Abstract
Membrane peeling is a standard vitreoretinal procedure, where the surgeon delaminates a very thin membrane from retina surface using surgical picks and forceps. This requires extremely delicate manipulation of the retinal tissue. Applying excessive forces during the surgery can cause serious complications leading to vision loss. For successful membrane peeling, most of the applied forces need to be very small, well below the human tactile sensation threshold. In this paper, we present a robotic system that combines a force sensing forceps tool and a cooperatively-controlled surgical robot. This combination allows us to measure the forces directly at the tool tip and use this information for limiting the applied forces on the retina. This may prevent many iatrogenic injuries and allow safer maneuvers during vitreoretinal procedures. We show that our system can successfully eliminate hand-tremor and excessive forces in membrane peeling experiments on the inner shell membrane of a chicken embryo.
I. Introduction
Peeling translucent and very thin membranes from the retina is a standard procedure in vitreoretinal surgery. In current practice, the surgeons first create an edge in the membrane with a pick, then elevate and peel the membrane using forceps. Nevertheless, there are severe challenges associated with this procedure, such as the poor visualization and inconsistent tissue properties. In addition, limitations of human capabilities considering hand tremor, fatigue and lack of force feedback have great impact on the outcome of the surgery. For this reason, atraumatic and complete internal limiting membrane (ILM) removal is extremely difficult for surgeons without extensive surgical experience. Inadvertent pinches to the nerve fiber layer, retinal oedema and focal hemorrhages are some of the common complications [1].
There are various studies approaching the challenges of vitreoretinal surgery from different aspects. Special instruments have been developed for faster membrane peeling with fewer attempts [2]. ILM staining dyes have been used as visualization aid allowing easy and controlled maneuvers [1]. Furthermore, robotic assistance systems have been developed to enhance surgeon’s dexterities. Among these systems, one implemented approach has been to scale the hand motion of the surgeon by using a master slave robot to improve the control and minimize hand tremor [3]. Other approaches have focused on canceling hand tremor by compensating undesirable tool motion with an active handheld instrument [4] or by stabilizing the surgeon’s hand with a cooperatively-controlled surgical robot [5].
Despite the benefits of aforementioned studies, iatrogenic injuries by the instrumentation may still occur due to excessive forces applied on the retina. An example for this is reported in [6] where the retina was damaged with forceps although the ILM was stained. The experiments of Gupta et al. may clarify the cause of this injury, where they claimed that most of the forces that are applied to the retina during surgery are below human tactile sensation [7]. Addressing this issue, Berkelman et al. has designed a microsurgical tool that can measure the tip forces [8] and later on Jagtap et al. used this tool for in vivo experiments [9]. However Jagtap et al. have reported that discrimination between forces applied at the tool tip and forces due to contact with the sclera is a significant challenge in the development of useful force feedback. Therefore a family of instruments with force sensing was developed at Johns Hopkins University, which can measure the force directly at the tool tip. First, a 1-DOF force sensing prototype [10], then a 2-DOF pick like instrument [11] were built with fiber Bragg grating (FBG) sensors. The 2-DOF pick was also used in combination with the steady-hand robot [12]. This was followed by a manual pair of 2-DOF force sensing forceps [13], since membrane peeling is mostly done with forceps.
In this paper, we report the next step of our 2-DOF force sensing forceps which can be used in combination with the steady-hand robot. In the following sections, we will first present the design, fabrication and calibration steps of our new tool. This will be followed by the experiment results and performance assessment of the inner shell membrane peeling of a chicken embryo with tremor canceling and force scaling features of our steady-hand robot. The paper concludes with discussion of the results.
II. Force Sensing Micro-Forceps
A. Design and Fabrication
The design concept of our micro-forceps aims not only at achieving technical functionality but also at meeting the requirements of the surgical environment (see Fig. 1). Reusable forceps must be cleaned from protein remains and sterilized after every operation. As the tool goes through many cycles of operation, cleaning and sterilization, material fatigue may occur and surface properties may change. Consequently, forceps cannot grasp the membrane as required and in worst case they break during the surgery. In order to avoid such problems and reduce the associated cost, current trend in vitreoretinal surgery is to use disposable forceps. Another important advantage of disposable forceps is the axially symmetric handle which is very convenient for rotating the tool around the tool shaft, since rotation is crucial for membrane peeling surgery to grasp the membrane with the right angle.
