Abstract
Apathy is the most common neuropsychiatric symptom in mild cognitive impairment (MCI) and Alzheimer’s disease (AD) dementia. We sought to determine whether apathy is associated with cortical amyloid burden measured by Pittsburgh Compound B (PiB) positron emission tomography (PET) and regional hypometabolism measured by 18F-fluorodeoxyglocuse (FDG) PET in MCI. We found a significant association between increased apathy (lower Apathy Evaluation Scale score) and greater cortical PiB retention independent of age (prs=−0.46, p=0.03), but no significant association between apathy and regional FDG metabolism. These results suggest that increased apathy is associated with greater amyloid burden but not regional hypometabolism in MCI.
Keywords: Alzheimer’s disease, amyloid, apathy, 18F-flourodeoxyglucose, mild cognitive impairment, Pittsburgh Compound B, positron emission tomography
Introduction
Apathy is characterized by loss of interest, lack of motivation, and social withdrawal. Apathy is the most common neuropsychiatric symptom in Alzheimer’s disease (AD) dementia and among the most common in amnestic mild cognitive impairment (MCI), and it increases in frequency as the disease progresses {1–3}. In AD patients, apathy has been associated with executive dysfunction and impairment in activities of daily living and is a major source of frustration for caregivers {4–6}. In MCI, apathy has also been shown to herald the development of AD dementia {7, 8}.
It has been hypothesized that apathy is mediated by a frontal-subcortical circuit {9, 10}. Functional imaging studies have shown that apathy relates most consistently to hypometabolism and hypoperfusion in the anterior cingulate and orbitofrontal region in mild to moderate AD dementia {11, 12}. Apathy has also been associated with increased anterior cingulate neurofibrillary tangle burden in moderate to severe AD dementia at post-mortem and with elevated cerebrospinal fluid (CSF) total and phospho-tau in vivo earlier in the disease course in mild AD dementia {13, 14}.
To our knowledge, apathy has yet to be linked to amyloid burden. In vivo investigation of amyloid pathology is now possible using Pittsburgh Compound B (PiB) positron emission tomography (PET) {15}. Multiple studies in MCI have demonstrated a bimodal distribution of PiB retention, with one group exhibiting PiB binding similar to that seen in AD dementia patients and the other exhibiting non-specific binding similar to that seen in PiB-negative clinically normal elderly subjects {16–19}. Similar findings have been reported with autopsy studies as well {20, 21}. Therefore, further clinical characterization of MCI might help better define individuals who are likely to have underlying AD pathology. Neuropsychiatric symptoms, such as apathy, can serve this purpose.
The objective of this study was to determine whether apathy is associated with cortical amyloid burden measured by PiB PET and regional hypometabolism measured by 18F-fluorodeoxyglocuse (FDG) PET in MCI.
Methods
Subjects
Twenty four MCI subjects participating in an investigator-initiated imaging study underwent clinical assessments and PET imaging with PiB and FDG. Subjects were ages 54–85 (inclusive), were medically stable, and did not have significant cofounding neurological conditions, recent substance or alcohol abuse, or current primary psychiatric diagnoses. Subjects had a Modified Hachinski Ischemic Score {22} ≤ 4 and a Geriatric Depression Scale (long form) {23} ≤ 10. Subjects had a study partner who provided collateral information about their mood, behavior, and daily functioning.
Subjects met criteria for amnestic MCI, single or multiple domain {24}. These criteria included a memory complaint (reported by the subject or study partner); objective memory impairment assessed with the Logical Memory IIa (story recall) of the Wechsler Memory Scale-Revised; essentially intact activities of daily living; and no evidence of dementia. They also had a Mini-Mental State Examination (MMSE) {25} score of 24–30 (inclusive), a Clinical Dementia Rating (CDR) {26} global score of 0.5, and a Memory Box score ≥ 0.5.
The study was approved by the Partners (local) Institutional Review Board. Informed consent was obtained from all subjects and their study partners before any of the study procedures were carried out.
Clinical assessments
The Apathy Evaluation Scale (AES) {27} was used to assess apathy severity based on an informant interview. The AES consists of 18 items relating to apathy. Each item was rated on a 4-point Likert-type scale. Lower scores indicated greater apathy (range 42–72 in this analysis; full range 18–72). The AES examines apathy in greater depth than the Neuropsychiatric Inventory {28, 29}, which was employed in many prior studies.
