Abstract
Human papillomaviruses (HPV) infect stratified epithelia and link their life cycles to epithelial differentiation. The HPV E5 protein plays a role in the productive phase of the HPV life cycle but its mechanism of action is still unclear. We identify a new binding partner of E5, A4, using a membrane associated yeast two hybrid system. The A4 protein co localizes with HPV 31 E5 in perinuclear regions and forms complexes with E5 and Bap31. In normal keratinocytes, A4 is found primarily in basal cells while in HPV positive cells high levels of A4 are seen in both undifferentiated and differentiated cells. Reduction of A4 expression by shRNAs, enhanced HPV genome amplification and increased cell proliferation ability following differentiation but this was not seen in cells lacking E5. Our studies suggest that the A4 protein is an important E5 binding partner that plays a role in regulating cell proliferation ability upon differentiation.
Keywords: Keratinocyte, Endoplasm, Amplification, Differentiation, A4, Bap31, E5
Introduction
Human papillomaviruses (HPVs) are small DNA viruses that infect stratified epithelia at various body locations(Hebner and Laimins 2006). Over 120 HPV types have been identified and nearly one-third of these infect epithelia in the genital tract. Genital HPVs viruses are further subdivided into the high risk types (such as HPV-16, -18, -31) that are associated with cancer development, and low-risk types (such as HPV-6, -11) which cause benign lesions (Lowy, Kirnbauer et al. 1994; zur Hausen and de Villiers 1994).
The HPV productive life cycle is coupled to the differentiation of the host cell. Following entry, HPV genomes are established in the nucleus at about 50 to 100 copies per cell that replicate in synchrony with cellular chromosomes. When normal basal cells divide, one of the daughter cells detaches from the basement membrane, stops proliferating and undergoes differentiation in suprabasal layers (Watt 1998). In contrast, in HPV infections, the daughter cell that migrates away from the basement membrane remains active in the cell cycle as it undergoes differentiation in suprabasal layers. HPV genomes are replicated to high levels in highly differentiated cells in a process called amplification that depends on host cell replication “machinery” (Dollard, Wilson et al. 1992; Hummel, Hudson et al. 1992; Frattini, Lim et al. 1996; Ruesch and Laimins 1998).
In the high-risk HPV types, three viral proteins have been reported to promote oncogenic activities: E6, E7 and E5. The high-risk E6 proteins are approximately 18 kDa in size, and are localized to both the nuclear and cytoplasmic compartments. (Howie, Katzenellenbogen et al. 2009). E6 interacts with a variety of cellular factors including the tumor suppressor p53 in complex with the cellular ubiquitin ligase E6AP leading to rapid degradation of p53 (Scheffner, Werness et al. 1990; Werness, Levine et al. 1990). The E7 oncoprotein, which is similar in size to E6, is localized primarily to the nucleus (McLaughlin-Drubin and Munger 2009) and targets the retinoblastoma (Rb) proteins for degradation (Dyson, Howley et al. 1989). The combination of E6 and E7 can efficiently immortalize a variety cell types (Hawley-Nelson, Vousden et al. 1989; Munger, Phelps et al. 1989).
While the transforming activities of high risk HPV E6 and E7 are well characterized, the role of E5 in tumor progression remains poorly understood. The high-risk E5 proteins are small hydrophobic peptides of approximately 83 amino acids in size that localize to the membranes of the endoplasmic reticulum (ER) and nuclear envelope (Leechanachai, Banks et al. 1992; Conrad, Bubb et al. 1993). E5 has been shown to potentiate the immortalization activity of E6 and E7 in in vitro assays (Foulard, Matlashewski et al. 1994; Valle and Banks 1995; Stoppler, Straight et al. 1996). Importantly, targeted expression of HPV16 E5 to the skin of transgenic mouse model rapidly induces cutaneous tumors (Maufort, Williams et al. 2007). In estrogen treated E5 transgenic mice, E5 expression alone was sufficient to induce cervical tumors indicating that it can act as an oncogene (Maufort, Shai et al. 2010). In addition, E5 has been shown to transform several rodent fibroblast cell lines and this appears linked to enhanced EGFR (epidermal growth factor receptor) activity in a ligand-dependent manner (Straight, Hinkle et al. 1993). In keratinocytes transfected with either wildtype or E5 mutant HPV 31 genomes, no change in the levels of phosphorylated or total EGFR was seen suggesting that E5 has additional targets in these cells. The HPV 16 E5 oncoprotein has also been shown to interact with the 16 kDa subunit of vacuolar ATPase proton pump, however, HPV E5 proteins are localized to endoplasmic reticulum membranes and not endosomes suggesting this may not be significant interaction in keratinocytes (Straight, Herman et al. 1995; Disbrow, Hanover et al. 2005). Recent studies have shown that HPV 31 E5 interacts with Bap31 (B-cell-associated protein 31) (Ladasky, Boyle et al. 2006; Regan and Laimins 2008), a ubiquitously expressed 28-kDa membrane protein that is highly enriched in the ER (Annaert, Becker et al. 1997; Ng, Nguyen et al. 1997). Importantly, this interaction is important for regulation of differentiation-dependent late viral functions (Regan and Laimins 2008).
In this study we identify another binding partner of E5, the A4 protein. A4 is a small transmembrane lipoprotein of 152 amino acids that was initially found in differentiated human colonic epithelium (Oliva, Wu et al. 1993). This hydrophobic lipoprotein is localized in the endoplasmic reticulum and A4 has been reported to interact with Bap31. HPV E5 enhances the differentiation-dependent expression of A4 and this contributes to regulating the proliferation ability of cells upon differentiation which is necessary for the viral life cycle.
Materials and Methods
Cell culture
Human foreskin keratinocytes (HFKs) were derived from neonatal human foreskin epithelia as described in (Wilson and Laimins 2005) and were maintained in serum-free keratinocytes growth medium supplemented with bovine pituitary extract, insulin, hydrocortisone and epidermal growth factor (Lonza, Walkersville, MD). Stable cell lines expressing HPV E6 and E7 were generated by transduction of retroviral vectors and selected in G418 (Sigma) as previously described (Melar-New and Laimins 2010).
To create cell lines containing HPV31 genomes (HFKg31) and E5 knockout HPV31 genomes (HFKg31E5KO), HFKs were transfected with HPV type 31 genomes and genomes of HPV type 31 lacking the E5 gene followed by selection with antibiotics as described previously (Wilson and Laimins 2005).
CIN 612 cells are derived from a biopsy and maintain HPV 31 episomes while LKP cells are derived from normal human foreskin keratinocytes transfected with HPV 31 DNA and maintain episomes (Frattini, Lim et al. 1996) HFKs, all stably transfected HFK cell lines along with LKP and CIN612 cell lines that maintain HPV 31 episomes were grown in the presence of 3T3 J2 fibroblast feeders treated with mitomycin C and cultured in E medium supplemented with mouse epidermal growth factor (EGF) (5 ng/(Frattini, Lim et al. 1996)mL; BD Biosciences, San Jose, CA) as described (Fehrmann and Laimins 2005).
293TT, Cos7 and HaCat cells were grown in DMEM medium supplemented with 10% fetal bovine serum (Life Technologies, Grand Island, NY). For transient transfection assays, 293TT cells were transfected using ployethylenimine reagent at a final concentration of 0.2 mg/mL (Polyscience, Inc., Warrington, PA) at 30% confluence. Cells were harvested 48h postransfection for further analyses.
