cathepsin K is consistently diffusely overexpressed in ASPS.19 However, the usefulness of cathepsin K immunohistochemistry (IHC) in the differential diagnosis of ASPS is not known, because a comprehensive analysis of cathepsin K expression in human neoplasms has not been performed. The purpose of the current study is to survey a broad range of human neoplasms for cathepsin K immunohistochemical labeling, focusing on the lesions in the differential diagnosis of ASPS.
Materials and Methods
Source of Tissue Specimens
This study was approved by the institutional review board of the Johns Hopkins Hospital, Baltimore, MD. We investigated 1,554 neoplasms spanning 65 different subtypes, which covers a broad range of neoplasms from various organs Table 1 and Table 2. The vast majority of these cases were resected and processed at the Johns Hopkins Hospital and were arrayed in tissue microarray (TMA) format. This resulted in 71 TMAs containing multiple 0.6- to 1.5-mm cores (mean, 5.9 cores/case) of each tumor specimen and, in most instances, adjacent normal tissue. Although RCC and ASPS cases were used as positive controls for IHC, we did not formally include them in the current study, because they were analyzed previously.17,19 We also labeled whole sections of 8 additional cases of clear cell sarcoma contributed by one of the authors (C.A.) from Memorial Sloan-Kettering Cancer Center, New York, NY.
Table 1.
Cathepsin K Expression in Epithelial Neoplasms
| Cathepsin K IHC | ||||||
|---|---|---|---|---|---|---|
| Organ System | Tumor Type | Total Cases | 0 | 1+ | 2+ | 3+ |
| Adrenal gland | Adrenal cortical adenoma | 15 | 15 | 0 | 0 | 0 |
| Adrenal cortical carcinoma | 21 | 20 | 1 | 0 | 0 | |
| Bladder | Bladder micropapillary carcinoma | 15 | 15 | 0 | 0 | 0 |
| In-situ and invasive urothelial carcinoma | 26 | 25 | 1 | 0 | 0 | |
| Breast | Breast ductal adenocarcinoma | 74 | 74 | 0 | 0 | 0 |
| Breast micropapillary carcinoma | 80 | 75 | 5 | 0 | 0 | |
| Primary breast carcinoma and paired metastases | 2 | 2 | 0 | 0 | 0 | |
| Cervix | Cervical squamous intraepithelial lesion | 59 | 59 | 0 | 0 | 0 |
| Colon | Colonic adenocarcinoma | 52 | 52 | 0 | 0 | 0 |
| Esophagus | Esophageal adenocarcinoma | 49 | 48 | 1 | 0 | 0 |
| Kidney | Collecting duct carcinoma | 27 | 25 | 1 | 1 | 0 |
| Sarcomatoid RCC | 43 | 41 | 2 | 0 | 0 | |
| Liver | Fibrolamellar carcinoma | 10 | 9 | 0 | 0 | 1 |
| Hepatocellular carcinoma | 26 | 24 | 2 | 0 | 0 | |
| Mixed hepatoblastoma in adults | 1 | 1 | 0 | 0 | 0 | |
| Lung | Atypical carcinoid | 1 | 1 | 0 | 0 | 0 |
| Bronchioloalveolar carcinoma | 6 | 6 | 0 | 0 | 0 | |
| Carcinoid | 1 | 1 | 0 | 0 | 0 | |
| Lung adenocarcinoma | 23 | 23 | 0 | 0 | 0 | |
| Lung papillary adenocarcinoma | 28 | 28 | 0 | 0 | 0 | |
| Lung poorly differentiated carcinoma | 9 | 9 | 0 | 0 | 0 | |
| Lung squamous cell carcinoma | 35 | 34 | 0 | 1 | 0 | |
| Ovary | High-grade serous carcinoma | 178 | 177 | 0 | 0 | 1 |
| Biliary tract | Cholangiocarcinoma | 78 | 78 | 0 | 0 | 0 |
| Pancreas | Intraductal papillary mucinous neoplasm | 2 | 2 | 0 | 0 | 0 |
| Mucinous cystic neoplasm | 2 | 2 | 0 | 0 | 0 | |
| Pancreatic adenocarcinoma | 41 | 40 | 1 | 0 | 0 | |
| Pancreatoblastoma | 1 | 1 | 0 | 0 | 0 | |
| Solid pseudopapillary neoplasm | 12 | 12 | 0 | 0 | 0 | |
| Prostate | Small cell carcinoma | 29 | 28 | 0 | 1 | 0 |
| Prostate adenocarcinoma | 22 | 21 | 0 | 1 | 0 | |
| Salivary gland | Acinic cell carcinoma | 4 | 4 | 0 | 0 | 0 |
| Mucoepidermoid carcinoma | 3 | 3 | 0 | 0 | 0 | |
| Adenoid cystic carcinoma | 39 | 31 | 6 | 2 | 0 | |
| Stomach | Gastric adenocarcinoma | 126 | 123 | 2 | 1 | 0 |
IHC, immunohistochemistry; RCC, renal cell carcinoma.
