Abstract
This study examined whether chronic Δ9-THC during early adulthood would produce the same hormonally-dependent deficits in learning that are produced by chronic Δ9-THC during adolescence. To do this, either sham-operated (Intact) or ovariectomized (OVX) female rats received daily saline or 5.6 mg/kg of Δ9-THC i.p. for 40 days during early adulthood. Following chronic administration, and a drug-free period to train both a learning and performance task, acute dose-effect curves for Δ9-THC (0.56–10 mg/kg) were established in each of the four groups (intact/saline, intact/THC, OVX/saline and OVX/THC). The dependent measures of responding under the learning and performance tasks were the overall response rate and the percentage of errors. Although the history of OVX and chronic Δ9-THC in early adulthood did not significantly affect non-drug or baseline behavior under the tasks, acute administration of Δ9-THC produced both rate-decreasing and error-increasing effects on learning and performance behavior, and these effects were dependent on their hormone condition. More specifically, both intact groups were more sensitive to the rate-decreasing and error-increasing effects of Δ9-THC than the OVX groups irrespective of chronic Δ9-THC administration, as there was no significant main effect of chronic treatment and no significant interaction between chronic treatment (saline or Δ9-THC) and the dose of Δ9-THC administered as an adult. Post mortem examination of 10 brain regions also indicated there were significant differences in agonist-stimulated GTPγS binding across brain regions, but no significant effects of chronic treatment and no significant interaction between the chronic treatment and cannabinoid signaling. Thus, acute Δ9-THC produced hormonally-dependent effects on learning and performance behavior, but a period of chronic administration during early adulthood did not alter these effects significantly, which is contrary to what we and others have shown for chronic administration during adolescence.
Keywords: Δ9-tetrahydrocannabinol, young adults, ovariectomy, behavior, learning, rat
1. Introduction
Previous research from this laboratory has demonstrated that chronic Δ9-THC in adolescent female rats resulted in long-term behavioral and pharmacodynamic effects and that these effects can depend on ovarian hormone status (Winsauer et al. 2011). A reasonable explanation for these long-term effects is that adolescence is a significant period of development and maturation (Crews et al. 2007; Trezza et al. 2008), and this may enhance an individual’s vulnerability to the effects of illicit drugs. Among the illicit drugs, cannabis is also one of the most widely abused by this age group (Gruber and Pope, Jr. 2002; Substance Abuse and Mental Health Services Administration 2005), and the psychoactive component of cannabis, Δ9-THC, has been shown to produce lasting behavioral changes in animals (O’Shea et al. 2006; O’Shea et al. 2004; Quinn et al. 2008; Stiglick and Kalant 1983) and humans (Ehrenreich et al. 1999; Pope, Jr. et al. 2003). However, the extent to which the effects of chronic Δ9-THC in females is age dependent is not known, and there is the possibility that chronic Δ9-THC alone may produce long-term effects regardless of the age at which it is administered (O’Shea et al. 2006).
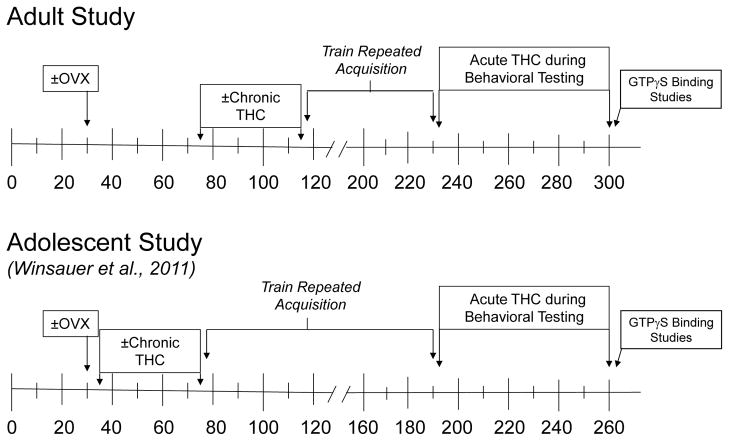
The present study was conducted as a systematic replication of an earlier study (Winsauer et al. 2011) to determine if chronic Δ9-THC in female rats during early adulthood produces the same long-term effects on non-spatial learning as chronic Δ9-THC during adolescence (see Figure 1). The presence or absence of ovarian hormones was again of interest as a cofactor because ovarian hormones have direct and indirect influences on maturation (Charmandari et al. 2003), and published data indicate that the ovarian hormone estradiol can attenuate the disruptive effects of Δ9-THC on non-spatial learning in adult female rats (Daniel et al. 2002). Ovarian hormones have also been shown to alter the binding of cannabinoid ligands in the limbic system (Riebe et al. 2010) and brain areas that are critical for learning, such as the hippocampus (Riebe et al. 2010; Winsauer et al. 2011) and striatum (Rodriguez et al. 1994; Winsauer et al. 2011). There is also substantial evidence supporting a bidirectional interaction between the endocannabinoids and gonadal hormones (for a review, see Gorzalka et al. 2012).
Fig. 1.
Timeline of manipulations for the present study and those for a study by Winsauer et al. in 2011. As shown, after behavioral training, the subjects treated chronically as young adults were re-administered Δ9-THC acutely to establish dose-effects curves.
As in the previous study, a baseline of repeated acquisition and performance was used to assay non-spatial learning and performance behavior. One advantage of this particular procedure is that responding in the performance component can serve as a behavioral control for the non-specific effects of either drug or hormone status. Having a stable baseline of acquisition (learning) behavior was also critical because of the longitudinal nature of the study. Baseline levels of responding under this procedure were characterized by two separate dependent measures, response rate and the percentage of errors. When these measures were stable for the subjects in each treatment group (i.e., intact/saline, intact/THC, OVX/saline and OVX/THC), Δ9-THC was re-administered acutely to establish dose-effect curves. Thus, differences in baseline behavior in the acquisition and performance components, as well as differences in the dose-effect curves among the groups following re-administration Δ9-THC, could be analyzed for the residual or long-term effects of chronic Δ9-THC during early adulthood.
2. Materials and methods
2.1. Subjects
Twenty-nine female Long-Evans rats served as subjects for this experiment in which chronic Δ9-THC was administered during early adulthood. To maintain the comparability with the previous study involving adolescents, all of these female rats were purchased from a commercial vendor (Harlan Sprague Dawley, Indianapolis, IN) as pups and arrived at the Animal Care facility on PD 21. Following their arrival, the pups were group housed and provided a standard diet of rodent chow (Rodent Diet 5001, PMI Inc. St. Louis, MO) and water ad libitum until PD 30 when all the subjects underwent either ovariectomy or a sham surgery. After these procedures, the subjects were individually housed in polypropylene plastic cages with hardwood chip bedding. Food restriction was also instituted at this time to maintain the compatibility of the treated groups; in this case, subjects were maintained at approximately 90% of their free-feeding weights while allowing for a gain of 5 grams per week to control for normal growth.