Differentiation of keratinocytes
To induce differentiation in high calcium LKP, CIN612 and HPV 31 stably transfected HFKs were cultured in the absence of 3T3 J2 feeders in EGF free E medium for 24 h prior to differentiation. After 24 h cells were switched to EGF free E medium containing CaCl2 at a final concentration 1.5 mM for 48h, 72h and/or 96h. Alternatively, keratinocytes were differentiated in semisolid media as described in (Melar-New and Laimins 2010).
Western Blot analyses
Total cell protein extracts were prepared with RIPA lysis buffer containing a cocktail of protease inhibitors (Roche, Branchburg, NJ) and quantitated using the NanoDrop ND2000 Spectrophotometer (Thermo Fisher, Suwanee, GA). Protein samples were electrophoresed on 15% sodium dodecyl sulfate-polyacrilamide (SDS) gels and subsequently transferred to polyvinylidene difluoride membranes (EMD Milipore, Darmstadt, Germany). The membrane was then blocked in a 5% nonfat milk solution (0.1% Tween 2 in PBS), followed by incubation with primary antibodies: anti-A4, anti-Flag (Sigma-Aldrich, St. Louis, MO), anti-AU1 (Covance, Madison, WI), anti-GAPDH (Santa Cruz, Dallas, TX), anti-Involucrin (Santa Cruz), anti-Cytokeratin (Santa Cruz) and anti-Bap31 (AbCam,, Cambridge, MA). Proteins were visualized via enhanced chemiluminescence (GE, Healthcare, Waukesha, WI).
Immunoprecipitation analyses
Cell lysates of transiently transfected 293TT cells expressing various proteins (A4 and/or E5 and/or Bap31) were prepared with CHAPS (15 mM 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate) buffer solution (CHAPS, 30mM NaCl, 1mM EDTA, 50mM TRIS-HCl [pH7.4]) (Sigma-Aldrich) 48h posttransfection as described in (Regan and Laimins 2008). Antibodies used in the analyses include: anti-AU1 (Covance) and anti-Bap31 (AbCam).
Immunofluorescence
The expression pattern of A4 proteins were examined by immunofluorescence of cross sections of raft tissue. Paraformaldehyde-fixed, paraffin-embedded tissue on silanized slides were heated at 50°C for 30 min, washed in Xylenes, 100% and 95% Ethanol and finally in PBS. The slides were then incubated in 10mM Citrate Solution (pH 6.0) at 95°C for 30 min, followed by an additional 20 min incubation at room temperature. Subsequently slides were washed with PBS and blocked in Normal Goat Serum (Life Technologies) and 1% Triton x-100 in humid chamber. Incubation with primary antibodies was performed at room temperature for 2h. Slides were then washed with PBS solution containing 1% triton and incubated with secondary Alexa-Fluor antibodies in light protected humid chamber for 30 min. After staining with DAPI, sections were mounted and analyzed under inverted fluorescent microscope.
Yeast two-hybrid screen
To detect possible protein interactions with E5 protein a yeast two-hybrid screen that is based on the split-ubiquitin technique was performed as described (Johnsson and Varshavsky 1994; Regan and Laimins 2008).
Plasmids
To construct A4 expression plasmids, the A4 open reading frame isolated from the yeast two-hybrid positive clones was PCR amplified and cloned into the pSG5. The Bap31 and HPV31 E5 expression vectors were previously described (Regan and Laimins 2008). A codon optimized HPV16 E5* with an AU1 tag was a generous gift from R. Schlegel and was subcloned in a pSG5 vector (Disbrow, Sunitha et al. 2003).
Southern Blot
Stable A4 knockdown cell line expressing shRNA against A4 were established as described (Mighty and Laimins 2011). Cells were differentiated in high calcium as described above and total DNA was isolated from harvested cells using DNeasy Blood & Tissue Kit (Qiagen Hilden, Germany). Ten micrograms of each total DNA sample was digested with DpnI and XhoI and electrophoresed in a 0.8% agarose gel overnight and transferred to Gene Screen nylon membranes (Bio-Rad, Des Plaines, IL) using vacuum transfer according to the manufacturer’s protocols. The DNA HPV31 probe was labeled with [32P]dCTP using Ready-To-Go DNA-labeling beads (Amersham Biosciences, Buckinghamshire, England) and column purified. The membrane was hybridized with labeled probe in hybridization solution overnight, washed and visualized by autoradiography.
Proliferation test
HPV positive cells were grown in the presence of mitomycin C-treated J2 3T3 fibroblasts. At the approximately 80 % confluence, feeder cells were removed, keratinocytes were harvested and number of cells was determined by TC10 automated cell counter (BioRad). Two and a half of millions of cells were resuspended in 1.5% methylcellulose for 48 h to induce differentiation. The keratinocytes were then collected, washed with PBS, and replated as monolayer cultures into new dishes. The total number of cells was determined after 5 days in culture by TC10 automated cell counter.
Results
A4 is a novel target of HPV E5 identified by yeast two-hybrid screen
To identify novel binding partners of HPV 31 E5, we used a membrane-associated yeast two hybrid system (Regan and Laimins 2008) (DUAL membrane system) screen initially developed by Johnsson and Varshavsky (Johnsson and Varshavsky 1994). In this system, the C-terminal half of ubiquitin (Cub) is linked to the transcription factor LexA-VP16 fused to a codon-modified HPV16 E5 protein. The N-terminal half of ubiquitin (NubG) is fused to prey proteins from HeLa cDNA library and a positive lac Z signal identifies associations that occur on cell membranes. Using a quantitative LacZ assay, positive clones with the highest expression were isolated and A4, a membrane protein localized to the ER, was identified as a candidate for binding partner of E5.
A4 form complexes with HPV16 E5 and Bap31
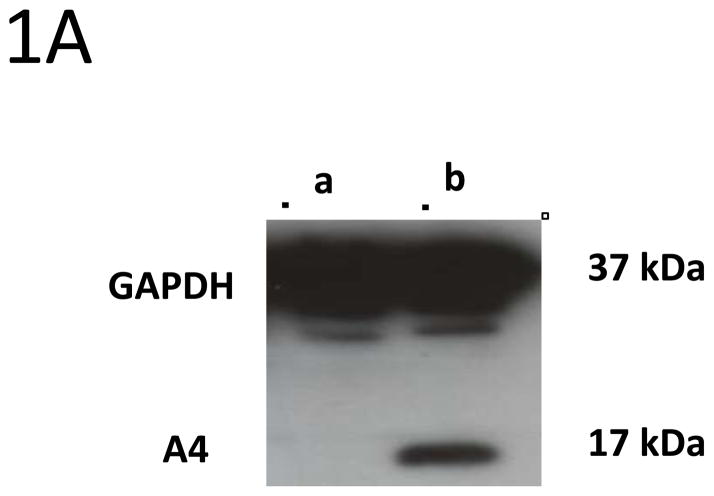
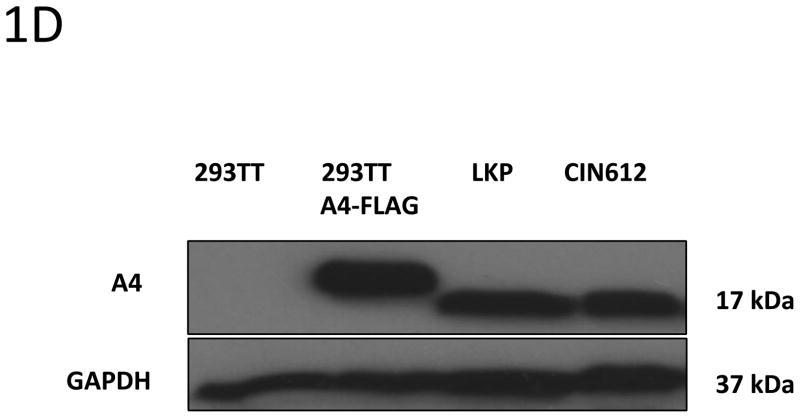
Prior to screening if E5 and A4 formed complexes in human cells, we first investigated if A4 protein was expressed in a series cell lines using Western blot analysis. The A4 protein was found to be expressed at high levels in Cos7, HFKs and HPV positive keratinocytes such as LKPs and CIN 612 cells that maintain HPV 31 as stable episomes. In contrast, no A4 protein was found expressed in 293TT cells that are derived from human embryonic kidney cells (Figure 1A).