Table 2.
Cathepsin K Expression in Mesenchymal Neoplasms
| Cathepsin K IHC | |||||
|---|---|---|---|---|---|
| Tumor Type | Total Cases | 0 | 1+ | 2+ | 3+ |
| Giant cell tumor of bone | 3 | 0 | 0 | 0 | 3 |
| Giant cell tumor of tendon sheath | 6 | 0 | 0 | 4 | 2 |
| Acral myxoinflammatory fibroblastic sarcoma | 4 | 0 | 0 | 3 | 1 |
| Juvenile xanthogranuloma | 9 | 1 | 1 | 7 | 0 |
| Kaposi sarcoma | 29 | 4 | 10 | 14 | 1 |
| Granular cell tumor | 14 | 2 | 4 | 5 | 3 |
| Melanoma | 85 | 14 | 15 | 27 | 29 |
| Liposarcoma | 13 | 3 | 3 | 6 | 1 |
| Chondrosarcoma | 4 | 1 | 0 | 2 | 1 |
| Gastrointestinal stromal tumor | 38 | 10 | 8 | 17 | 3 |
| Undifferentiated pleomorphic sarcoma (MFH) | 23 | 6 | 5 | 8 | 4 |
| Leiomyosarcoma | 12 | 4 | 3 | 4 | 1 |
| Low-grade sarcoma, NOS | 3 | 1 | 1 | 1 | 0 |
| Angiosarcoma | 9 | 4 | 3 | 2 | 0 |
| Langerhans histiocytosis | 9 | 4 | 2 | 3 | 0 |
| Epithelioid sarcoma | 10 | 5 | 2 | 3 | 0 |
| Low-grade fibromyxoid sarcoma | 5 | 3 | 1 | 1 | 0 |
| Myxoid sarcoma, NOS | 6 | 5 | 0 | 1 | 0 |
| Rosai-Dorfman disease | 3 | 2 | 1 | 0 | 0 |
| Rhabdomyosarcoma | 11 | 10 | 0 | 1 | 0 |
| Clear cell sarcoma | 12 | 9 | 1 | 2 | 0 |
| Ewing sarcoma | 28 | 23 | 4 | 1 | 0 |
| Paraganglioma | 19 | 19 | 0 | 0 | 0 |
| Pheochromocytoma | 47 | 46 | 1 | 0 | 0 |
| Angiomatoid fibrous histiocytoma | 1 | 1 | 0 | 0 | 0 |
| Chordoma | 4 | 4 | 0 | 0 | 0 |
| Low-grade fibrosarcoma | 1 | 1 | 0 | 0 | 0 |
| MPNST | 4 | 4 | 0 | 0 | 0 |
| Myxofibrosarcoma | 2 | 2 | 0 | 0 | 0 |
IHC, immunohistochemistry; MFH, malignant fibrous histiocytoma; MPNST, malignant peripheral nerve sheath tumor; NOS, not otherwise specified.
IHC Technique and Scoring of Cathepsin K Labeling
All tissue samples were fixed in formalin and embedded in paraffin according to standard methods. The TMA or the whole section slides were immunolabeled with cathepsin K antibody (clone 3F9, Abcam, Cambridge, England) using previously described methods.17 Heat-induced antigen retrieval was performed using a microwave oven and 0.01 mol/L of citrate buffer, pH 6.0, for 30 minutes. All samples were processed using a “Bond Polymer Refine” detection system in an automated Bond immunostainer (Vision Biosystem, Menarini, Florence, Italy). The results of the IHC labeling were expressed quantitatively with an H score. The intensity of labeling was graded on a scale of 0 to 3 (0 = none; 1 = weak; 2 = moderate; 3 = strong), and the percentage of labeling was recorded on a scale of 0 to 100%. An H score (0–300) was calculated by multiplying intensity and percentage scores. H scores were broken into 4 categories (0: H score=0; 1+: 0< H score <20; 2+: 20 ≤H score <100; 3+: H score ≥100). For the purpose of scoring, an H score of 0 was considered negative, H scores of less than 20 (1+) were considered focally positive, and H scores of 20 or more (2+ and 3+) were considered nonfocally positive. The positive controls included 10 specimens of remodeling bone, which showed strong diffuse labeling in osteoclasts, and a case of ASPS, which showed strong diffuse labeling (H score =300).