Figure 1.
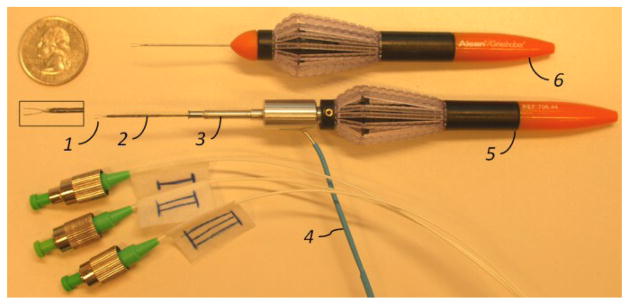
1: The graspers of 23Ga disposable forceps, 2:Nitinol tube with FBGs, 3: Easy-release mechanism, 4: Optical fibers, 5: Handle of the disposable forceps, 6: Original 23Ga disposable forceps (Alcon, USA).
The idea of integrating force sensing to disposable forceps is a challenging design task since using new sensors for every operation would not be a cost effective solution. Another challenge is integrating the rotation functionality: FBG sensors could easily be damaged or could block surgeon’s maneuvers during rotation. We have solved these problems by building two mechanically decoupled functional parts: the force sensing module and the forceps mechanism.
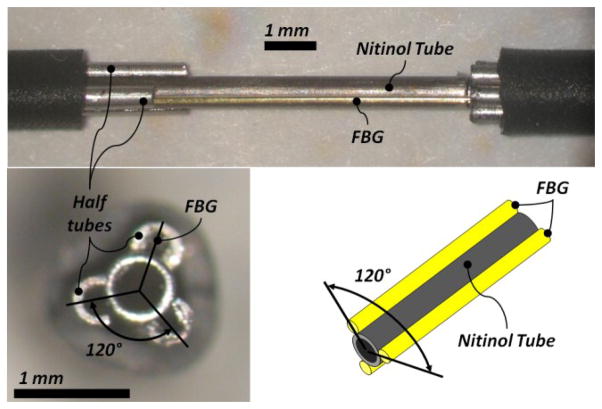
The force sensing module, shown in Fig. 2, is a 23Ga nitinol tube with three embedded FBG strain sensors. FBG strain sensors (Smart Fibers, UK) are preferred mainly due to their small dimension, high sensitivity, biocompatibility, sterilizability and immunity from electro-static and electromagnetic noise. For axially symmetric FBG positioning around the nitinol tube, first we built a small apparatus that could place three half tubes on the nitinol tube. Then we inserted the FBGs through these half tubes. After the FBGs were glued the half tubes were removed. The nitinol tube with force sensors was mounted into a custom quick-release mechanism that connected the steady-hand robot tool holder. In order to monitor the FBG strain sensors, an optical sensing interrogator, sm 130–700 from Micron Optics Inc. (Atlanta GA), was used.
Figure 2.
The force sensing module and its fabrication steps. First, three half tubes were located on the nitinol tube (upper). Second, FBGs were inserted through half tubes to achieve axi-symmetric configuration (lower).
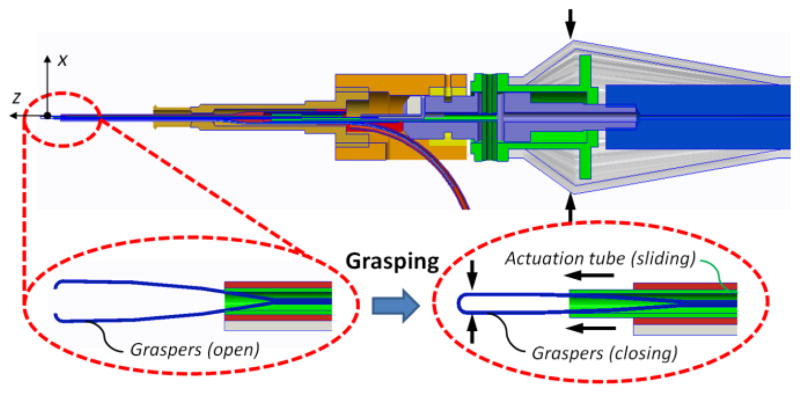
Our forceps mechanism consists of an actuating tube and graspers. For this mechanism we have customized disposable forceps from Alcon, Inc. (Fort Worth, TX) as shown in Fig. 1. The graspers were taken off from a 23Ga disposable forceps and actuation tube was built with a 27Ga stainless steel hypodermic needle. The handle of a disposable forceps tool was customized, so that the graspers and the actuation tube could easily be replaced after each operation. As shown in Fig. 3, the mechanism can be actuated by squeezing the tool handle. The squeezing motion causes the actuation tube to slide in the distal direction so that the graspers are closed. The graspers open again if the handle is released. Fig. 4 shows the rotation functionality, where the tool tip can be rotated by rotating the tool handle. In either case, the force sensing module remains stationary relative to the easy-release mechanism.