Figure 1. HPV E5 interacts with human A4 protein.

1A: Expression of A4 protein in different cell types. In order to determine the expression of A4 proteins in different cell lines the cell lysates from (a) 293TT and (b) Cos7 cells were prepared and analyzed by Western blot using antibodies against A4. Similar levels of A4 are seen in HPV positive cells.
1B: HPV16 E5 binds the human A4 protein. Total protein lysates were isolated from 293TT cells transfected with expression plasmids for HPV16 E5 and A4, and 1 mg of protein lysate was immunoprecipitated with an AU1 tag E5 antibody. Precipitates were analyzed by Western blot using FLAG tag A4 antibody.
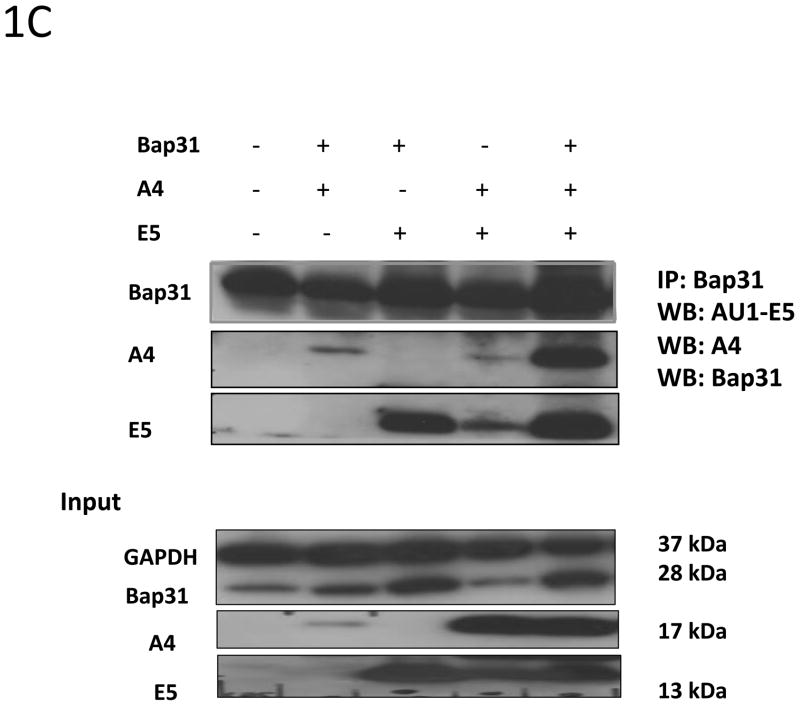
1C: HPV16 E5 interacts with A4 and Bap31. 293TT cells were transiently co-transfected with pSG5 empty vector and DNA vectors expressing HPV16 E5, A4 and Bap31 genes. Cell lysates were immunoprecipitated with an anti-Bap31 antibody and further analyzed by Western blotting using AU1, A4 and Bap31 antibodies.
1D: Levels of A4 proteins are similar in transfected and stable lines. Cell lysates from 293TT cells transfected with expression vector for A4 were compared to endogenous levels in LKP and CIN 612 cell lines by Western blot analysis.
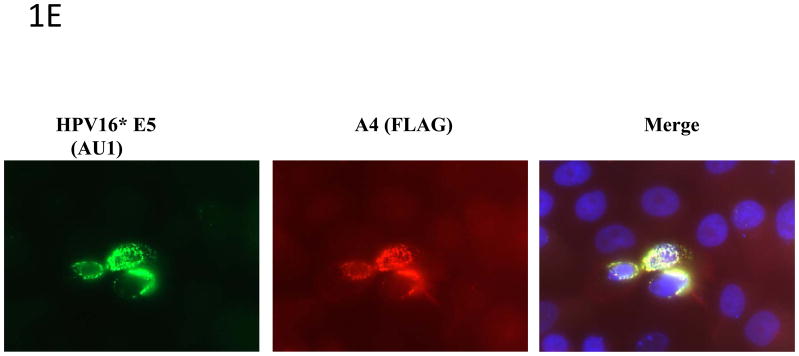
1E: Colocalization of A4 and HPV16 E5. HPV31 positive keratinocytes (CIN612) were transiently co-transfected with FLAG-tagged A4 and AU1-tagged HPV16 E5 expression vectors. Cells were immunostained with an anti-AU1 (FITC) and anti-FLAG (Texas Red) antibodies. Nuclei are visualized by staining with DAPI (blue). Yellow signals in merged image represent co-localization of A4 and HPV16 E5 within the ER. Panels in middle show E5 alone and A4 alone localization in the absence of transfection of the other vector.
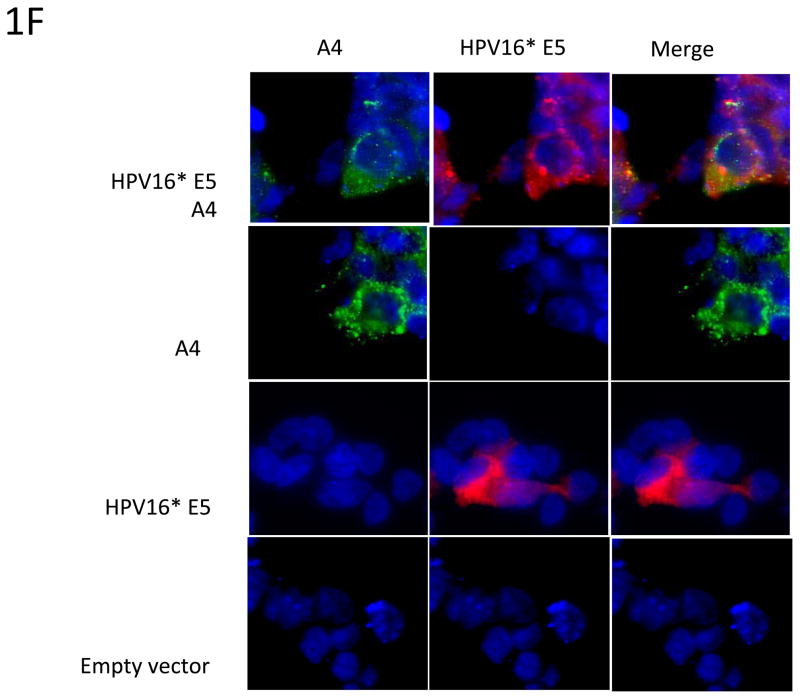
1F: Colocalization of A4 and transfected HPV16 E5. 293TT cells were transiently transfected with AU1-tagged HPV16 E5 and FLAG-tagged A4 expression vectors by themselves selves and in combination as indicated. Top panels show co-transfections while middle panels show A4 and E5 by themselves. Cells were immunostained with an anti-FLAG and anti-AU1 (Texas Red) antibodies. Nuclei are visualized by staining with DAPI (blue). Yellow signals in merged image represent co-localization of A4 and HPV16 E5 within the ER.