Results
Cathepsin K Expression in Epithelial Neoplasms
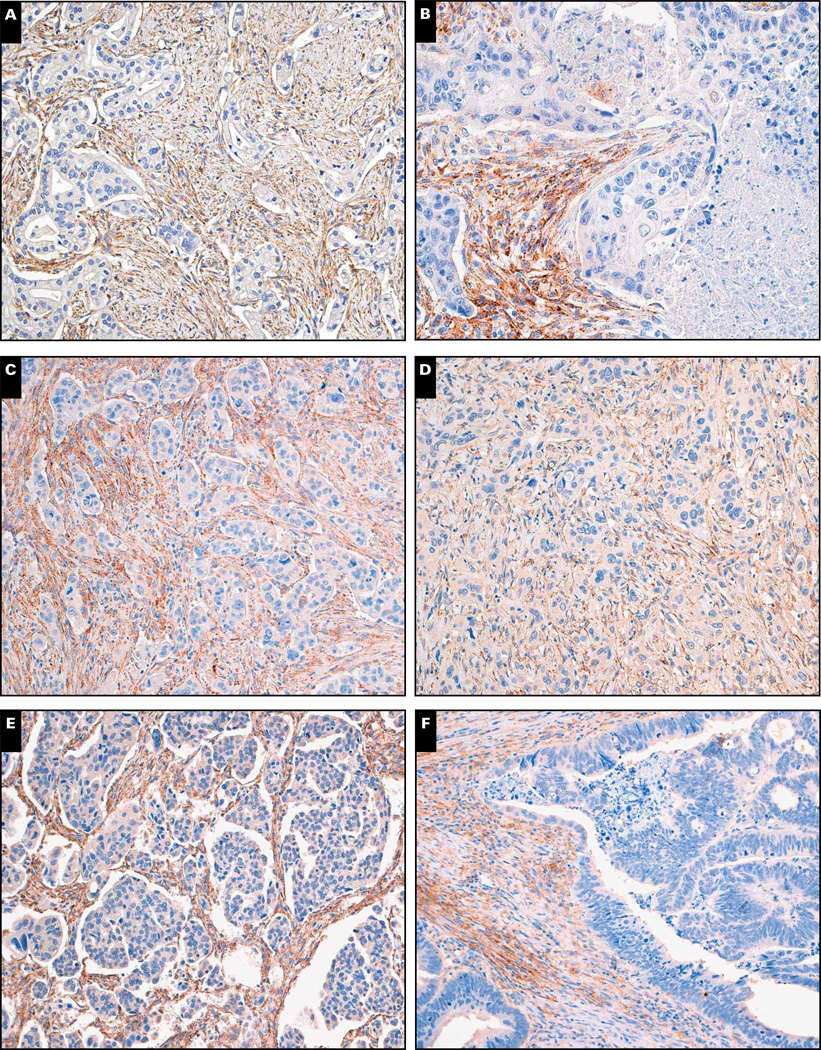
To broadly survey cathepsin K labeling in epithelial neoplasms, we assessed 1,140 neoplasms of 36 different types. This encompassed a broad range of epithelial neoplasms arising from the adrenal gland, bladder, breast, cervix, colon, esophagus, kidney, liver, lung, ovary, biliary tract, pancreas, prostate, salivary gland, and stomach (Table 1). Only 31 neoplasms exhibited cathepsin K (22 focally) labeling, thus yielding a prevalence of 2.7% (0.8% positive, 1.9% focally positive). The distribution of focal vs nonfocal labeling for each epithelial neoplasm is listed in Table 1. Overall, cathepsin K labeling was present in 1 (4.76%) of 21 cases of adrenal cortical carcinoma, 1 (3.84%) of 26 cases of urothelial carcinoma, 5 (6.3%) of 80 cases of breast micropapillary carcinoma, 1 (2.0%) of 49 cases of esophageal adenocarcinoma, 2 (7.4%) of 27 cases of collecting duct carcinoma of kidney, 2 (4.7%) of 43 cases of sarcomatoid RCC, 1 (10.0%) of 10 cases of fibrolamellar carcinoma of liver, 2 (7.7%) of 26 cases of hepatocellular carcinoma, 1 (2.9%) of 35 cases of squamous cell carcinoma of lung, 1 (0.6%) of 178 cases of ovarian serous carcinoma, 1 (2.4%) of 41 cases of pancreatic adenocarcinoma, 1 (3.4%) of 29 cases of small cell carcinoma of