Throughout testing, the colony room was maintained at 21 ± 2° C with 50 ± 10% relative humidity on a 14L:10D light/dark cycle (lights on 06:00 h, lights off 20:00 h). The subjects used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, Louisiana State University Health Sciences Center, and in compliance with the recommendations of the National Research Council in the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
2.2. Adolescent Ovariectomies
Subjects were ovariectomized while under general anesthesia induced by intraperitoneal (i.p.) injection of ketamine (50 mg/kg) and xylazine (10 mg/kg). During the ovariectomy procedure, the subjects were shaved and ovaries were removed through bilateral flank incisions. Ovarian blood vessels were tied off with 4-0 silk and muscle walls were closed with absorbable 3-0 vicryl suture (Ethicon, Inc., Somerville, NJ). Skin incisions were then closed with wound clips. Female subjects that were not ovariectomized underwent sham surgeries as a control for the ovariectomy. During sham surgeries, the subjects were anesthetized with ketamine/xylazine, shaved, and bilateral flank incisions were made, but the ovaries were not isolated or removed. Female rats generally recover fully within 2 days after surgery.
2.3. Administration of Saline or Δ9-THC to Adults
Similar to the chronic regimen used with adolescents (Winsauer et al. 2011), both ovariectomized and intact (sham surgery) subjects received a single i.p. injection of either saline or 5.6 mg/kg of Δ9-THC each day for 40 days. However, in contrast to the study involving adolescents, subjects in the present study received their chronic injections from PD 75 to 115 (i.e., the beginning of sexual maturity to adulthood for rats (Spear 2000; Waynforth 1992). This yielded 4 treatment groups with respect to hormone status and chronic Δ9-THC administration (i.e., intact/saline, intact/THC, OVX/saline and OVX/THC). The Δ9-THC was obtained from the National Institute on Drug Abuse (Research Technical Branch, Rockville, MD), and arrived in a 100% ethanol solution at a concentration of either 100 or 200 mg/ml. These concentrations were then partitioned into smaller aliquots (e.g., 50 mg), lyophilized by high-speed vacuum, and then stored at −20° C. When needed, the aliquots of Δ9-THC were reconstituted for injection as an emulsion using ethanol, emulphor (Alkamuls EL-620, Rhodia, Inc., Cranbury, NJ), and saline in a proportion of 1:1:18, respectively. The volume for both saline and Δ9-THC injections was 0.1 ml/100 g body weight. As shown in Figure 1, the four groups chronically treated during adulthood began training to respond under a multiple schedule of repeated acquisition and performance of response sequences (Thompson and Moerschbaecher 1978) on PD 116.
2.4. Apparatus for Behavioral Testing
Twelve identical modular test chambers (Coulbourn Instruments, Allentown, PA, Model E10-10TC) configured specifically for rodents were used. Located on the front wall of each chamber were a houselight, speaker, auditory feedback relay, pellet trough (5.5 cm above the floor and centered), and three response keys aligned horizontally (8 cm apart, center to center, and 14.5 cm above the floor). Each response key could be transilluminated by three Sylvania 28PSB indicator lamps, one with a red plastic cap, one with a yellow cap, and one with a green cap. Response keys required a minimum force of 0.15 N for activation and correct responses produced an audible click of the feedback relay. Each chamber was enclosed within a sound-attenuating cubicle equipped with a fan for ventilation and white noise to mask extraneous sounds. All test chambers were connected to a computer programmed in MED-PC for Windows, Version IV (MED Associates, Inc., St. Albans, VT), and to cumulative recorders (Gerbrands, Arlington, MA) located within the same room.
2.5. Behavioral Procedure
As diagramed in Figure 1, the groups treated chronically as young adults were trained to respond under the behavioral procedure between PD 116–230. Preliminary training of the subjects to nose press a key in the apparatus, and to acquire a 3-response sequence on a daily basis (i.e., repeated acquisition), has been described previously (Gerak et al. 2004). After this preliminary training was completed, a multiple schedule with repeated acquisition and performance components was instituted. During the acquisition components, the three response keys in the apparatus were illuminated at the same time with one of three colors: green, red or yellow. Each subject’s task was to respond on the correct key in the presence of each color, such that a correct response in the presence of one color changed the color of the key lights as well as the position for the next correct response (e.g., keys green, center correct; keys red, left correct; keys yellow, right correct). When the response sequence was completed by emitting three correct responses (i.e., one correct response in the presence of each color), the key lights were extinguished and the stimulus light in the pellet trough was illuminated for 0.4 s. Subsequently, the response keys were illuminated with the first stimulus (i.e., green) and the sequence was reset. Within a given session, the correct response and its association with a particular color did not change, and the same sequence (in this case, center–left–right or C–L–R) was repeated during all of the acquisition components for that session. Responding on this sequence was maintained by food presentation under a second-order fixed-ratio (FR) 3 schedule such that every three completions of the sequence resulted in the presentation of a 45-mg food pellet (Purina Mills TestDiet, Richmond, VA). When rats responded on an incorrect key (in the example above, the left or right key when the green lights were illuminated), the error was followed by a 5-s period in which the keylights were extinguished and responses had no programmed consequence (i.e., timeout). An incorrect response did not reset the three-response sequence (i.e., the stimuli and the position of the correct response were the same before and after a timeout).
To establish a steady state of repeated acquisition, the sequence was changed from session to session (i.e., daily). An example of sequences for five consecutive sessions was: C–L–R, L–R–C, C–R–L, R–L–C, and L–C–R. The sequences were carefully selected to be equivalent in several ways, and there were restrictions on their ordering across sessions. Briefly, each sequence was scheduled with equal frequency and consecutive correct responses within a sequence were scheduled on different keys. Occasionally, a correct sequence position for a given color was the same for two consecutive sessions (as in the list of sequences above, L–R–C and C–R–L).
During performance components, the houselight and the response keys were illuminated. The houselight served as a discriminative stimulus for responding during this component, and unlike the acquisition component, the sequence in this component remained the same from session to session (i.e., R-C-L). In all other aspects (colored stimuli for each response in the sequence, second-order FR 3 schedule of food presentation, 5-s timeout, etc.), the performance components were identical to the acquisition components. Experimental sessions always began with an acquisition component, which then alternated with a performance component after 40 reinforcers or 20 min, whichever occurred first. Each session terminated after 150 reinforcers or 80 min, whichever occurred first. Throughout testing, sessions were generally conducted 5 days per week, Monday through Friday.