Since A4 was identified as a potential binding partner of E5 by yeast two hybrid analyses, we examined whether these two proteins could form a complex in human cells. For this analysis, 293TT cells, which do not express A4, were transfected with plasmids expressing A4 and AU1 tagged HPV16 E5 genes. At 48 hours posttransfection, cells were harvested and suspended in CHAPS lysis buffer followed by immunoprecipitation with an antibody to the AU1 tag. Precipitates were then analyzed by Western blot analyses for A4 protein and showed that E5 protein forms a complex with the human A4 protein (Figure 1B). Our previous studies identified the Bap31 protein as a binding partner of E5 (Regan and Laimins 2008), and it has also been reported that Bap31 can associate with A4 (Wang, Nguyen et al. 2003) as both are localized to the endoplasmic reticulum. We therefore investigated if E5 protein could potentially bind both, A4 and Bap31. To address this question, several transient transfections were performed in 293TT cells using HPV16 E5, A4 and Bap31 expression vectors. Protein lysates were prepared 48 hours posttransfection as described above, and tested by immunoprecipitation with an antibody to Bap31 following by Western blot analyses for A4, AU1 tag of E5 and Bap31 proteins. Our data (Figure 1C) demonstrate that E5 binds A4 in the presence of endogenous and exogenous Bap31, indicating that all three proteins can associate in complexes. Similar levels of A4 proteins are seen in 293T cells transfected with A4 expression vectors as are present in cells such as LKP or CIN 612 (Figure 1D). In our yeast two-hybrid assays, no endogenous Bap31 are expressed yet we detected binding of A4 to E5. This indicates that E5 can bind directly to A4 in the absence of Bap31. Finally, we consistently observed an increase in A4 levels due to the presence of E5 suggesting it may regulate levels of its expression (Figure 1C).
A4 and HPV16 E5 co-localize to perinuclear regions consistent with the endoplasmic reticulum
Having confirmed that HPV E5 binds to the A4 protein, it was important to determine whether these two proteins localize to the same region inside the cell. The E5 protein has been shown to localize to the perinuclear region consistent with the endoplasmic reticulum and A4 has been reported to exhibit a similar a perinuclear localization pattern (Goldstein and Schlegel 1990; Conrad, Bubb et al. 1993; Breitwieser, McLenithan et al. 1997; Disbrow, Sunitha et al. 2003; Wang, Nguyen et al. 2003; Disbrow, Hanover et al. 2005; Regan and Laimins 2008). Since it was possible that other viral proteins affected localization, HPV-31 positive CIN 612 cells were transiently co-transfected with vectors expressing AU1-tagged HPV16 E5 and FLAG-tagged A4. At 48 hours post transfection immunofluorescence analyses were performed and showed that E5 and A4 co-localized in the same perinuclear regions (Figure 1E). Similar perinuclear localization of E5 and A4 is seen in T cells transfected with expression vectors (Figure 1F).
A4 expression is downregulated upon differentiation of HFKs but is maintained at high levels in HPV positive cells
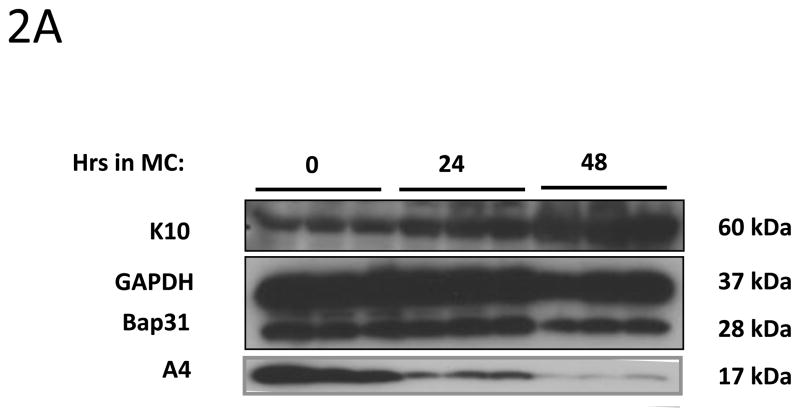
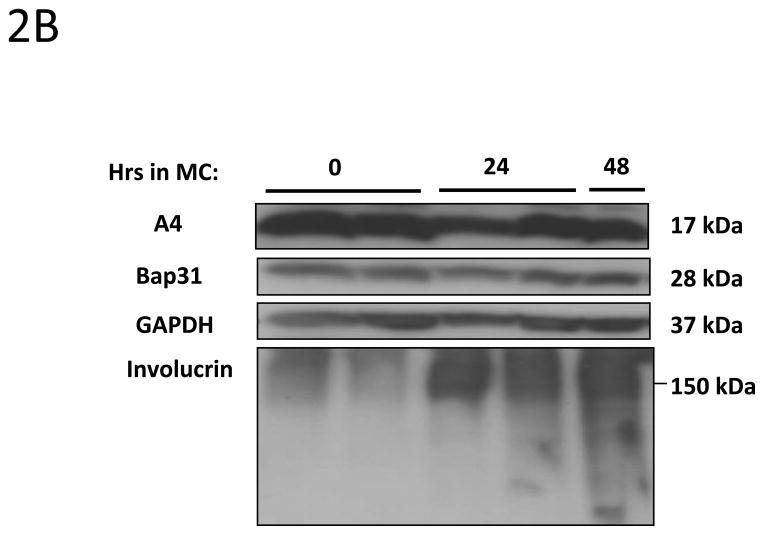
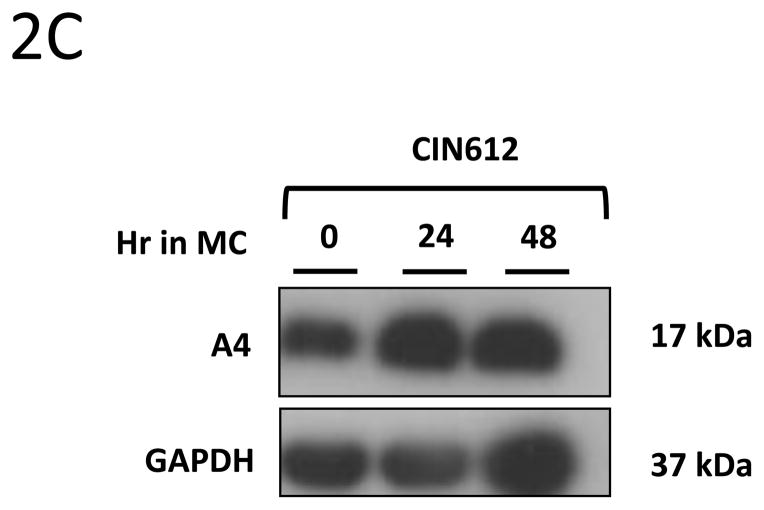
We next investigated whether expression of A4 protein changes upon differentiation of normal human keratinocytes. For this analysis, HFKs were differentiated in methylcellulose for 24h and 48h as this allows for isolation of uniformly differentiated populations of cells. Cell lysates were harvested and screened for A4 levels by Western blot analyses. As shown in Figure 2A, A4 proteins are expressed at high levels in undifferentiated normal keratinocytes (HFKs) but expression decreases dramatically upon differentiation. Similar effects were seen in HFKs isolated from different donors (not shown). We next sought to investigate if A4 expression was altered in HPV positive keratinocytes. For these studies, HFKs that stably maintain HPV31 genomes (LKP) were differentiated in 1.5% methylcellulose for 24h and 48h. Western blot analyses of whole cell lysates demonstrated high and constant levels of A4 upon differentiation (Figure 2B). Similar high levels of expression following differentiation were seen other human epithelial cell lines that maintain HPV31 episomes generated either by transfection (HFKg31) or derived from a cervical biopsy of a CIN lesion (CIN612). After differentiation in semi-solid media for 24h and 48h we again observed high expression of A4 protein upon differentiation (Figure 2C). The same expression pattern of A4 in normal and HPV31 positive keratinocytes was also observed in cells differentiated in high calcium (data not shown).