PET imaging
PiB was synthesized and dynamic PiB PET imaging acquisition was performed as previously described {15, 30–34}. Data were acquired using a Siemens/CTI (Knoxville, TN) ECAT HR+ scanner (3D mode; 63 image planes; 15.2cm axial field of view; 5.6mm transaxial resolution and 2.4mm slice interval; 69 frames: 12x15 seconds, 57x60 seconds). Following a transmission scan, 8.5–15mCi 11C-PiB was injected as a bolus and followed immediately by a 60-minute dynamic acquisition. PiB PET data were reconstructed with ordered set expectation maximization, corrected for attenuation. Each frame was evaluated to verify adequate count statistics and absence of head motion. The Logan graphical analysis method with cerebellar cortex as the reference tissue input function was used to evaluate specific PiB retention expressed as the distribution volume ratio (DVR) {34, 35}. PiB DVR was calculated in an aggregate cortical region of interest defined using the Automated Anatomic Labeling (AAL) template following Statistical Parametric Mapping (SPM) spatial transformation, as described previously {36, 37}. This aggregate of cortical PiB regions consisted of regions that typically have elevated PiB retention in AD dementia and was used in all analyses.
FDG PET data was acquired according to the Alzheimer’s Disease Neuroimaging Initiative protocol {38}. Briefly, a bolus of 5 mCi of FDG was injected in a quiet, dimly lit room, with subjects in the eyes-open state. FDG acquisition began 30 minutes after injection and lasted 30 minutes. Cortical FDG metabolism was expressed as the standardized uptake value and normalized to the cerebellum for each region of interest. Based on previous functional imaging studies in AD dementia {11, 12}, two regions highly correlated with apathy were selected from the AAL template for these analyses: the anterior cingulate and orbitofrontal cortices. Three additional regions typically affected in MCI an AD dementia regardless of symptoms of apathy were also selected: supramarginal, precuneus, and inferior temporal cortices.
Statistical analyses
All analyses in this study were carried out using SPSS version 20.0. The AES distribution was not normal (it was left-skewed). Therefore, non-parametric tests were employed. The AES was related to subject demographics and characteristics using Spearman’s correlations for continuous variables and Mann-Whitney U test for categorical variables (two-tailed p values were reported).
Partial Spearman’s correlations, controlling for age, were used to assess the association between apathy and the aggregate of cortical PiB regions, as well as the 5 FDG regions (correlation coefficients and two-tailed p values were reported). For the association of apathy with cortical PiB, additional analyses were done to control for age, as well as disease severity, using either the Rey Auditory Verbal Learning Test (RAVLT) {39} total learning score (a measure of memory), the MMSE (a measure of global cognition), or the CDR sum of boxes (a measure of global functioning).
Exploratory whole-brain voxel-based analyses were performed using SPM8 (Wellcome Department of Cognitive Neurology), a UNIX-based software package using custom routines in MATLAB (Mathworks, Inc.), to further determine if there is any regional association between apathy and FDG metabolism. Analyses were adjusted for age and data were smoothed using an 8 mm kernel.
Results
Subject demographics and characteristics are displayed in the Table. Increased apathy (lower AES score) was significantly associated with greater global functioning impairment (higher CDR sum of boxes) (rs=−0.45, p=0.03). Greater cortical PiB retention was also significantly associated with higher CDR sum of boxes (rs=0.57, p=0.003). Apathy was not significantly associated with any other subject demographics and characteristics.
Table.
Demographics and characteristics of subjects.
| MCI subjects | |
|---|---|
| n | 24 |
| Age (years) | 73.6±9.2 (54–85) |
| Sex (% male) | 72.0 |
| Education (years) | 17.3±2.4 (12–20) |
| RAVLT total learning | 32.7±8.9 (18–55) |
| MMSE | 27.4±1.9 (24–30) |
| CDR sum of boxes | 1.8±1.0 (0.5–3.5)* |
| AES | 61.4±8.3 (42–72) |
AES (Apathy Evaluation Scale), CDR (Clinical Dementia Rating), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), RAVLT (Rey Auditory Verbal Learning Test).
All values (except n and sex) represent mean ± standard deviation (range).