2.6. Post-Training Δ9-THC Administration during Adulthood
Following behavioral training, Δ9-THC was administered acutely to all four groups (i.e., intact/saline, intact/THC, OVX/saline and OVX/THC) between PD 231–300 to establish dose-effect curves for response rate and the percentage of errors in each group. Essentially, during these 70 days of acute testing, doses of Δ9-THC were administered in a mixed order every 3 or 4 days. The presession administration time for Δ9-THC was always 30 minutes. The dose-effect curves for each group ranged from an ineffective dose to a dose that substantially decreased overall response rate or eliminated responding entirely. As a control for these acute Δ9-THC injections, saline or vehicle injections were also administered every 4 or 5 days, 30 minutes prior to the start of the session. On days when the subjects were not receiving Δ9-THC or saline, baseline sessions (no injection) were conducted to maintain stable responding under the behavioral procedure.
2.7. WIN 55,212-2-stimulated [35S]GTPγS binding
WIN 55,212-2-stimulated activation of [35S]GTPγS binding was determined in brain slices from a subgroup of subjects from each treatment group (i.e., intact/saline, n=5; intact/THC, n=6; OVX/saline, n=7; OVX/THC, n=5). For these analyses, frozen coronal sections, 20 μm thick, were cut on a microtome cryostat, thaw-mounted on gelatinized slides, and stored at −80° C. Ten consecutive sections (alternating across two slides) were taken from each of six levels of each brain corresponding to bregma 4.20, 1.60, 0.48, −0.92, −2.80, −5.60 (Paxino and Watson, 1998). The specific regions were the prefrontal cortex (PFC), motor-1 (M1), nucleus accumbens (NAc), cingulated gyrus (Cg), striatum (Str), sensory-1 (S1), globus pallidus (GP), hippocampus (CA), basolateral amygdale (BLA), and substantia nigra (SN). Slide-mounted sections were thawed, dried and preincubated in slide mailers containing 2 mM GDP (Sigma, St. Louis, MO) in assay buffer (50 mM Tris, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, and 0.5% bovine serum albumin fatty acid-free, pH 7.4) at 25°C for 20 min to reduce basal levels of [35S]GTPγS binding. Sections were incubated in slide mailers for 2 h at 25°C in assay buffer with 2 mM GDP and 0.05 nM [35S]GTPγS (Amersham Biosciences, Piscataway, NJ) with the CB-1 agonist WIN 55,212-2 (50 μM, Sigma) to determine stimulated levels of binding. Alternate sections were incubated in assay buffer with GDP and [35S]GTPγS without WIN 55,212-2 to determine basal levels of binding. Nonspecific binding was determined in the presence of 10 μM unlabeled GTPγS (Sigma, St. Louis, MO) without WIN 55,212-2. Tissue sections were exposed to Tritium Storage Phosphor Screens (GE Healthcare Bio-Sciences Corp, Piscataway, NJ) for 12 hours, and the screens were scanned following this time period using Typhoon Scanner software (Fujitsu America, Inc., Sunnyvale, CA). Relative optical densities for regions of interest were then quantified using MCID software (InterFocus Imaging Ltd, Cambridge, England), and the experimenter was blind to treatment conditions during quantification. Values were averaged across hemispheres and across sections for each brain. Data were expressed as a percent increase in the optical density of stimulated sections from basal sections.
2.8. Data Analyses
The body-weight data from the 4 treatment groups were compared using a three-way ANOVA with hormone condition and chronic Δ9-THC administration considered to be between-group factors and the phase of the experiment as a repeated measure (SigmaStat Statistical Software, SYSTAT Software, Inc. Point Richmond, CA, USA). If significant interactions occurred, two-way and one-way ANOVA tests were conducted where appropriate and these tests were followed by Holm-Sidak post hoc tests, especially to determine differences between the treated groups and the untreated control group. Significance was accepted at α level ≤ 0.05 for all statistical tests.
Responding in the acquisition and performance components of the multiple-schedule procedure was analyzed in terms of: (1) the overall response rate (total responses/min, excluding timeouts), and (2) the overall accuracy, expressed as the percentage of errors [(incorrect responses/total responses) × 100]. However, when a dose of Δ9-THC reduced the overall response rate to less than 5 responses per minute, the percentage of errors produced by that dose was not calculated due to the small number of responses involved. To determine if there were any statistical differences among the groups in terms of their baseline behavior, data obtained after saline administration (i.e., the control data) were analyzed separately from the drug data using a two-way ANOVA with hormone status and chronic treatment serving as factors. Differences in adult sensitivity to Δ9-THC were determined by statistically comparing the dose-effect curves resulting from the acute Δ9-THC administrations during adulthood, which occurred after chronic treatment and after drug-free behavioral training. These dose-effect curves were analyzed using a two-way ANOVA with chronic treatment and dose serving as factors. Significant main effects for dose were analyzed further by Holm-Sidak post hoc tests that compared the effects of various doses on response rate and the percentage of errors with the respective control values.
Effects of hormonal status and chronic Δ9-THC were also quantified by comparing the ED50 values of the dose-effect curves established during adulthood. These ED50 values were determined by linear regression using two or more data points reflecting the slopes of the descending curve for response rate or the ascending curve for the percentage of errors. For response rate, the ED50 represented the estimated dose of Δ9-THC that decreased responding from control levels by 50%. For the percentage of errors, the ED50 represented the estimated dose of Δ9-THC that increased the percentage of errors from control levels by 50%.
WIN 55,212-2-stimulated activation of GTPγS binding was analyzed using a two-way repeated-measures ANOVA, with treatment group serving as a between-groups factor and brain region serving as a within-group, repeated measure. Multiple comparisons among the brain regions were made using Holm-Sidak post hoc tests.
3. Results
3.1. Effects on Body Weight
As shown in Table 1, the mean weights for all of the groups increased during the experiment and were unaffected by either hormone condition or chronic Δ9-THC treatment. This was verified by a three-way ANOVA, which indicated that there was only a main effect for the phase of the experiment (F(2,65)=295.26, p<0.001), but no other significant main effects (hormone condition: F(1,65)=3.69, p=0.059; chronic treatment: F(1,65)=0.4, p>0.05) or interactions (hormone condition x chronic treatment: F(1,65)=1.34; p>0.05; hormone condition x experimental phase: F(2,65)=0.33, p>0.05; chronic administration x experimental phase: F(2,65)=0.45, p>0.05; hormone condition x chronic administration x experimental phase: F(2,65)=1.17, p>0.05).