Figure 3.
Squeezing the handle slides the actuation tube (green) in distal direction and closes the graspers (blue).
Figure 4.
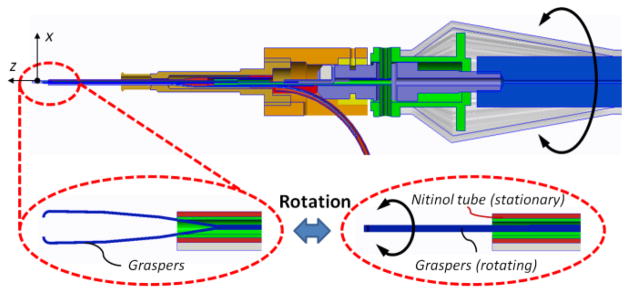
The graspers (blue) and the actuation tube (green) can be rotated together in the nitinol tube (red) by rotating the handle.
Finally the customized forceps mechanism was inserted through the force sensing module. In this design, the optical fibers leave the tool right after the easy-release mechanism as shown in Fig. 1. This inhibits any interference with the surgeon’s hand at the handle during the operation. Furthermore, the fibers never get twisted or bent since they are separated from all of the rotating components. The complete design can be actuated and rotated just like the original tool.
B. Calibration and force calculation
The calibration setup, calibration protocol, and the force computation steps follow [11]. Due to the interaction between the actuation tube and the nitinol tube with FBGs, a constant offset in force measurement exists when the micro-forceps is open. Since measuring the tool-to-tissue interaction forces after grasping the membrane is critical, the micro-forceps was kept closed during the calibration. A linear reproducible behavior was observed for all FBGs during both x and y axis calibration procedure as shown in Fig 5. The following calibration matrix was determined:
Figure 5.
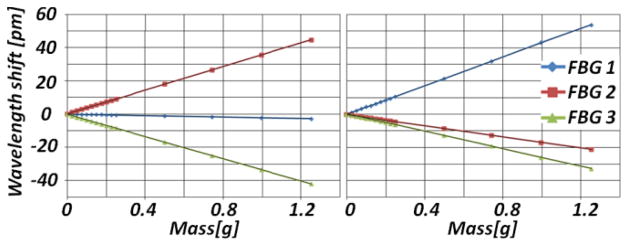
Calibration results in x-axis direction (left) and in y-axis direction (right). Linear behavior is observed for all FBGs in both axes.
In order to verify our calibration results, we rotated the forceps mechanism and the tool shaft relative to the force sensing module while monitoring the change in measured forces under constant loading on tool tip. The rotation caused a shift in the force measurement, but did not affect the linear trend of the calibration functions except for the starting points. Since the initial linear trend is preserved, recalibration is not required during the operation. The shift in the starting points can easily be eliminated by rebiasing the system during use. After the system rebias, the errors in our tests were less than 0.3 mN in the relevant force range of 0–12.5mN. This shows that our tool is able to measure the forces in any direction in the xy-plane and in any orientation of the graspers relative to the tool, with an accuracy of 0.3 mN and precision of 0.25 mN.