prostate, 1 (4.6%) of 22 cases of prostatic adenocarcinoma, 8 (20.5%) of 39 cases of adenoid cystic carcinoma, and 3 (2.4%) of 126 cases of gastric adenocarcinoma. Other carcinomas were completely negative. Interestingly, peritumoral stromal expression of cathepsin K was far more prevalent, as found in 34 (45.9%) of 74 cases of breast carcinoma, 24 (46.2%) of 52 cases of colonic adenocarcinoma, 8 (16.3%) of 49 cases of esophageal adenocarcinoma, 4 (17.4%) of 23 cases of lung adenocarcinoma, 38 (21.3%) of 178 cases of ovarian serous carcinoma, 16 (20.5%) of 78 cases of cholangiocarcinoma, 20 (48.8%) of 41 cases of pancreatic adenocarcinoma, and 13 (10.3%) of 126 cases of gastric adenocarcinoma. This labeling was primarily seen in elongated stromal cells, consistent with reactive myofibroblasts. Representative examples are shown in Image 1.
Image 1.
Peritumoral stromal expression of cathepsin K from carcinomas from stomach (A), lung (B), pancreas (C), esophagus (D), ovary (E), and colon (F) (×160).
Cathepsin K Labeling in Mesenchymal Neoplasms
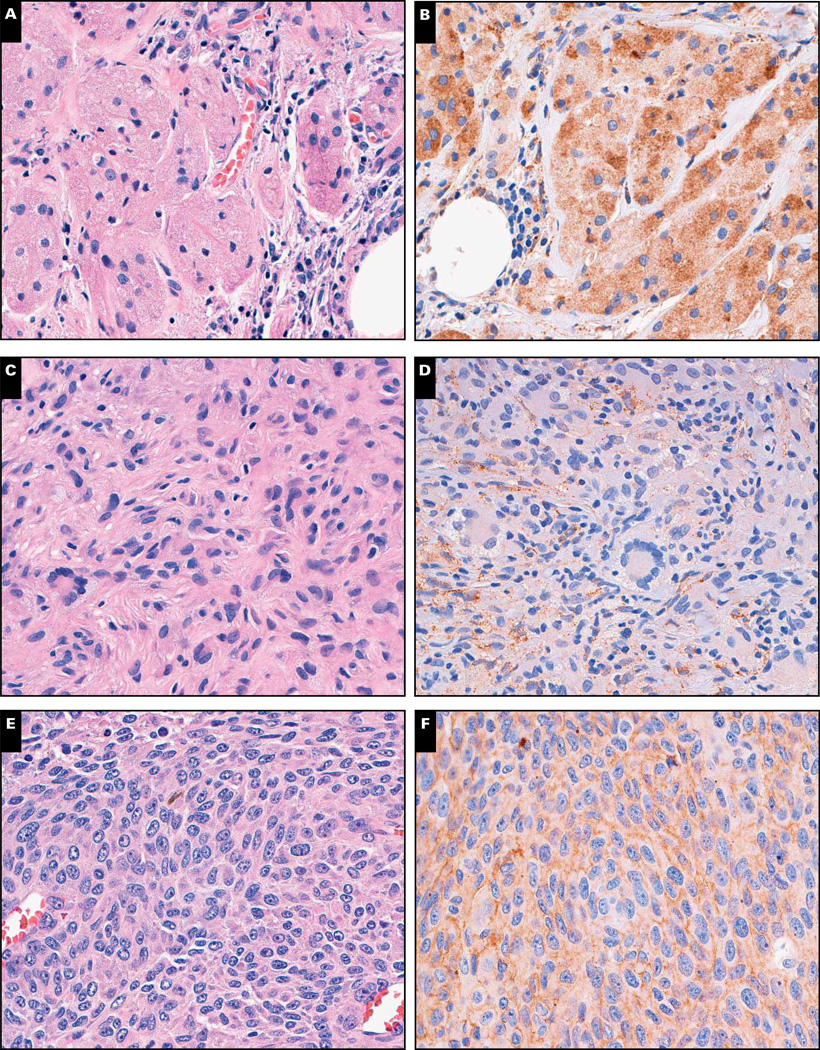
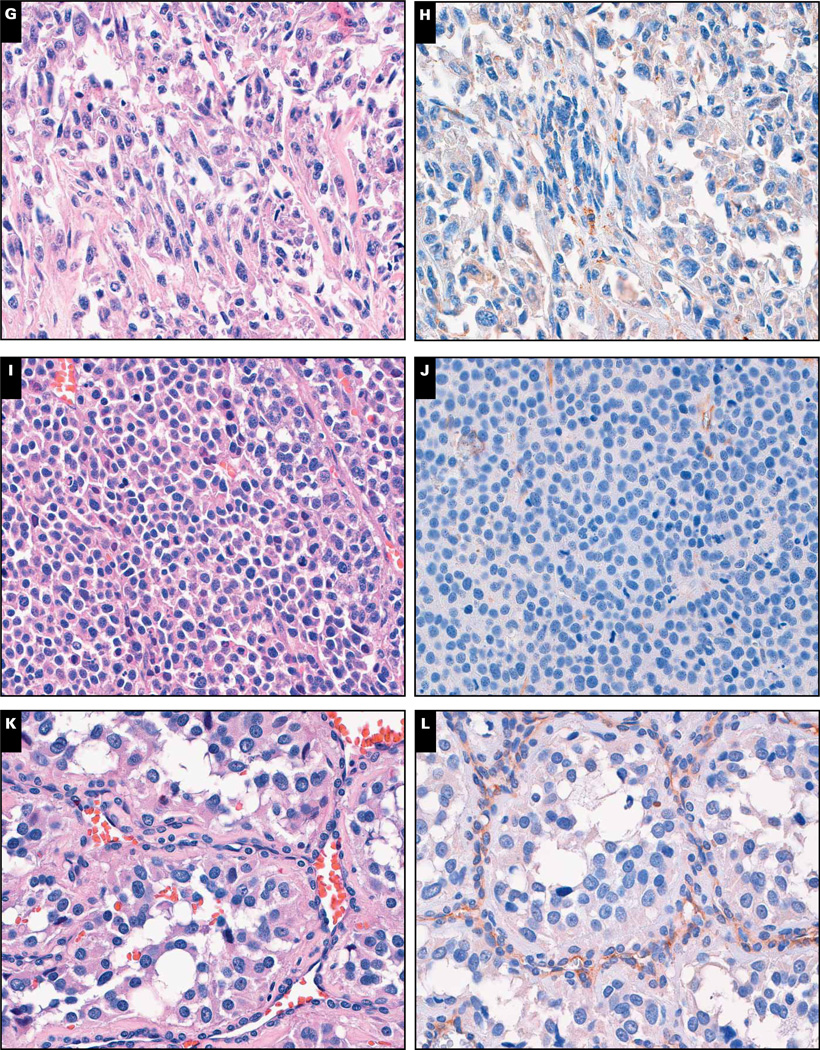
We also investigated cathepsin K expression in 414 mesenchymal neoplasms of 29 different types (Table 2). A total of 226 neoplasms were identified as cathepsin K positive (65 focally positive), yielding a prevalence of 