Figure 2. HPV increases the levels of A4 protein in HPV positive keratinocytes upon differentiation.


2A, 2B, 2C: A4 expression in keratinocytes induced to differentiate by suspension in methylcellulose for 24 and 48 hours. (2A) Normal human keratinocytes (HFK) (2B) HPV 31 positive LKP cells and (2C) biopsy derived HPV 31 positive CIN612. Cell lysates were prepared and analyzed for protein expression by Western Blot. Legend: MC: Methylcellulose. Experiments shown in panels 2A and 2B were reproduced in three independent exeperiments.
2D and 2E: Immunostaining of organotypic raft cultures of normal (2D) and LKP HPV 31 positive keratinocytes (2E). Cell differentiation was induced by growing keratinocytes in organotypic raft cultures for two weeks. Histological cross-sections were examined by immunohistochemistry using an antibody to A4 (FITC) and Cytokeratin (Texas Red). Left panel: A4 staining; middle panel: K10 staining; right panel: merge. Nuclear staining is DAPI (4′,6-diamidino-2-phenylindole).
To confirm the activation of A4 expression A4 in monolayer and differentiated HPV positive cells, we grew organotypic raft cultures and screened for expression by immunofluoresence using an anti-A4 antibody combined with an anti-Cytokeratin antibody (Figure 2D, 2E). These immunofluoresence analyses confirmed high level expression of A4 protein in the basal layer but low expression in the upper differentiated layers of the normal keratinocyte raft cultures. In contrast, high levels of the A4 protein were observed throughout all layers of HPV positive raft cultures. These data confirm our findings that HPV31 increases expression of A4 protein during epithelial differentiation.
HPV31 E5 oncoprotein may modulate A4 levels in human epithelium
Since HPV31 E5 forms complexes with the A4 protein, we investigated if E5 was responsible for the increased levels of A4 upon differentiation. Initially, we attempted to establish stable cell lines overexpressing codon modified 16E5 or 31E5 from retroviral vectors but in multiple experiments the cells failed to survive selection, which contrasted with cells transfected with control plasmids. This indicated that high-level expression of E5 could be toxic in our cells. As an alternate approach to study the effect of E5 on A4 levels, we generated HFKs stably transfected with HPV31 genomes in which a translation terrmination stop codon had been inserted into the E5 ORF (HPVg31 E5KO) and compared expression to that seen in normal keratinocytes or cells with wild type genomes. All sets of cells were examined for A4 expression by Western blot analyses using cell lysates of undifferentiated and differentiated keratinocytes. As shown in Figure 3A, the amount of A4 protein decreases upon differentiation in HPVg31E5KO cells, similar to that seen in differentiated normal keratinocytes. We have previously demonstrated that effects seen in keratinocytes induced to differentiate in high-calcium media accurately recapitulate differentiation seen in raft cultures (Moody and Laimins 2009; Bodily, Mehta et al. 2011; Gunasekharan and Laimins 2013). We conclude that E5 is necessary for enhanced levels of A4 upon differentiation.
Figure 3. HPV31 E5 viral oncoprotein modulates expression of A4 protein in differentiated keratinocytes.
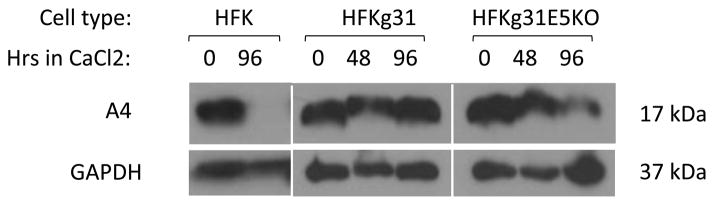
A4 levels are increased by HPV E5 oncoproteins in human keratinocytes upon differentiation. The protein levels of A4 protein were analyzed by Western blot methods using the following cell lines: wild type human foreskin cells (HFKs), HFKs infected with HPV31 (HFKg31) and HFKs infected with E5 knockout HPV31 genomes (HFKg31E5KO). All sets of cells were differentiated in high calcium and harvested for detection of A4 protein levels using an antibody to A4.