III. Experiments
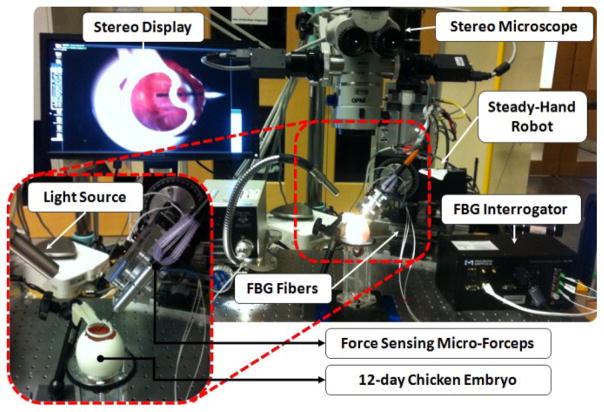
In order to assess the performance of our micro-forceps in robot assisted membrane peeling, we conducted four types of experiments on the setup shown in Fig. 6. In all tests, we used the inner shell membrane (ISM) and the chick chorioallantoic membrane (CAM) as a biological phantom since it has previously been reported to be a suitable model for vitreoretinal microsurgical instrument studies [14].
Figure 6.
Setup for membrane peeling experiments on inner shell membrane in chicken embryo.
After removing the eggshell gently, ISM of the chicken embryo becomes accessible. Attached under this thin layer is the CAM. In our experiments, the ISM simulates the ILM while the CAM serves as a phantom for the retina. The task is to peel off the ISM without breaking the CAM using the force sensing micro-forceps. In order to assist the subject, force sensor output is translated into auditory signals. Depending on the tool tip force level, the operator can hear three different tempos of audio “beeps” representing three safety zones. The borders of these zones were defined based on typical vitreoretinal operations. The audio remains silent until 1 mN or greater force is measured. Between 1 mN and 3.5 mN, a constant slow beeping is emitted, which is designated to be a “safe zone”. The second zone is between 3.5mN and 7 mN [12]. A proportionally increasing tempo is generated to indicate this “caution zone”. Beyond 7 mN is a “danger zone” for potential retinal breaks and tears, which is represented by a constant high beeping. In order to assess the effect of such auditory force feedback and robotic assistance, we studied four different cases:
Free-hand peeling with auditory force feedback
Robot assisted peeling with auditory force feedback
Robot assisted peeling with force scaling
Robot assisted peeling with auditory force feedback and force scaling
For all cases, the 2-DOF force sensing forceps was held perpendicular to the direction of the peeling motion to minimize axial forces. The tool tip force and video were recorded. Based on the video timestamp, starting and ending points of the peeling motion were identified in the acquired data. The assessment was based on the applied forces during delaminating motion.
IV. Results And Discussion
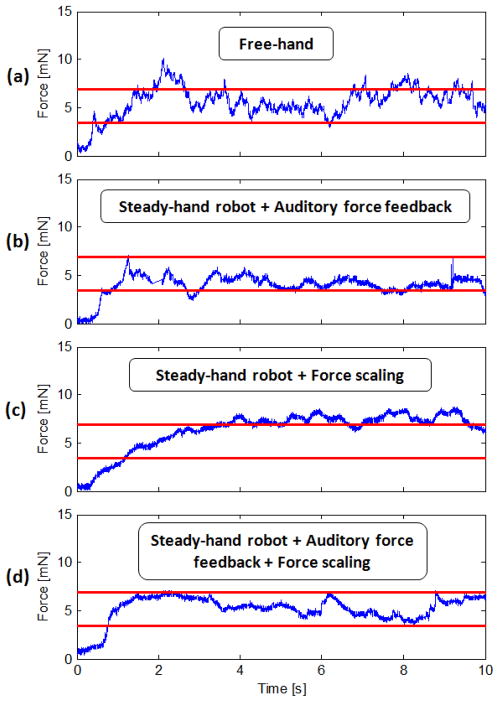
Typical force measurements for the tested cases are presented in Fig. 7. According to Fig. 7.a, free hand membrane peeling forces exhibit two main types of variations during the delaminating period: (1) low frequency changes due to varying peeling speed and heterogeneous tissue structure, (2) high frequency oscillations due to hand tremor. Using the steady-hand robot, most of the hand-tremor based components were eliminated as shown in Fig. 7.b.
Figure 7.