54.6% (38.9% positive, 15.7% focally positive). The distribution of focal vs nonfocal labeling for each mesenchymal neoplasm is listed in Table 2. Cathepsin K expression was present in 3 (100%) of 3 cases of giant cell tumor of bone, 6 (100%) of 6 cases of giant cell tumor of tendon sheath, 8 (88.9%) of 9 cases of juvenile xanthogranuloma, 25 (86.2%) of 29 cases of Kaposi sarcoma, 12 (85.7%) of 14 cases of granular cell tumor, 71 (83.5%) of 85 cases of melanoma, 10 (76.9%) of 13 cases of liposarcoma, 3 (75.0%) of 4 cases of chondrosarcoma, 28 (73.7%) of 38 cases of gastrointestinal stromal tumor, 17 (73.9%) of 23 cases of undifferentiated pleomorphic sarcoma (malignant fibrous histiocytoma [MFH]), 8 (66.7%) of 12 cases of leiomyosarcoma, 2 (66.7%) of 3 cases of low-grade sarcoma, not otherwise specified, 5 (55.6%) of 9 cases of angiosarcoma, 5 (55.6%) of 9 cases of Langerhans cell histiocytosis, 5 (50.0%) of 10 cases of epithelioid sarcoma, 2 (40.0%) of 5 cases of low-grade fibromyxoid sarcoma, 1 (16.7%) of 6 cases of myxoid sarcoma, not otherwise specified, 1 (33.3%) of 3 cases of Rosai-Dorfman disease, 1 (9.1%) of 11 cases of rhabdomyosarcoma, 3 (25.0%) of 12 cases of clear cell sarcoma, 5 (17.9%) of 28 cases of Ewing sarcoma, and 1 (2.1%) of 47 cases of paraganglioma/pheochromocytoma. Representative examples are shown in Image 2.