HPV31 E5 induces high A4 levels to support cell proliferative ability upon differentiation
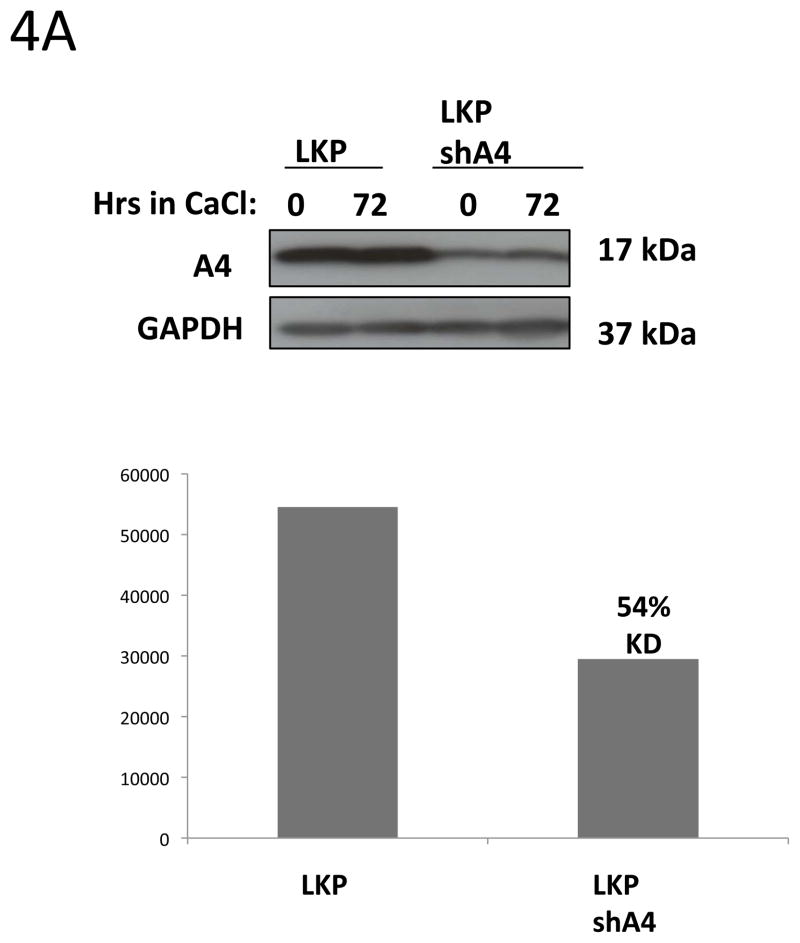
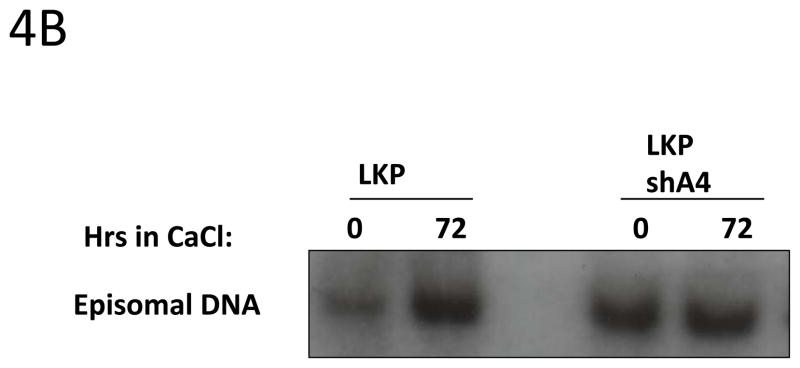
Our data indicate that E5 may control A4 levels upon differentiation and it was previously shown that cells that maintained HPV31 genomes in which E5 was mutated exhibited decreased viral amplification as well as cell proliferation ability in differentiating cells (Fehrmann, Klumpp et al. 2003). The knockdown of Bap31 knockdown similarly inhibits cell proliferation ability upon differentiation of HPV positive keratinocytes (Regan and Laimins 2008), but has no effect on HPV amplification of stable replication. We therefore investigated whether A4 as a binding partner of E5 had any effect on HPV amplification and proliferation ability. To examine the effect of reducing A4 levels, we infected HPV 31 positive cells with lentiviruses expressing shRNAs against A4 (Fig 4A) and screened for HPV31 genome amplification upon differentiation by Southern Blot. Interestingly, cells in which A4 was knocked down (LKPshA4) keratinocytes showed increased levels of viral episomes in undifferentiated cells and these were maintained at a high levels upon differentiation. The level of HPV episomes in undifferentiated and differentiated cells in which A4 was knocked down was increased over that seen in undifferentiated wt cells and similar to that seen upon amplification (Figure 4B). This suggests that knock down of A4 has a positive effect on HPV replication. In contrast to stable knockdowns in HPV positive cells, we were unable to obtain viable cells following knockdown of A4 in normal keratinocytes.
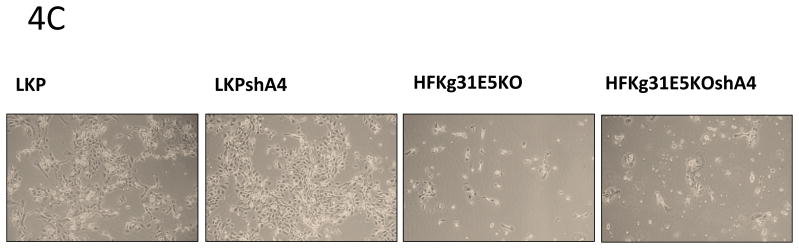
Figure 4. HPV31 replication and cell proliferation are enhanced in A4 shRNA knock down keratinocytes.
4A: Western blot analyses of A4 stable knock down in keratinocytes.
HPV31 positive keratinocytes LKP were infected with lentiviral particles carrying shRNAs against the A4 gene. Infected cells were selected with puromycin for two weeks. ImageJ program was used to quantify knockdown of A4 in cell line LKPshA4.
4B: Southern blot analysis of LKP and LKPshA4 keratinocytes.
Total genomic DNA was harvested from monolayer and differentiated cultures and digested with DpnI to remove residual input DNA and XbaI to linearize the HPV31 genomes. The Southern blot was hybridized with a probe that contains the complete HPV31 genome.
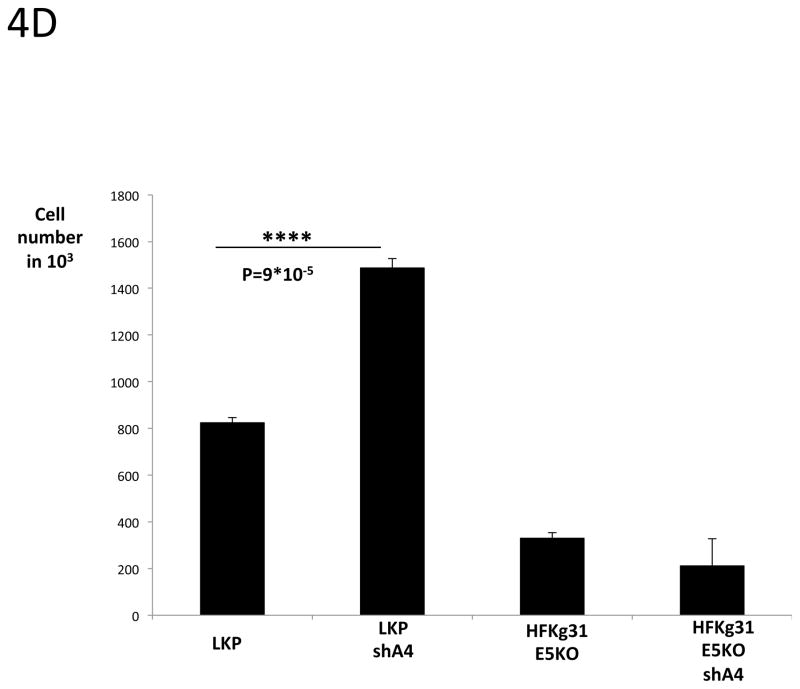
4 C, D: Cell proliferation assay.
LKP, LKPshA4, HFKg31E5KO and HFKg31E5KOshA4 cell lines were differentiated by suspension culture in methylcellulose. 48 hours later cells were re-plated into new dishes and cultured for few days. Colonies were visible under microscope on day 5 (4C). The total cell number was estimated on day 5 using TC10 automated cell counter (BioRad) (4D). Data are means ± standard deviations (error bars), n=3. ****P < 0.0001 indicates values significantly different from the control (LKP).
It was next important to investigate if A4 knockdown had any effect on cell proliferation of HPV positive keratinocytes following differentiation. Wildtype and shA4 knockdown HPV 31 positive cell lines were differentiated in 1.5% methylcellulose for 48h and cells were then re-plated to new dishes. After 5 days the number of cells was determined and we consistently observed a significant increase of cell growth in the A4 knock down cells as compared to the wild type HPV 31 positive cells (Fig 4C and 4D). Since HPV31 genomes containing mutated E5 exhibit decreased cell proliferation following differentiation (Fehrmann, Klumpp et al. 2003) we wanted to test whether A4 knockdown could restore this ability in cells containing mutant E5 proteins. For this analysis, we knocked down A4 with shRNAs in cells that maintain HPV 31 genomes with mutated E5 (HFKg31E5KOshA4). In these cells only partial (54%) A4 knockdown was achieved as determined by Western blot assay (Figure 4A). The same re-plating assay described above was performed following cell differentiation in semi-solid media and showed that knockdown of A4 did not restore cell proliferation ability to that seen in wt cells (Fig 4C, D). This suggests that the effects of A4 on proliferative ability are significant only in the presence of a functional E5 protein.