Significantly correlated with AES, p<0.05
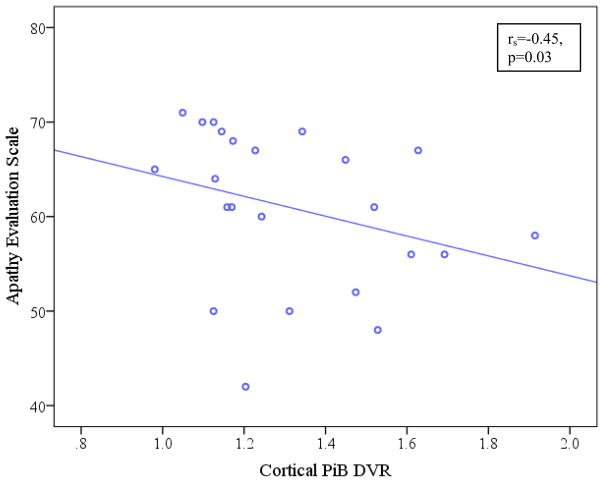
There was a significant association between increased apathy (lower AES score) and greater cortical PiB retention after adjusting for age (prs=−0.46, p=0.03), see Figure. After adjusting for age and measures of disease severity, the relationship between apathy and cortical PiB retention was retained with RAVLT total learning (prs=−0.44, p=0.05) and MMSE (prs=−0.43, p=0.05) but not with CDR sum of boxes (prs=−0.19, p=0.42).
Figure.
Scatter plot of cortical PiB DVR vs. AES. Unadjusted Spearman’s correlation coefficient and p value are reported here, while the main analyses used partial Spearman’s correlations adjusted for age. Lower scores on AES indicate greater apathy. AES (Apathy Evaluation Scale), PiB DVR (Pittsburgh Compound B distribution volume ratio).
There was no significant association between apathy and any of the FDG regions after adjusting for age: anterior cingulate (prs=0.03, p=0.89), orbitofrontal (prs=0.12, p=0.61), supramarginal (prs=0.11, p=0.65), precuneus (prs=0.14, p=0.55), and inferior temporal (prs=0.23, p=0.31). No significant associations were seen with the SPM analyses even when using an inclusive voxel-level threshold of 0.05 and small volume correction.
Discussion
The results of our study suggest that after adjusting for age, increased apathy is associated with greater amyloid burden in MCI but not with regional hypometabolism. To our knowledge, this is the first study to show a relationship between apathy and amyloid pathology in the AD spectrum. It suggests that apathy, which has been shown to be an early and salient symptom of MCI and AD dementia {1, 3}, can help more accurately characterize the heterogeneous clinical diagnosis of MCI due to AD {40}. Impairment in instrumental activities of daily living has been associated with increased amyloid burden in MCI as well {33} and can similarly help better clinically characterize these individuals that are primarily diagnosed based on their cognitive performance.
Prior studies assessing the relationship between apathy and functional and pathological markers of AD at later stages of AD dementia have found associations with medial frontal metabolism, perfusion, and tau pathology rather than amyloid burden {11–14}. As such, the lack of association between apathy and anterior cingulate and orbitofrontal hypometabolism in the current study was unexpected. However, this study focused on subjects with MCI in whom these associations have not been previously explored. It is possible that at the stage of MCI decreased cerebral activity is not as prominently linked to apathy. In order to determine whether this lack of association was due to an a priori selection of the wrong regions of interest, we also looked at parietal and temporal regions, which are typically affected in MCI and AD dementia regardless of the presence of apathy. However, we did not find an association with these regions either. Finally, we performed an exploratory whole-brain voxel-based analysis in order to find potentially smaller regional metabolic associations with apathy, but again we did not find a significant association. This question may be more definitively answered if a larger subject population is assessed.
This study had several limitations. First, the sample size of 24 was small. However, this is the first study with MCI subjects and sensitive imaging biomarkers that used a specialized assessment of apathy, the AES, which allowed us to measure numerous apathy-related symptoms with a continuous scale. Second, the primary analyses performed in the current study controlled for age but no other covariates. Due to the small sample size, we could not afford to control for many covariates. However, of the covariates assessed initially, only CDR sum of boxes was significantly related to apathy. This was expected since the CDR, a measure of global functioning, heavily relies on assessment of executive function and activities of daily living, both of which have been shown to relate to apathy {4–6}. We also found that CDR sum of boxes was significantly associated with amyloid burden as previously reported with the CDR and other measures of activities of daily living {16, 33}. Consequently, we repeated the analyses after adjusting for age and CDR sum of boxes, and the association between apathy and amyloid burden was no longer significant. The sample size and cross-sectional design of our analyses limited our ability to examine the reciprocal relations and causal associations between apathy, CDR sum of boxes, and amyloid burden. Future larger studies can address these clinically important associations. Aside from the CDR, we also controlled for other measures of disease severity, including a measure of memory (RAVLT total learning) and a measure of global cognition (MMSE). Controlling for these cognitive measures did not influence the relationship between apathy and amyloid burden which remained significant. Finally, for the association between apathy and regional hypometabolism, we focused on two regions, the anterior cingulate and orbitofrontal cortices, which have been shown to be most closely related to apathy in AD dementia. However, even when we extended our analyses to include other regions typically affected in AD dementia, as well as performed a whole-brain exploratory SPM analyses, we did not find a relationship with apathy. This could be due to the small sample size or due to the milder severity of apathy at the stage of MCI, making it more difficult to detect an association with FDG metabolism.