Image 2.
Cathepsin K expression in histologic mimickers of alveolar soft part sarcoma. H&E stain and cathepsin K labeling of granular cell tumor (A and B), juvenile xanthogranuloma (C and D), and melanoma (E and F).
Clear cell sarcoma (G and H), adrenal cortical carcinoma (I and J), and paraganglioma (K and L) (×160).
Discussion
In the current study, we surveyed cathepsin K expression using IHC in a broad range of human neoplasms arising from various organs, including adrenal gland, bladder, breast, cervix, colon, esophagus, kidney, liver, lung, ovary, biliary tract, pancreas, prostate, salivary gland, stomach, skin, bone, and soft tissue. In contrast to translocation RCCs,17 most carcinomas in our study were negative for cathepsin K (2.9% prevalence of labeling). Moreover, in most carcinomas with labeling, it was focal. This suggests that cathepsin K can be helpful in distinguishing translocation RCCs from other carcinomas, and that positive labeling for cathepsin K in an epithelial neoplasm is highly suggestive of an MiTF family– related carcinoma. Although negative in most carcinoma cells, cathepsin K was expressed in the peritumoral stroma of adenocarcinomas from different organs, including breast, esophagus, stomach, colon, pancreas, biliary tract, lung, and ovary, among which stromal labeling was previously described in lung adenocarcinoma20 and breast carcinoma.21 Similar to other studies, we found cathepsin K reactivity in the desmoplastic stroma of adenocarcinomas to be mainly in elongated spindle cells morphologically consistent with reactive myofibroblasts.20,21 It has been suggested that stromal tissue expressing cathepsin K actively contributes to stromal remodeling, tumor proliferation, and invasion.22 Our study expands the list of carcinomas in which the peritumoral stroma expresses cathepsin K, suggesting a broader role of cathepsin K in the growth and invasion of human carcinomas. As cathepsin K inhibitors are being developed for cancer therapy (mainly for bone metastases),23 our study expands the spectrum of cancers that potentially could be responsive.
Consistent with the notion that MiTF-TFE family transcription factors regulate cathepsin K expression, melanoma and giant cell tumor of bone, both of which express MiTF, frequently exhibited cathepsin K labeling, consistent with previously published literature.8–10 However, depending on the cellular context, the presence of MiTF-TFE family transcription factors alone may not be sufficient to induce cathepsin K expression. For example, the ASPL-TFE3 gene fusion is present in both ASPS and a subset of translocation RCCs; however, in contrast to consistent diffuse cathepsin K positivity of ASPS, ASPL-TFE3 translocation RCCs are almost always negative for cathepsin K.19 Along these lines, clear cell sarcoma harbors a unique chromosome translocation t(12;22)(q13;q13), causing fusion of the Ewing sarcoma– associated gene (EWS) to the activity transcription factor 1 (ATF1) gene.24 EWS-ATF1 occupies the MiTF promoter and induces its expression, leading to melanocytic differentiation.25 However, in contrast to the high frequency of cathepsin K expression in melanoma, cathepsin K was expressed in only 3 of 12 cases of clear cell sarcoma in our study, again suggesting that cellular context may determine if cathepsin K expression is induced by MiTF.
The differential diagnosis of ASPS is broad, including neoplasms with nested or organoid patterns of growth and neoplastic cells with abundant eosinophilic cytoplasm. The neoplasms in this differential diagnosis include RCC, adrenal cortical carcinoma, paraganglioma, granular cell tumor, alveolar rhabdomyosarcoma, and melanoma. We previously showed that ASPS is typically diffusely positive for cathepsin K (mean, 76% strong labeling).19 Nontranslocation RCCs and (as shown in this study) adrenal cortical neoplasms are almost always negative for cathepsin K, suggesting that cathepsin K is helpful in distinguishing them from ASPS. Similarly, cathepsin K may also be used in distinguishing ASPS from paraganglioma, which is almost always completely negative for cathepsin K. In contrast to the consistent diffuse positivity of ASPS, only 3 of 12 cases of clear cell sarcoma exhibited cathepsin K labeling (one focally), suggesting that diffuse cathepsin K labeling favors ASPS in this differential diagnosis. However, for other histologic mimickers of ASPS, such as melanoma, granular cell tumor, and histiocytic lesions like juvenile xanthogranuloma, which were frequently positive for cathepsin K in our study, cathepsin K is not discriminatory. Thus, other markers, such as HMB45 for melanoma, S-100 protein or inhibin for granular cell tumor, and CD68 or CD163 for juvenile xanthogranuloma, should be used in this differential diagnosis. It is worth mentioning that TFE3 labeling may be found in a subset of granular cell tumors,18,26 which may be a potential pitfall in its differential diagnosis with ASPS.