Discussion
Our studies identify the proteolipid protein, A4, as a binding partner of HPV 31 E5. The 17 kd A4 protein has characteristics of a four transmembrane ion channel (Breitwieser, McLenithan et al. 1997) and co-localizes with E5 to the endoplasmic reticulum. A4 was identified as a binding partner of HPV 16 E5 through the use of split-ubiquitin membrane associated yeast two-hybrid screen (Johnsson and Varshavsky 1994; Regan and Laimins 2008) and complex formation was confirmed in human cells through co-immunoprecipitation studies. The A4 protein can also form complexes with another endoplasmic reticulum protein, Bap31, that we previously reported also bound to E5 (Regan and Laimins 2008). Since yeast cells do not contain a Bap31 homologue, this indicated the interaction of A4 with E5 maybe direct. In our studies we attempted to confirm that the two factors bound independently to E5 using human cell lines. All cells we examined were found to express Bap31 and this is consistent with previous reports (Quistgaard, Low et al. 2013). While many cells express A4, 293 embryonic kidney cells were found to be A4 deficient. Using these cells Bap31 binding to E5 was found to be independent of A4 binding. Since all human cells express Bap31, we tried to abrogate BAP31 expression by use of shRNAs but were never able to reduce amounts by more than 50% suggesting Bap31 may be an essential protein. Future studies involving a detailed mutagenesis of E5 binding sequences may help to determine if these factors bind the same sequences or act through different binding sites on E5.
The A4 protein is normally expressed in a differentiation-dependent manner. In primary keratinocytes, A4 expression is restricted to undifferentiated basal cells and is absent from differentiated suprabasal layers. This contrasts with Bap31 expression, which is expressed in both basal as well as suprabasal cells and its expression is not changed by HPV proteins. Using organotypic rafts as well as methylcellulose induced differentiation, we observed that A4 was uniformly expressed in both undifferentiated and differentiated layers of cells that maintain HPV episomes. The E5 protein is responsible for inducing expression in suprabasal layers. At present we are unsure if this effect is mediated at the level of transcription or through post-transcriptional effects. In epithelia cancers that lack the ability to differentiate yet retain stratification ability, A4 is also found in suprabasal cells suggesting that A4 expression is linked to proliferative ability of cells (Oliva, Wu et al. 1993; Breitwieser, McLenithan et al. 1997).
Upon differentiation of cells that maintain HPV viral DNA as episomes, genome amplification is induced in suprabasal layers. This process requires differentiating cells to remain active in the cell cycle as host replication proteins such as polymerases and PCNA are needed for viral replication (Bodily and Laimins 2011). HPV genome amplification occurs as cells transit through S phase into G2/M. This ability to retain proliferative capability upon differentiation is mediated by both E7 and E5 proteins (Fehrmann, Klumpp et al. 2003; Regan and Laimins 2008; Banerjee, Wang et al. 2011). Our studies demonstrate that A4 expression in suprabasal cells is important to regulate this proliferative capability in differentiating cells. In contrast to studies with Bap31, knockdown of A4 enhanced proliferative ability rather than decreased it. Our studies suggest that A4 can modulate proliferative capacity and that is dependent on the presence of a functional E5 protein. A4 has been shown to associate with PI3K resulting in activation of Akt phosphorylation, which is important cell proliferation and future studies will determine if this is the critical target of A4 action. Our studies indicate that the action of A4 is central to regulating this activity, however, it appears to act in an opposite manner to Bap31. We also observed that knockdown of A4 lead to an increase in genome copy number in undifferentiated cells and that this enhanced level was maintained throughout differentiation. A4 may thus be important for helping to restrict high level viral replication to differentiated suprabasal cells. Our studies indicate that the interactions of both A4 and Bap31 are critical to E5 functions.
A4 and Bap31 are both co-localized to the endoplasmic reticulum together with E5 proteins. Previous studies had suggested that E5 could form a complex with the subunits of the vacuolar ATPAse (V-ATPase), however, the V-ATPAse is localized to late endosomes and not the endoplasmic reticulum indicating it is likely not a physiological target of E5 (Suprynowicz, Krawczyk et al. 2010). Interestingly, A4 shares an amino acid sequence similarity with the V-ATPAse in the first transmembrane region that could be a potential interacting domain with E5 and explain why previous studies implicated V-ATPase as a binding partner of E5. Future mutational analyses of A4 should elucidate if this is the E5 interacting region (Breitwieser, McLenithan et al. 1997).
In summary, our studies demonstrate that high risk HPV E5 proteins bind A4 and maintains high levels of A4 during differentiation. The binding of A4 as well as interactions of Bap31 are necessary to regulate proliferation ability of HPV positive cells upon differentiation. Further analyses of A4 and Bap31 functions will help to provide critical new insights in the role of the E5 in HPV pathogenesis.
Highlights.
A4 associates with HPV 31 E5 proteins.
A4 is localized to endoplasmic reticulum.
HPV proteins induce A4 expression in suprabasal layers of stratified epithelium.
E5 is important for proliferation ability of differentiating HPV positive cells.