Table 1.
Mean body weight for each treatment group at three different time points during the study.
| Treatment Group | Pre-Chronic (PD 35) | Post-Chronic (PD 115) | Final (~PD 300) |
|---|---|---|---|
| Intact/Saline | 132.6 ± 2.3a | 226.6 ± 4.8b | 272.6 ± 5.5c |
| Intact/THC | 127.2 ± 3.6a | 236.7 ± 6.2b | 275.6 ± 5.4c |
| OVX/S0aline | 132.8 ± 4a | 244.3 ± 5.2b | 299.3 ± 7c |
| OVX/THC | 138.7 ± 3.8a | 233.8 ± 9.6b | 278 ± 9.6c |
For each treatment group, superscript letters indicate significant differences among the three time points (e.g., mean group weights designated with a superscript “a” are similar to each other, but significantly different from “b” and “c”).
3.2. Effects of Chronic Saline or Δ9-THC during Early Adulthood on the Behavioral Responding of Mature Females
Each of the experimental groups was successfully trained to respond under the behavioral procedure, which included both learning and performance components. In general, responding in each component was considered stable when the response rate and the percentage of errors varied by less than 20% of the mean for 10 consecutive days. The pattern of responding in the acquisition or learning component was also characterized by a decrease in the number of errors and an increase in consecutive correct completions of the response sequence each day, as within-session error reduction essentially defined sequence acquisition. The pattern of responding in the acquisition components also differed from the pattern in the performance component as sequence acquisition (learning) was not required in the performance component (i.e., the percentage of errors was typically larger in the acquisition component than in the performance component). The mean number of days required to establish stable responding under the behavioral procedure prior to re-administering Δ9-THC as an adult averaged 89 days. In addition, the mean number of days of training required for each group to achieve stable responding did not differ significantly among the experimental groups (F(3,25)=0.4, p>0.05).
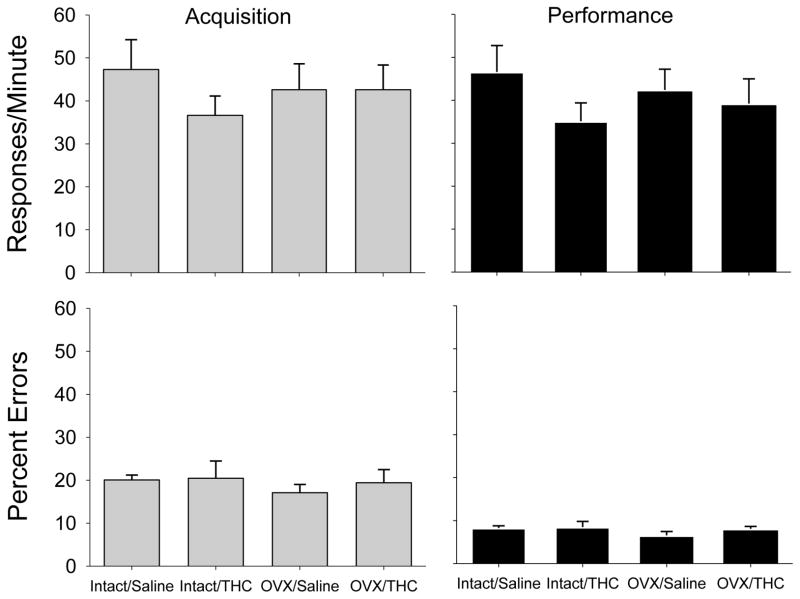
Just as the average number of days required to achieve stable responding in each behavioral component did not vary significantly among the groups, the baseline rates of responding attained by the treatment groups under baseline (no injection) and control (saline injection) conditions did not vary significantly among groups as indicated by a two-way ANOVA with hormone condition and chronic treatment serving as factors. As shown in Figure 2, for example, there were no significant main effects for hormone condition (F(1,25)=0.01, p>0.05) or chronic treatment (F(1,25)=0.67, p>0.05) and no significant interaction between factors for response rate in the acquisition component, despite the comparatively low rate emitted by the intact/THC group (hormone condition x chronic treatment, F(1,25)=0.67, p>0.05). There were also no effects of hormone condition (F(1,25)=0.00, p>0.05) or chronic treatment (F(1,25)=1.60, p>0.05) for response rate in the performance components, and no interaction between factors in the performance component (F(1,25)=0.51, p>0.05).
Fig. 2.
Bar graphs showing the effects of hormonal status and chronic Δ9-THC administration during early adulthood on baseline behavior under a multiple schedule of repeated acquisition and performance of response sequences. These data were collected after acute injections of saline during adulthood, at least 200 days post OVX (or sham) and 115 days post-chronic Δ9-THC (or saline). There were no significant main effects of hormonal status or chronic treatment, and no significant interactions between these two factors on either dependent measure (p>0.05).
Similar to the analyses conducted on the baseline rates of responding, a two-way ANOVA indicated that there was no significant effect of hormone condition (acquisition: F(1,25)=0.50, p>0.05; performance: F(1,25)=1.24, p>0.05) or chronic treatment (acquisition: F(1,25)=0.23, p>0.05; performance: F(1,25)=0.85, p>0.05) on the baseline percentage of errors for the different experimental groups. The two-way ANOVA tests also indicated that there were no significant interactions on percent errors in either component (acquisition: F(1,25)=0.12, p>0.05; performance: F(1,25)=0.47, p>0.05).
3.3. Effects of Acute Δ9-THC on Mature Intact Females Treated Chronically with Saline or Δ9-THC during Early Adulthood
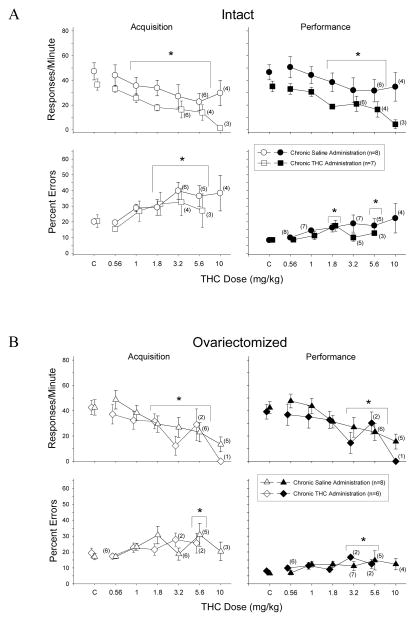
Figure 3A shows the acute effects of Δ9-THC in mature intact females that received either chronic saline or Δ9-THC as young adults. In both the saline- and Δ9-THC-treated groups, 0.56–10 mg/kg of Δ9-THC produced dose-dependent rate-decreasing and error-increasing effects in the acquisition and performance components of the behavioral procedure. More specifically, a two-way repeated-measures ANOVA on the response rate data from each component indicated that there was a main effect of dose (acquisition, F(5,59)=10.97, p<0.001; performance, F(5,59)=10.53, p<0.001), but no effect of chronic treatment (acquisition, F(1,59)=1.62, p>0.05; performance, F(1,59)=2.47, p>0.05) and no interaction between chronic treatment and dose (acquisition, F(5,59)=0.30, p>0.05; performance, F(5,59)=0.46, p>0.05). Another indication that there was little difference in sensitivity between these two chronically treated groups of intact females was that the dose-effect curves decreased in parallel fashion, suggesting a comparable rate-decreasing effect at each dose. Finally, with the two groups of intact females combined due to the absence of a treatment effect, Holm-Sidak post hoc tests indicated that doses of Δ9-THC from 1–5.6 mg/kg were significantly different from acute saline (control) injections in the acquisition component and doses from 1.8–5.6 mg/kg in the performance component. The data for 10 mg/kg were not analyzed due to the small number of subjects responding in each group at this dose.