Despite the large number of cases and tumor types tested, this study has limitations. Because of the limited sampling inherent in TMA methodology, it is possible that we may have underestimated the true prevalence of cathepsin K labeling in some tumor types, because labeling could potentially be focal and missed in a relatively small TMA sample. However, focal labeling for cathepsin K by one of its mimickers is not likely to be confused with diffuse labeling typically seen in ASPS. In addition, because of the small number of cases for some tumor types, the estimates for prevalence of cathepsin K expression in our study may be somewhat imprecise. Nonetheless, this is the first report, to our knowledge, of a systematic analysis of cathepsin K expression in human neoplasms; these findings may help future studies determine cathepsin K immunoreactivity with greater precision.
Upon completion of this activity you will be able to:
discuss the difference in cathepsin K expression between carcinoma and mesenchymal lesions.
list neoplasms included in the morphological differential diagnosis of alveolar soft part sarcoma and discuss their cathepsin K expression.
outline the cellular role of cathepsin K and the transcription factors that affect its expression.
The ASCP is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The ASCP designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™ per article. Physicians should claim only the credit commensurate with the extent of their participation in the activity. This activity qualifies as an American Board of Pathology Maintenance of Certification Part II Self-Assessment Module.
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Questions appear on p 255. Exam is located at www.ascp.org/ajcpcme.
References
- 1.Kiviranta R, Morko J, Uusitalo H, et al. Accelerated turnover of metaphyseal trabecular bone in mice overexpressing cathepsin K. J Bone Miner Res. 2001;16:1444–1452. doi: 10.1359/jbmr.2001.16.8.1444. [DOI] [PubMed] [Google Scholar]
- 2.Runger TM, Quintanilla-Dieck MJ, Bhawan J. Role of cathepsin K in the turnover of the dermal extracellular matrix during scar formation. J Invest Dermatol. 2007;127:293–297. doi: 10.1038/sj.jid.5700535. [DOI] [PubMed] [Google Scholar]
- 3.Buhling F, Rocken C, Brasch F, et al. Pivotal role of cathepsin K in lung fibrosis. Am J Pathol. 2004;164:2203–2216. doi: 10.1016/S0002-9440(10)63777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motyckova G, Weilbaecher KN, Horstmann M, et al. Linking osteopetrosis and pycnodysostosis: regulation of cathepsin K expression by the microphthalmia transcription factor family. Proc Natl Acad Sci U S A. 2001;98:5798–5803. doi: 10.1073/pnas.091479298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gowen M, Lazner F, Dodds R, et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res. 1999;14:1654–1663. doi: 10.1359/jbmr.1999.14.10.1654. [DOI] [PubMed] [Google Scholar]
- 6.Reghellin D, Poletti V, Tomassett S, et al. Cathepsin-K is a sensitive immunohistochemical marker for detection of micro-granulomas in hypersensitivity pneumonitis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:57–63. [PubMed] [Google Scholar]
- 7.Buhling F, Reisenauer A, Gerber A, et al. Cathepsin K: a marker of macrophage differentiation? J Pathol. 2001;195:375–382. doi: 10.1002/path.959. [DOI] [PubMed] [Google Scholar]
- 8.Quintanilla-Dieck MJ, Codriansky K, Keady M, et al. Cathepsin K in melanoma invasion. J Invest Dermatol. 2008;128:2281–2288. doi: 10.1038/jid.2008.63. [DOI] [PubMed] [Google Scholar]
- 9.Seethala RR, Goldblum JR, Hicks DG, et al. Immunohistochemical evaluation of microphthalmia-associated transcription factor expression in giant cell lesions. Mod Pathol. 2004;17:1491–1496. doi: 10.1038/modpathol.3800211. [DOI] [PubMed] [Google Scholar]