Acknowledgments
This work was supported by grants from the NIH to LAL. (R37CA74202; RO1CA142861)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annaert WG, Becker B, et al. Export of cellubrevin from the endoplasmic reticulum is controlled by BAP31. J Cell Biol. 1997;139(6):1397–1410. doi: 10.1083/jcb.139.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee NS, Wang HK, et al. Human papillomavirus (HPV) E7 induces prolonged G2 following S phase reentry in differentiated human keratinocytes. J Biol Chem. 2011;286(17):15473–15482. doi: 10.1074/jbc.M110.197574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodily J, Laimins LA. Persistence of human papillomavirus infection: keys to malignant progression. Trends Microbiol. 2011;19(1):33–39. doi: 10.1016/j.tim.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodily JM, Mehta KP, et al. The E7 open reading frame acts in cis and in trans to mediate differentiation-dependent activities in the human papillomavirus type 16 life cycle. J Virol. 2011;85(17):8852–8862. doi: 10.1128/JVI.00664-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard V, Matlashewski G, et al. The human papillomavirus type 16 E5 gene cooperates with the E7 gene to stimulate proliferation of primary cells and increases viral gene expression. Virology. 1994;203(1):73–80. doi: 10.1006/viro.1994.1456. [DOI] [PubMed] [Google Scholar]
- Breitwieser GE, McLenithan JC, et al. Colonic epithelium-enriched protein A4 is a proteolipid that exhibits ion channel characteristics. Am J Physiol. 1997;272(3 Pt 1):C957–965. doi: 10.1152/ajpcell.1997.272.3.C957. [DOI] [PubMed] [Google Scholar]
- Conrad M, V, Bubb J, et al. The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J Virol. 1993;67(10):6170–6178. doi: 10.1128/jvi.67.10.6170-6178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow GL, Hanover JA, et al. Endoplasmic reticulum-localized human papillomavirus type 16 E5 protein alters endosomal pH but not trans-Golgi pH. J Virol. 2005;79(9):5839–5846. doi: 10.1128/JVI.79.9.5839-5846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow GL, Sunitha I, et al. Codon optimization of the HPV-16 E5 gene enhances protein expression. Virology. 2003;311(1):105–114. doi: 10.1016/s0042-6822(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Dollard SC, Wilson JL, et al. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. OFF. Genes Dev. 1992;6(7):1131–1142. doi: 10.1101/gad.6.7.1131. [DOI] [PubMed] [Google Scholar]
- Dyson N, Howley PM, et al. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Fehrmann F, Klumpp DJ, et al. Human papillomavirus type 31 E5 protein supports cell cycle progression and activates late viral functions upon epithelial differentiation. J Virol. 2003;77(5):2819–2831. doi: 10.1128/JVI.77.5.2819-2831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrmann F, Laimins LA. Human papillomavirus type 31 life cycle: methods for study using tissue culture models. Methods Mol Biol. 2005;292:317–330. doi: 10.1385/1-59259-848-x:317. [DOI] [PubMed] [Google Scholar]
- Frattini MG, Lim HB, et al. In vitro synthesis of oncogenic human papillomaviruses requires episomal genomes for differentiation-dependent late expression. Proc Natl Acad Sci U S A. 1996;93(7):3062–3067. doi: 10.1073/pnas.93.7.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DJ, Schlegel R. The E5 oncoprotein of bovine papillomavirus binds to a 16 kd cellular protein. EMBO J. 1990;9(1):137–145. doi: 10.1002/j.1460-2075.1990.tb08089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekharan V, Laimins LA. Human papillomaviruses modulate microRNA 145 expression to directly control genome amplification. J Virol. 2013;87(10):6037–6043. doi: 10.1128/JVI.00153-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P, Vousden KH, et al. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8(12):3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebner CM, Laimins LA. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol. 2006;16(2):83–97. doi: 10.1002/rmv.488. [DOI] [PubMed] [Google Scholar]
- Howie HL, Katzenellenbogen RA, et al. Papillomavirus E6 proteins. Virology. 2009;384(2):324–334. doi: 10.1016/j.virol.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M, Hudson JB, et al. Differentiation-induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. J Virol. 1992;66(10):6070–6080. doi: 10.1128/jvi.66.10.6070-6080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N, Varshavsky A. Split ubiquitin as a sensor of protein interactions in vivo. Proc Natl Acad Sci U S A. 1994;91(22):10340–10344. doi: 10.1073/pnas.91.22.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladasky JJ, Boyle S, et al. Bap31 enhances the endoplasmic reticulum export and quality control of human class I MHC molecules. J Immunol. 2006;177(9):6172–6181. doi: 10.4049/jimmunol.177.9.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leechanachai P, Banks L, et al. The E5 gene from human papillomavirus type 16 is an oncogene which enhances growth factor-mediated signal transduction to the nucleus. Oncogene. 1992;7(1):19–25. [PubMed] [Google Scholar]
- Lowy DR, Kirnbauer R, et al. Genital human papillomavirus infection. Proc Natl Acad Sci U S A. 1994;91(7):2436–2440. doi: 10.1073/pnas.91.7.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maufort JP, Shai A, et al. A role for HPV16 E5 in cervical carcinogenesis. Cancer Res. 2010;70(7):2924–2931. doi: 10.1158/0008-5472.CAN-09-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maufort JP, Williams SM, et al. Human papillomavirus 16 E5 oncogene contributes to two stages of skin carcinogenesis. Cancer Res. 2007;67(13):6106–6112. doi: 10.1158/0008-5472.CAN-07-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Munger K. The human papillomavirus E7 oncoprotein. Virology. 2009;384(2):335–344. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melar-New M, Laimins LA. Human papillomaviruses modulate expression of microRNA 203 upon epithelial differentiation to control levels of p63 proteins. J Virol. 2010;84(10):5212–5221. doi: 10.1128/JVI.00078-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mighty KK, Laimins LA. p63 is necessary for the activation of human papillomavirus late viral functions upon epithelial differentiation. J Virol. 2011;85(17):8863–8869. doi: 10.1128/JVI.00750-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody CA, Laimins LA. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog. 2009;5(10):e1000605. doi: 10.1371/journal.ppat.1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K, Phelps WC, et al. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng FW, Nguyen M, et al. p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum. J Cell Biol. 1997;139(2):327–338. doi: 10.1083/jcb.139.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva MM, Wu TC, et al. Isolation and characterization of a differentiation-dependent gene in the human colonic cell line HT29-18. Arch Biochem Biophys. 1993;302(1):183–192. doi: 10.1006/abbi.1993.1197. [DOI] [PubMed] [Google Scholar]
- Quistgaard EM, Low C, et al. Structural and Biophysical Characterization of the Cytoplasmic Domains of Human BAP29 and BAP31. PLoS One. 2013;8(8):e71111. doi: 10.1371/journal.pone.0071111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JA, Laimins LA. Bap31 is a novel target of the human papillomavirus E5 protein. J Virol. 2008;82(20):10042–10051. doi: 10.1128/JVI.01240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruesch MN, Laimins LA. Human papillomavirus oncoproteins alter differentiation-dependent cell cycle exit on suspension in semisolid medium. Virology. 1998;250(1):19–29. doi: 10.1006/viro.1998.9359. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Stoppler MC, Straight SW, et al. The E5 gene of HPV-16 enhances keratinocyte immortalization by full-length DNA. Virology. 1996;223(1):251–254. doi: 10.1006/viro.1996.0475. [DOI] [PubMed] [Google Scholar]
- Straight SW, Herman B, et al. The E5 oncoprotein of human papillomavirus type 16 inhibits the acidification of endosomes in human keratinocytes. J Virol. 1995;69(5):3185–3192. doi: 10.1128/jvi.69.5.3185-3192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight SW, Hinkle PM, et al. The E5 oncoprotein of human papillomavirus type 16 transforms fibroblasts and effects the downregulation of the epidermal growth factor receptor in keratinocytes. J Virol. 1993;67(8):4521–4532. doi: 10.1128/jvi.67.8.4521-4532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprynowicz FA, Krawczyk E, et al. The human papillomavirus type 16 E5 oncoprotein inhibits epidermal growth factor trafficking independently of endosome acidification. J Virol. 2010;84(20):10619–10629. doi: 10.1128/JVI.00831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle GF, Banks L. The human papillomavirus (HPV)-6 and HPV-16 E5 proteins co-operate with HPV-16 E7 in the transformation of primary rodent cells. J Gen Virol. 1995;76(Pt 5):1239–1245. doi: 10.1099/0022-1317-76-5-1239. [DOI] [PubMed] [Google Scholar]
- Wang B, Nguyen M, et al. Uncleaved BAP31 in association with A4 protein at the endoplasmic reticulum is an inhibitor of Fas-initiated release of cytochrome c from mitochondria. J Biol Chem. 2003;278(16):14461–14468. doi: 10.1074/jbc.M209684200. [DOI] [PubMed] [Google Scholar]
- Watt FM. Epidermal stem cells: markers, patterning and the control of stem cell fate. Philos Trans R Soc Lond B Biol Sci. 1998;353(1370):831–837. doi: 10.1098/rstb.1998.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werness BA, Levine AJ, et al. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Wilson R, Laimins LA. Differentiation of HPV-containing cells using organotypic “raft” culture or methylcellulose. Methods Mol Med. 2005;119:157–169. doi: 10.1385/1-59259-982-6:157. [DOI] [PubMed] [Google Scholar]
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]