Fig. 3.
Acute effects of Δ9-THC in intact (A) and OVX (B) adult female rats responding under a multiple schedule with acquisition and performance components. Data points and vertical lines above C in each panel indicate the grand mean and standard error of the mean (SEM) for 7–16 saline (control) injections. The data points and vertical lines in the dose-effect curves represent a grand mean and SEM for that dose, as the majority of the doses were determined at least twice in each subject. Asterisks and brackets indicate significant differences from acute saline (control) injections. Numerical values in parentheses and adjacent to a data point indicate the number of subjects represented by that point when it differed from the total number of subjects for that group (e.g., instances in which responding was eliminated entirely and the percentage of errors could not be calculated, or simply differences in the potency of Δ9-THC across subjects).
As shown in the bottom panels of Figure 3A, acute injections of Δ9-THC significantly increased the percentage of errors in both the acquisition (F(5,53)=5.36, p<0.001) and performance (F(5,55)=4.00, p=0.004) components, but there was no significant effect of chronic treatment (acquisition, F(1,53)=0.21, p>0.05; performance, F(1,55)=1.54, p>0.05) and no significant interaction between chronic treatment and dose of Δ9-THC in either component (acquisition, F(5,53)=0.29, p>0.05; performance, F(5,55)=1.04, p>0.05). Subsequent post-hoc tests indicated that the 1.8–5.6 mg/kg doses were significantly different from acute saline (control) injections in the acquisition component, whereas only the 1.8- and 5.6-mg/kg doses were significantly different from control in the performance component.
In agreement with the statistical data, the ED50 values for response rate and the percentage of errors in each behavioral component were not markedly different (i.e., more than 2-fold) between the two intact groups (see Table 2). For example, the ED50 for response rate in the acquisition component for the intact females that were treated with Δ9-THC was 2.59, whereas the ED50 for the intact females that received saline was 4.88.
Table 2.
Effective dose (ED) for decreasing response rate by 50% or increasing the percentage of errors by 50% in female rats responding under a behavioral procedure with both acquisition and performance components.
| ED50 (mg/kg) | Acquisition Component | Performance Component | ||
|---|---|---|---|---|
|
| ||||
| Group | Resp. Rate | Percent Error | Resp. Rate | Percent Error |
| Intact/Saline | 4.88 | 1.44 | - | 0.76 |
| Intact/THC | 2.59 | 2.04 | 3.35 | 1.04 |
| OVX/Saline | 5.15 | 2.59 | 5.90 | 0.94 |
| OVX/THC | 4.85 | - | 3.12 | 1.49 |
(−) indicates instances where the ED50 could not be calculated due to a less than 50% change.
3.4. Effects of Acute Δ9-THC on Mature OVX Females Treated Chronically with Saline or Δ9-THC during Early Adulthood
Figure 3B shows the acute effects of Δ9-THC in mature OVX females that received chronic saline or Δ9-THC as young adults. When the data from these groups were analyzed, the effects were very similar across the behavioral components for both chronically-treated groups. More specifically, in both the acquisition and performance components, acute Δ9-THC produced dose-dependent rate-decreasing effects and small, significant error-increasing effects. The similarity of the effects across the two chronically-treated groups and across the two behavioral tasks was also evident in the statistical analyses of the grouped data, which found only a significant effect of dose on response rate (acquisition, F(5,54)=12.68, p<0.001; performance, F(5,54)=11.92, p<0.001) and the percentage of errors (acquisition, F(5,46)=3.24, p=0.014; performance, F(5,47)=2.76, p=0.029), but no significant effect of chronic treatment (acquisition rate, F(1,54)=0.45, p>0.05; acquisition error, F(1,46)=0.0, p>0.05; performance rate, F(1,54)=0.67, p>0.05; performance error, F(1,47)=0.03, p>0.05) and no significant interaction between dose and chronic treatment on either dependent variable (acquisition rate, F(5,54)=1.28, p>0.05; acquisition error, F(5,46)=1.46, p>0.05; performance rate, F(5,54)=0.86, p>0.05; performance error, F(5,47)=1.08, p>0.05). Subsequent post hoc analysis of the effects of dose on response rate indicated that 1.8–5.6 mg/kg of Δ9-THC were significantly different from control injections for both groups in the acquisition component, whereas 3.2–5.6 mg/kg were significantly different from control for both groups in the performance component. With respect to the percentage of errors, post hoc analysis of dose indicated that only the 5.6-mg/kg dose was significantly different from control in the acquisition component, whereas the 3.2- and 5.6-mg/kg doses were significantly different from control in the performance component.
Similar to the ED50 values for the intact groups, the ED50 values for the chronically-treated OVX groups did not differ by more than two-fold for response rate and the percentage of errors in each behavioral component (see Table 2). In general, the overlap of the dose-effect curves (Figure 3B) and these small differences in the ED50 values support the absence of a significant main effect for chronic Δ9-THC or saline administration, and the absence of a significant interaction between chronic treatment and the dose of Δ9-THC in the OVX subjects.