In conclusion, this initial study showed an association between apathy and increased in vivo amyloid burden but not regional hypometabolism in MCI for the first time. This association was independent of age, memory, and global cognition, but not of global functioning, which has been previously associated with apathy. Future larger cross-sectional and longitudinal studies of subjects across the AD spectrum assessing additional AD biomarkers, such as CSF abeta 1–42, total and phospho-tau, will help further clarify these associations.
Acknowledgments
This study was supported by R01 AG027435, K23 AG033634, K24 AG035007, the Rosalinde and Arthur Gilbert Foundation/AFAR New Investigator Awards in Alzheimer’s Disease, the Massachusetts Alzheimer’s Disease Research Center (P50 AG005134), and the Harvard Aging Brain Study (P01 AGO36694). Authors have also received research salary support from Janssen Alzheimer Immunotherapy (DMR, GAM, REA), Wyeth/Pfizer Pharmaceuticals (DMR, GAM, REA), and Bristol-Myers-Squibb (RAS).
Footnotes
This research was previously presented at the 6th International Human Amyloid Imaging Meeting, Miami, Florida, January 12–13, 2012.
References
- 1.Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25:115–126. doi: 10.1159/000112509. [DOI] [PubMed] [Google Scholar]
- 2.Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 3.Peters ME, Rosenberg PB, Steinberg M, et al. Prevalence of neuropsychiatric symptoms in CIND and its subtypes: the Cache County Study. Am J Geriatr Psychiatry. 2012;20:416–424. doi: 10.1097/JGP.0b013e318211057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle PA, Malloy PF, Salloway S, et al. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:214–221. [PubMed] [Google Scholar]
- 5.Marshall GA, Rentz DM, Frey MT, et al. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2011;7:300–308. doi: 10.1016/j.jalz.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPherson S, Fairbanks L, Tiken S, et al. Apathy and executive function in Alzheimer’s disease. J Int Neuropsychol Soc. 2002;8:373–381. doi: 10.1017/s1355617702813182. [DOI] [PubMed] [Google Scholar]
- 7.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis. 2010;20:175–183. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 8.Robert PH, Berr C, Volteau M, et al. Apathy in patients with mild cognitive impairment and the risk of developing dementia of Alzheimer’s disease: a one-year follow-up study. Clin Neurol Neurosurg. 2006;108:733–736. doi: 10.1016/j.clineuro.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 10.Mega MS, Cummings JL, Salloway S, et al. The limbic system: an anatomic, phylogenetic, and clinical perspective. J Neuropsychiatry Clin Neurosci. 1997;9:315–330. doi: 10.1176/jnp.9.3.315. [DOI] [PubMed] [Google Scholar]
- 11.Benoit M, Clairet S, Koulibaly PM, et al. Brain perfusion correlates of the apathy inventory dimensions of Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19:864–869. doi: 10.1002/gps.1163. [DOI] [PubMed] [Google Scholar]
- 12.Marshall GA, Monserratt L, Harwood D, et al. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch Neurol. 2007;64:1015–1020. doi: 10.1001/archneur.64.7.1015. [DOI] [PubMed] [Google Scholar]
- 13.Marshall GA, Fairbanks LA, Tekin S, et al. Neuropathologic correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21:144–147. doi: 10.1159/000090674. [DOI] [PubMed] [Google Scholar]
- 14.Skogseth R, Mulugeta E, Jones E, et al. Neuropsychiatric correlates of cerebrospinal fluid biomarkers in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25:559–563. doi: 10.1159/000137671. [DOI] [PubMed] [Google Scholar]
- 15.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 16.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 17.Sperling RA, Laviolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolk DA, Price JC, Saxton JA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 22.Rosen WG, Terry RD, Fuld PA, et al. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 23.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 27.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 28.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 29.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 30.Mathis CA, Wang Y, Holt DP, et al. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 31.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 32.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall GA, Olson LE, Frey MT, et al. Instrumental activities of daily living impairment is associated with increased amyloid burden. Dement Geriatr Cogn Disord. 2011;31:443–450. doi: 10.1159/000329543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker JA, Hedden T, Carmasin J, et al. Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 36.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 38.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 40.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]