Membrane peeling forces on chicken embryo from a test sample: (a) completely unaided, (b) robot assisted with only auditory force feedback, (c) robot assisted with only force scaling, (d) robot assisted with auditory force feedback and force scaling. High frequency oscillations in delaminating forces are reduced by steady-hand robot. Force scaling provides smoother force variations. Auditory feedback is beneficial for keeping the forces within the safe operation zone (red lines).
When force scaling feature of the steady-hand robot is activated, the force on the tool tip is multiplied by a cofactor (300x) and applied to the operator’s hand, which is further described in [12]. Practically, this provides a stiffer tool manipulation in the direction of the applied force. The results presented in Fig. 7.c indicate that force scaling feature provides enhanced stability and smoother force variations during the procedure as compared to the trials without force scaling. The force peaks in Fig. 7.b are eliminated upon activation of force scaling mode in Fig. 7.c and d. However force scaling alone cannot keep the forces within the safe zone (below 7 mN). Fig. 7.d. shows that only the combination of robot assistance, force scaling and auditory force feedback can provide relatively stable and limited forces during membrane peeling.
V. Conclusion
This paper has reported the development of a new 2-DOF force sensing micro-forceps. For force sensing capability, three FBG strain sensors were integrated into the distal part of the tool shaft. The tool design with separated force sensing module and forceps mechanism allows the FBGs to stay stationary with the robot, while the forceps mechanism is freely rotated and actuated by the surgeon. This feature minimizes both cabling and cleaning challenges, which is also advantageous for force scaling. Although the graspers are rotatable, the coordinate frame of the force sensing module remains fixed relative to the robot coordinates. This eliminates the need for a rotation sensor between the tool and the robot.
The developed instrument can measure forces with an accuracy of 0.3 mN and precision of 0.25 mN. Furthermore, it can easily be integrated with our steady-hand robot for improved assistance during robotic microsurgery. Our experiments have shown that our force sensing forceps with the steady-hand robot can help the surgeon keep peeling force stabilized and limited below 7 mN throughout the peeling maneuver.
In this study, we provided force sensing capabilities only for transverse loading on the tool tip. A limitation of this approach is the inability to measure axial forces. In order to minimize axial components, the operations need to be performed while holding the instrument almost perpendicular to the membrane surface, which is not always practical. This can be resolved by integrating a complete 3-DOF force sensing tool, which is currently in progress. After building such a tool, we aim to verify the performance through multiple-subject experiments.
Acknowledgments
This work was supported in part by the U.S. National Science Foundation under Cooperative Agreement EEC9731478, in part by the National Institutes of Health under BRP 1 R01 EB 007969-01 A1, and in part by Johns Hopkins internal funds.
The authors thank Alcon, Inc. (Fort Worth, TX) for their help with providing the tools.
Contributor Information
İsmail Kuru, Email: ismail.kuru@tum.de, Institute of Micro Technology and Medical Device Technology (MiMed), Technische Universität München, Boltzmannstr. 15, 85748 Garching b. München, Germany.
Berk Gonenc, Email: bgonenc1@jhu.edu, ERC for Computer Integrated Surgery at Johns Hopkins University, Baltimore, MD 21218 USA, phone: 360-975-1676.
Marcin Balicki, Email: marcin@jhu.edu, ERC for Computer Integrated Surgery at Johns Hopkins University, Baltimore, MD 21218 USA, phone: 360-975-1676.
James Handa, Email: jthanda@jhmi.edu, Wilmer Eye Institute, Johns Hopkins Hospital, Baltimore, MD 21287 USA.
Peter Gehlbach, Email: pgelbach@jhmi.edu, Wilmer Eye Institute, Johns Hopkins Hospital, Baltimore, MD 21287 USA.
Russell H. Taylor, Email: rht@jhu.edu, ERC for Computer Integrated Surgery at Johns Hopkins University, Baltimore, MD 21218 USA, phone: 360-975-1676.
Iulian Iordachita, Email: iordachita@jhu.edu, ERC for Computer Integrated Surgery at Johns Hopkins University, Baltimore, MD 21218 USA, phone: 360-975-1676.