3.5. Within-Session Effects of Acute Δ9-THC in both an Intact and OVX Female
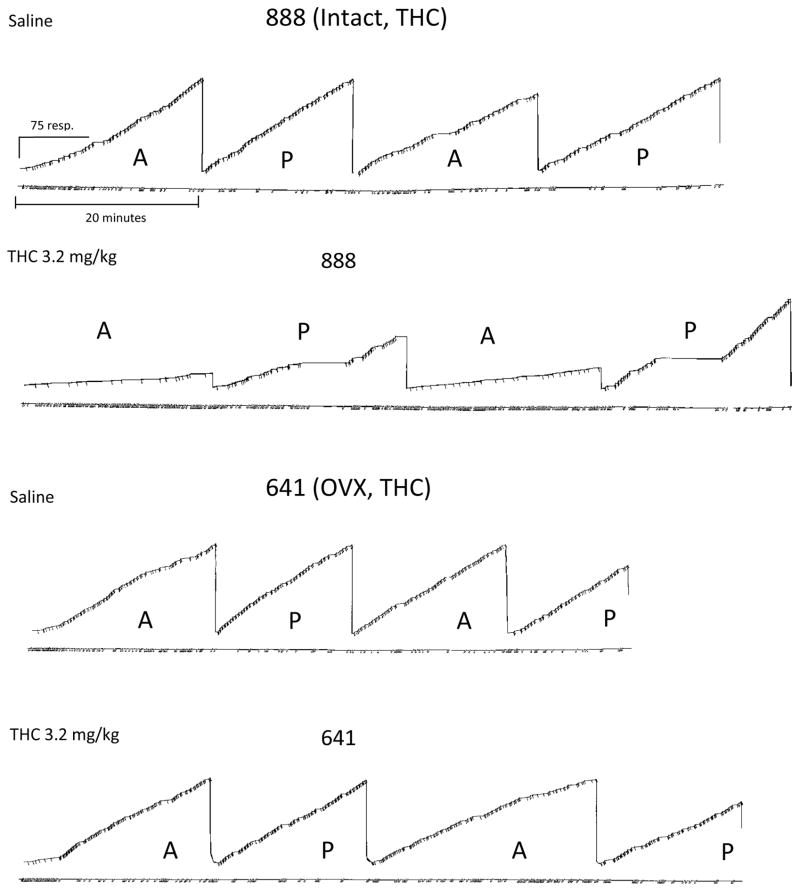
The cumulative records in Figure 4 are an illustration of the differences that were observed between intact and OVX subjects on the within-session patterns of responding after acute re-administration of Δ9-THC, which occurred long after these females had received chronic Δ9-THC as young adults. To best illustrate these differences, both a “control” record and a record from a session in which Δ9-THC was administered are shown for an intact (888) and OVX (641) subject. The purpose of showing the within-session pattern of responding is, in part, to illustrate the time course over which sequence acquisition occurred and the disruptions in the temporal pattern of responding after Δ9-THC administration. In general, the pattern of responding observed after a saline (control) injection was comparable to each subject’s daily, non-injection or baseline response pattern (not shown). During these sessions, subjects typically acquired the three-response sequence during the initial acquisition component shortly after the session began (i.e., errors occurred during the first few minutes of the component, but quickly transitioned to a pattern where fewer errors were emitted and there was a large number of consecutive errorless sequences). In subsequent acquisition components, responding was characterized by a comparatively low error rate and a high rate of correct responding compared to the initial acquisition component. The pattern of responding following sequence acquisition was also very similar to the pattern of responding in the performance component.
Fig. 4.
Representative cumulative records showing the within-session effects produced by saline (rows one and three) or 3.2 mg/kg of Δ9-THC (rows two and four) for both a intact and a OVX subject (641 and 888) responding in the acquisition (A) and performance (P) components of a multiple schedule. In each record, the response pen stepped upward with each correct response and was deflected downward each time the three-response sequence was completed. A food pellet was delivered after every three completions of the sequence. The response pen reset at the completion of each component. Downward deflections of the event pen (below each record) indicate incorrect responses in both components and the pen was deflected for the duration of the 5-sec timeout. Each session began with an acquisition component, which alternated with a performance component after 40 reinforcers or 20 min, whichever occurred first. Each session terminated after 150 reinforcers or 80 min, whichever occurred first.
As shown in the cumulative records in the second and fourth rows of Figure 4, the within-session effects of 3.2 mg/kg of Δ9-THC were different from those for a control injection and different between the intact and OVX subjects. In the intact female, this dose of Δ9-THC substantially increased the number of errors and decreased the number of consecutive correct responses, particularly in the acquisition components where there was little or no evidence that the sequence was acquired during the session. There were also instances in the performance components where the subject paused for an extended period of time, which likely contributed to the significant difference in response rate from control sessions. In contrast, in the OVX female, 3.2 mg/kg of Δ9-THC produced only small rate-decreasing effects as indicated by the longer session, and small increases in errors in both components compared to the control session. Moreover, this subject was able to acquire the sequence during the first acquisition component, despite a somewhat higher density of errors throughout the session compared to the errors emitted under control conditions.
3.6. Effects of OVX on WIN 55,212-2-stimulated activation of GTPγS binding
WIN 55,212-2-activation of G-proteins was increased when compared with the basal levels of activation. In addition, little non-specific binding in the presence of excess unlabeled GTPγS was evident (not shown). Table 3 shows the mean values for the relative amount of stimulation across brain areas when the saline- and Δ9-THC-treated subjects were combined by hormonal status (i.e., either intact and OVX). This was done because the behavioral data indicated that there was no significant effect of chronic Δ9-THC and no significant interaction between chronic Δ9-THC and hormone condition. With respect to GTPγS binding, the amount of activation significantly differed among the brain regions (F(9,176)=4.51, p<0.001), but there was no significant effect of hormonal condition (F(1,176)=0.27, p>0.05) and no significant interaction of hormonal condition and brain region (F(9,176)=1.68, p>0.05). Subsequent post hoc tests then indicated that the activation of GTPγS binding was similar between the GP and SN, and that binding in these regions was significantly different from signaling in the S1, striatum, and PFC. None of the other comparisons between brain areas were significantly different.
Table 3.
Relative percent increase in WIN 55,212-2-stimulated activation of [35S]GTPγS binding in specific brain areas after either a sham operation or OVX.
| Group | Brain Region | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PFCb | M1ab | NAcab | Cgab | Strb | S1b | GPa | CAab | BLAab | SNa | |
| Intact | 6.63 | 8.26 | 10.40 | 12.50 | 9.54 | 2.89 | 26.38 | 8.90 | 9.97 | 30.61 |
| OVX | 10.22 | 12.58 | 16.29 | 15.93 | 3.56 | 3.63 | 14.97 | 5.85 | 9.11 | 10.44 |
PFC=prefrontal cortex, M1-motor area 1, NAc=nucleus accumbens, Cg=cingulate gyrus, Str=striatum, S1=sensory area 1, GP=globus pallidus, CA=hippocampus, BLA=basolateral amygdala, SN=substantia nigra. The superscript letters indicate significant differences among the various brain regions (e.g., a superscript “a” is significantly different from b, but not different from an “ab”).