- 10.Lindeman JH, Hanemaaijer R, Mulder A, et al. Cathepsin K is the principal protease in giant cell tumor of bone. Am J Pathol. 2004;165:593–600. doi: 10.1016/S0002-9440(10)63323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558–1575. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 12.Martignoni G, Bonetti F, Chilosi M, et al. Cathepsin K expression in the spectrum of perivascular epithelioid cell (PEC) lesions of the kidney. Mod Pathol. 2012;25:100–111. doi: 10.1038/modpathol.2011.136. [DOI] [PubMed] [Google Scholar]
- 13.Chilosi M, Pea M, Martignoni G, et al. Cathepsin-k expression in pulmonary lymphangioleiomyomatosis. Mod Pathol. 2009;22:161–166. doi: 10.1038/modpathol.2008.189. [DOI] [PubMed] [Google Scholar]
- 14.Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- 15.Argani P, Olgac S, Tickoo SK, et al. Xp11 translocation renal cell carcinoma in adults: expanded clinical, pathologic, and genetic spectrum. Am J Surg Pathol. 2007;31:1149–1160. doi: 10.1097/PAS.0b013e318031ffff. [DOI] [PubMed] [Google Scholar]
- 16.Argani P, Lae M, Hutchinson B, et al. Renal carcinomas with the t(6;11)(p21;q12): clinicopathologic features and demonstration of the specific alpha-TFEB gene fusion by immunohistochemistry, RT-PCR, and DNA PCR. Am J Surg Pathol. 2005;29:230–240. doi: 10.1097/01.pas.0000146007.54092.37. [DOI] [PubMed] [Google Scholar]
- 17.Martignoni G, Pea M, Gobbo S, et al. Cathepsin-K immunoreactivity distinguishes MiTF/TFE family renal translocation carcinomas from other renal carcinomas. Mod Pathol. 2009;22:1016–1022. doi: 10.1038/modpathol.2009.58. [DOI] [PubMed] [Google Scholar]
- 18.Folpe AL, Deyrup AT. Alveolar soft-part sarcoma: a review and update. J Clin Pathol. 2006;59:1127–1132. doi: 10.1136/jcp.2005.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martignoni G, Gobbo S, Camparo P, et al. Differential expression of cathepsin-K in neoplasms harbouring TFE3 gene fusions. Mod Pathol. 2011;24:1313–1319. doi: 10.1038/modpathol.2011.93. [DOI] [PubMed] [Google Scholar]
- 20.Rapa I, Volante M, Cappia S, et al. Cathepsin K is selectively expressed in the stroma of lung adenocarcinoma but not in bronchioloalveolar carcinoma: a useful marker of invasive growth. Am J Clin Pathol. 2006;125:847–854. doi: 10.1309/Q96A-YDAA-J3E1-TNWT. [DOI] [PubMed] [Google Scholar]
- 21.Kleer CG, Bloushtain-Qimron N, Chen YH, et al. Epithelial and stromal cathepsin K and CXCL14 expression in breast tumor progression. Clin Cancer Res. 2008;14:5357–5367. doi: 10.1158/1078-0432.CCR-08-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlagenhauff B, Klessen C, Teichmann-Dorr S, et al. Destruction of tumour parenchyma in basal cell carcinoma by tumour-associated neutral proteases: a histochemical study. Br J Dermatol. 1991;124:271–276. doi: 10.1111/j.1365-2133.1991.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 23.Le Gall C, Bonnelye E, Clezardin P. Cathepsin K inhibitors as treatment of bone metastasis. Curr Opin Support Palliat Care. 2008;2:218–222. doi: 10.1097/SPC.0b013e32830baea9. [DOI] [PubMed] [Google Scholar]
- 24.Antonescu CR, Tschernyavsky SJ, Woodruff JM, et al. Molecular diagnosis of clear cell sarcoma: detection of EWS-ATF1 and MITF-M transcripts and histopathological and ultrastructural analysis of 12 cases. J Mol Diagn. 2002;4:44–52. doi: 10.1016/S1525-1578(10)60679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis IJ, Kim JJ, Ozsolak F, et al. Oncogenic MITF dysregulation in clear cell sarcoma: defining the MiT family of human cancers. Cancer Cell. 2006;9:473–484. doi: 10.1016/j.ccr.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Argani P, Lal P, Hutchinson B, et al. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am J Surg Pathol. 2003;27:750–761. doi: 10.1097/00000478-200306000-00005. [DOI] [PubMed] [Google Scholar]