References
- 1.Kirchhof B, Wong D. Vitreo-retinal surgery. Springer; 2005. [Google Scholar]
- 2.Tano Y, Kamei M, Ooji M, Saitou Y, Won PI, Lewis JM. Membrane eraser. Jul, 1999. [Google Scholar]
- 3.Ueta, Yamaguchi Y, Shirakawa Y, Nakano T, Ideta R, Noda Y, Morita A, Mochizuki R, Sugita N, Mitsuishi M. Robot-assisted vitreoretinal surgery development of a prototype and feasibility studies in an animal model. Ophthalmology. 2009 Aug;116:1538–1543.e2. doi: 10.1016/j.ophtha.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Riviere C, Ang WT, Khosla P. Toward active tremor canceling in handheld microsurgical instruments. Robotics and Automation, IEEE Transactions on. 2003 Oct;19:793–800. [Google Scholar]
- 5.Uneri A, Balicki M, Handa J, Gehlbach P, Taylor R, Iordachita I. New steady-hand eye robot with micro-force sensing for vitreoretinal surgery. Biomedical Robotics and Biomechatronics (BioRob), 2010 3rd IEEE RAS and EMBS International Conference on; sept. 2010; pp. 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karacorlu M, Karacorlu S, Ozdemir H. Iatrogenic punctate chorioretinopathy after internal limiting membrane peeling. American Journal of Ophthalmology. 2003;135:178–182. doi: 10.1016/s0002-9394(02)01925-6. [DOI] [PubMed] [Google Scholar]
- 7.Gupta P, Jensen P, de Juan E. Surgical forces and tactile perception during retinal microsurgery. In: Taylor C, Colchester A, editors. Medical Image Computing and Computer-Assisted Intervention (MICCAI’99) vol 1679 of Lecture Notes in Computer Science. Springer; Berlin / Heidelberg: 1999. pp. 1218–1225. [Google Scholar]
- 8.Berkelman P, Whitcomb L, Taylor R, Jensen P. A miniature microsurgical instrument tip force sensor for enhanced force feedback during robot-assisted manipulation. Robotics and Automation, IEEE Transactions on. 2003 Oct;19:917–921. [Google Scholar]
- 9.Jagtap A, Riviere C. Applied force during vitreoretinal microsurgery with handheld instruments. Engineering in Medicine and Biology Society, 2004 IEMBS ’04 26th Annual International Conference of the IEEE. 2004 Sep;1:2771–2773. doi: 10.1109/IEMBS.2004.1403792. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Balicki M, Kang J, Handa J, Taylor R, Iordachita I. Development and preliminary data of novel integrated optical micro-force sensing tools for retinal microsurgery. Robotics and Automation. ICRA ’09. IEEE Int. Conf. on; 2009. pp. 1897–1902. [Google Scholar]
- 11.Iordachita I, Sun Z, Balicki M, Kang J, Phee S, Handa J, Gehlbach P, Taylor R. A sub-millimetric, 0.25 mn resolution fully integrated fiber-optic force-sensing tool for retinal microsurgery. International Journal of Computer Assisted Radiology and Surgery. 2009;4:383–390. doi: 10.1007/s11548-009-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balicki M, Uneri A, Iordachita I, Handa J, Gehlbach P, Taylor R. Micro-force sensing in robot assisted membrane peeling for vitreoretinal surgery. In: Jiang T, Navab N, Pluim J, Viergever M, editors. Medical Image Computing and Computer-Assisted Intervention (MICCAI 2010) vol 6363 of Lecture Notes in Computer Science. Springer; Berlin / Heidelberg: 2010. pp. 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He X, Balicki MA, Kang JU, Gehlbach PL, Handa JT, Taylor RH, Iordachita II. Force sensing micro-forceps with integrated fiber bragg grating for vitreoretinal surgery. Proceedings of SPIE; Feb 7, 2012. pp. 82180W–82180W. [Google Scholar]
- 14.Leng T, Miller JM, Bilbao KV, Palanker DV, Huie P, Blumenkranz MS. The chick chorioallantoic membrane as a model tissue for surgical retinal research and simulation. Retina. 2004;24(3):427. doi: 10.1097/00006982-200406000-00014. [DOI] [PubMed] [Google Scholar]