3.7. Uterine Weights
Uterine weight was analyzed using a two-way ANOVA with hormone condition (intact versus ovariectomized) and chronic treatment (saline versus Δ9-THC) serving as factors. This analysis indicated that there was a significant effect of hormone condition on uterine weight at sacrifice (F(1,25)=45.23, p<0.001), but no effect of chronic treatment (F(1,25)=1.88, p>0.05) and no interaction of chronic treatment and hormone condition (F(1,25)=1.43, p>0.05). The mean weight and SEM for the uteri of all of the gonadally-intact females (n=15) was 73.8 ± 9.3 mg, whereas the mean weight and SEM for the uteri of the ovariectomized females (n=13) was 10.6 ± 1.3 mg, confirming the effectiveness of the hormone manipulation (i.e., OVX).
4. Discussion
A comparison of the statistically significant rate-decreasing and error-increasing effects of acute Δ9-THC administration in the intact and OVX female rats indicated that the two intact groups (intact/saline, intact/THC) were more sensitive than the two OVX groups (OVX/saline, OVX/THC) as mature adults. These effects are consistent with those shown previously by our laboratory (Winsauer et al. 2011) and others (e.g., (Fattore et al. 2007; Rubino et al. 2008). However, unlike our previous study involving adolescent females, there were no main effects of chronic Δ9-THC administration or an interaction between chronic administration and hormonal condition on either the behavioral or GTPγS binding measures. In addition, neither OVX on PD 30 nor chronic Δ9-THC from PD 75–115 (early adulthood) significantly affected non-drug or baseline behavior of repeated-acquisition (learning) and performance, which contrasts with our previous study and suggests that age is an important variable in the long-term effects of chronic Δ9-THC.
As mentioned above, across the two studies from our laboratory, the intact females were more sensitive to the rate-decreasing and error-increasing effects of Δ9-THC than the OVX females. In the present study, for example, response rates in the acquisition and performance components were significantly decreased by 1 and 1.8 mg/kg in the intact groups, respectively; whereas response rates in these components were significantly decreased by doses one-quarter log unit higher in the OVX groups (i.e., 1.8 and 3.2 mg/kg). A similar difference in potency also occurred for the percentage of errors in that 1.8 mg/kg of Δ9-THC significantly disrupted accuracy of responding in both the acquisition and performance components in the intact groups, whereas 5.6 and 3.2 mg/kg, respectively, disrupted accuracy in these two components in the OVX groups.
Another similarity between the present study and our previous study was the overall sensitivity of cannabinoid signaling (i.e., GTPγS binding) in brain areas such as the globus pallidus, substantia nigra, and striatum. Interestingly, the globus pallidus was the brain region that showed the largest percent increase in agonist-stimulated GTPγS binding in both studies, whereas the striatum and substantia nigra showed the second largest percent increases during adolescence and early adulthood, respectively. These binding data also demonstrate the importance of the extrapyramidal system as a locus for the interaction of chronic Δ9-THC and the ovarian hormones. Although non-significant, GTPγS binding in the OVX rats was substantially reduced in all three areas compared with intact rats (i.e., 43% in GP, 63% in Str, and 66% in SN), which may, in part, explain the reduced sensitivity of the OVX groups to the rate-decreasing effects of Δ9-THC. Nonetheless, the relative amounts of GTPγS binding within the extrapyramidal system clearly point to the importance of cannabinoid signaling in behaviors requiring motor behavior, and indicate why cannabinoids have been tested for their clinical effectiveness against spasticity in movement disorders such as multiple sclerosis (Baker et al. 2000; Malfitano et al. 2008).
Probably the largest difference between the present study involving early adults and our previous study involving adolescents was the long-term effects of chronic Δ9-THC in the intact groups. In adult females that had received chronic Δ9-THC as adolescents, the rate-decreasing effects of acute Δ9-THC were attenuated, while the error-increasing effects were enhanced compared to the effects in adult females that received chronic saline as adolescents (Winsauer et al. 2011). Moreover, the long-term behavioral effects produced in adolescents were consistent with specific long-term pharmacodynamic effects. For example, the attenuation of Δ9-THC’s rate-decreasing effects in the intact/THC group was consistent with significant decreases in GTPγS binding in the globus pallidus, whereas the enhancement of Δ9-THC’s error-increasing effects was consistent with significant increases CB1R levels in the hippocampus. Although the explanation for the significant increase in CB1R in the hippocampus is unknown at this time, it was somewhat unexpected in that chronic treatment with a cannabinoid agonist typically leads to downregulation of receptors and a desensitization in response to agonist (Breivogel et al. 1999; Coutts et al. 2001; Delatte et al. 2002; Martin et al. 2004; Rubino et al. 2000a). Moreover, decreases in GTPγS binding can decrease CB1R signaling (Rubino et al. 2000b). Given that the brain levels of CB1R were assessed long after the chronic THC administration, there is the possibility that the increase was due to some form of compensatory response after chronic administration was terminated. Determining the events behind such an increase in receptors is also complicated by the reciprocal relationship between the endocannabinoids and the estrogens, particularly in intact female rats where endocannabinoid activity and CB1 receptor densities in brain appear to fluctuate across the different stages of their 4-day estrous cycle (Rodriguez et al. 1994). In any case, the long-term effects observed after chronic administration in adolescents suggested that CB1R in the hippocampus are as integral for Δ9-THC–induced disruptions in accuracy in repeated-acquisition procedures as they have been shown to be for Δ9-THC–induced disruptions in eight-arm radial-maze procedures (Lichtman et al. 1995) and delayed non-matching-to-sample procedures (Hampson and Deadwyler 1999; Hampson and Deadwyler 2000; Heyser et al. 1993). These data also extended previous findings from antagonism studies involving both monkeys (Winsauer et al. 1999) and rats (Brodkin and Moerschbaecher 1997) demonstrating that Δ9-THC-induced increases in the percentage of errors in repeated-acquisition procedures are mediated by CB-1 receptors.
Why fewer behavioral and pharmacodynamic effects were observed when chronic Δ9-THC was administered during early adulthood is unclear. Given that the experimental design replicated all of the conditions in the adolescent study, except the age of chronic Δ9-THC administration, the present data suggest that the intact females were less susceptible to the chronic effects of Δ9-THC as early adults than as adolescents. Age-dependent effects were also strongly suggested by the fact that chronic Δ9-THC did not produce long-term disruptions of baseline rates of responding among the groups when it was administered during early adulthood, but it did when administered during adolescence (Winsauer et al. 2011). Specifically, intact adolescent female rats that received chronic Δ9-THC had the lowest response rates of all of the groups, and the OVX groups had significantly higher response rates than both intact groups irrespective of the chronic regimen (i.e., saline or Δ9-THC). These data would also fit with the notion that estrogens lose their influential role after extended periods of time without them (i.e., a critical period). For example, estrogen replacement after an extended period of deprivation in female rodents is not very effective at restoring either reproductive behavior or deficits in declarative memory and attentional processes (for review, see Daniel and Bohacek 2010). With respect to the present data, both the age at which chronic Δ9-THC administration occurs and the age at which training occurs seem critically important for observing the interaction with ovarian hormones. Furthermore, the disruption of the baseline behavior in adolescent females that received chronic Δ9-THC would seem to be another indicator that Δ9-THC during this period of development has consequences above and beyond the change in adult sensitivity that was observed in both groups.
In summary, the present data complement and extend data in the literature on the hormonally-dependent behavioral and pharmacodynamic effects of Δ9-THC in female rats (Biscaia et al. 2003; O’Shea et al. 2006; Riebe et al. 2010; Stiglick and Kalant 1983). The present data also demonstrate that OVX prior to adolescence produces less sensitivity to the rate-decreasing and error-increasing effects of acute Δ9-THC as adults than intact females under the same conditions. Finally, these data along with previous data from this laboratory (Winsauer et al. 2011) demonstrate the age-dependence of this interaction as chronic Δ9-THC was less interactive with ovarian hormones during early adulthood than adolescence in female rats.
Highlights.
We administered chronic THC to intact and OVX female rats in early adulthood
We examined these subjects for changes in their capacity to learn as adults
We also examined their sensitivity to THC as adults
Sensitivity to THC as an adult was directly related to hormonal status
Chronic THC during early adulthood did not alter adult sensitivity to THC
Acknowledgments
This work was supported by USPHS grant DA019625 (P.J.W.) from the National Institute on Drug Abuse
Footnotes
Conflicts of interest
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- Biscaia M, Marin S, Fernandez B, Marco EM, Rubio M, Guaza C, Ambrosio E, Viveros MP. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology (Berl) 2003;170:301–308. doi: 10.1007/s00213-003-1550-7. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Brodkin J, Moerschbaecher JM. SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J Pharmacol Exp Ther. 1997;282:1526–1532. [PubMed] [Google Scholar]
- Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59:161–179. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Anavi-Goffer S, Ross RA, MacEwan DJ, Mackie K, Pertwee RG, Irving AJ. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J Neurosci. 2001;21:2425–2433. doi: 10.1523/JNEUROSCI.21-07-02425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta. 2010;1800:1068–1076. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Brauner IN, Moerschbaecher JM. Estrogen improves response accuracy and attenuates the disruptive effects of delta9-THC in ovariectomized rats responding under a multiple schedule of repeated acquisition and performance. Behav Neurosci. 2002;116:989–998. [PubMed] [Google Scholar]
- Delatte MS, Winsauer PJ, Moerschbaecher JM. Tolerance to the disruptive effects of Delta(9)-THC on learning in rats. Pharmacol Biochem Behav. 2002;74:129–140. doi: 10.1016/s0091-3057(02)00966-8. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol. 2007;152:795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Stevenson MW, Winsauer PJ, Moerschbaecher JM. Effects of pregnanolone alone and in combination with other positive GABAA modulators on complex behavior in rats. Psychopharmacology (Berl) 2004;173:195–202. doi: 10.1007/s00213-003-1717-2. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Dang SS. Minireview: endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behavior. Endocrinology. 2012;153:1016–1024. doi: 10.1210/en.2011-1643. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG., Jr Marijuana use among adolescents. Pediatr Clin North Am. 2002;49:389–413. doi: 10.1016/s0031-3955(01)00011-6. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life Sci. 1999;65:715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci. 2000;20:8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther. 1993;264:294–307. [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Malfitano AM, Proto MC, Bifulco M. Cannabinoids in the management of spasticity associated with multiple sclerosis. Neuropsychiatr Dis Treat. 2008;4:847–853. doi: 10.2147/ndt.s3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Sim-Selley LJ, Selley DE. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci. 2004;25:325–330. doi: 10.1016/j.tips.2004.04.005. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- O’Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol. 2006;20:611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- Paxino G, Watson C. The rat brain in stereotaxic coordinates. 4. New York, NY: Academic Press; 1998. [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mallet PE, Kashem MA, Matsuda-Matsumoto H, Iwazaki T, McGregor IS. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- Riebe CJN, Hill MN, Lee TTY, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–1269. doi: 10.1016/j.psyneuen.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez dF, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano’ D, Massi P, Spinello M, Zagato E, Giagnoni G, Parolaro D. Chronic delta-9-tetrahydrocannabinol treatment increases cAMP levels and cAMP-dependent protein kinase activity in some rat brain regions. Neuropharmacology. 2000a;39:1331–1336. doi: 10.1016/s0028-3908(99)00196-3. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano’ D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Costa B, Colleoni M, Parolaro D. Loss of cannabinoid-stimulated guanosine 5′-O-(3-[(35)S]Thiotriphosphate) binding without receptor down-regulation in brain regions of anandamide-tolerant rats. J Neurochem. 2000b;75:2478–2484. doi: 10.1046/j.1471-4159.2000.0752478.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Behavioral effects of prolonged administration of delta 9-tetrahydrocannabinol in the rat. Psychopharmacology (Berl) 1983;80:325–330. doi: 10.1007/BF00432114. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies. Rockville, MD: Dejpartment of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2005. Results from the 2004 National Survey on Drug Use and Health: National Findings. DHHS Publication No. SMA 05-4062. NSDUH Series H-28. [Google Scholar]
- Thompson DM, Moerschbaecher JM. Operant methodology in the study of learning. Environ Health Perspect. 1978;26:77–87. doi: 10.1289/ehp.782677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Cuomo V, Vanderschuren LJ. Cannabis and the developing brain: insights from behavior. Eur J Pharmacol. 2008;585:441–452. doi: 10.1016/j.ejphar.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Waynforth HB. Experimental and Surgical Technique in the Rat. London: Academic Press Inc; 1992. [Google Scholar]
- Winsauer PJ, Daniel JM, Filipeanu CM, Leonard ST, Hulst JL, Rodgers SP, Lassen-Greene CL, Sutton JL. Long-term behavioral and pharmacodynamic effects of delta-9-tetrahydrocannabinol in female rats depend on ovarian hormone status. Addict Biol. 2011;16:64–81. doi: 10.1111/j.1369-1600.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]