Abstract
OBJECTIVES
The aim of this study was to examine whether magnesium intake is associated with coronary artery calcification (CAC) and abdominal aortic calcification (AAC).
BACKGROUND
Animal and cell studies suggest that magnesium may prevent calcification within atherosclerotic plaques underlying cardiovascular disease. Little is known about the association of magnesium intake and atherosclerotic calcification in humans.
METHODS
We examined cross-sectional associations of self-reported total (dietary and supplemental) magnesium intake estimated by food frequency questionnaire with CAC and AAC in participants of the Framingham Heart Study who were free of cardiovascular disease and underwent Multi-Detector Computed Tomography (MDCT) of the heart and abdomen (n = 2,695; age: 53 ± 11 years), using multivariate-adjusted Tobit regression. CAC and AAC were quantified using modified Agatston scores (AS). Models were adjusted for age, sex, body mass index, smoking status, systolic blood pressure, fasting insulin, total-to-high-density lipoprotein cholesterol ratio, use of hormone replacement therapy (women only), menopausal status (women only), treatment for hyperlipidemia, hypertension, cardiovascular disease prevention, or diabetes, as well as self-reported intake of calcium, vitamins D and K, saturated fat, fiber, alcohol, and energy. Secondary analyses included logistic regressions of CAC and AAC outcomes as cut-points (AS >0 and AS ≥90th percentile for age and sex), as well as sex-stratified analyses.
RESULTS
In fully adjusted models, a 50-mg/day increment in self-reported total magnesium intake was associated with 22% lower CAC (p < 0.001) and 12% lower AAC (p = 0.07). Consistent with these observations, the odds of having any CAC were 58% lower (p trend: <0.001) and any AAC were 34% lower (p trend: 0.01), in those with the highest compared to those with the lowest magnesium intake. Stronger inverse associations were observed in women than in men.
CONCLUSIONS
In community-dwelling participants free of cardiovascular disease, self-reported magnesium intake was inversely associated with arterial calcification, which may play a contributing role in magnesium's protective associations in stroke and fatal coronary heart disease.
Keywords: abdominal aortic calcification, computed tomography, coronary artery calcification, diet, Framingham Heart Study, magnesium
Coronary artery calcification (CAC) (1–3) and abdominal aortic calcification (AAC) (3–5) are measures of advanced atherosclerosis that predict cardiovascular disease (CVD) morbidity and mortality independently of traditional CVD risk factors. CAC in particular has been shown to discriminate and reclassify future risk for clinical coronary events (6). Dietary magnesium, found in a broad range of foods including whole grains, green leafy vegetables, almonds, coffee, and dark chocolate, has been linked to many aspects of cardiovascular health (7–9), and this mineral may play a key role in vascular calci-fication. A protective role of magnesium in calci-fication may underlie previous observations of higher magnesium intake and lower risk of stroke (10,11), nonfatal myocardial infarction (MI), sudden cardiac death, and fatal coronary heart disease (CHD) (12–14).
In vitro (15–19) and animal (19–23) studies suggest biological mechanisms through which magnesium may prevent or reverse plaque formation and calcification. Magnesium may be acting as a calcium antagonist (24), and it may directly inhibit hydroxyapatite and crystal precipitation (25–27). In individuals with chronic kidney disease (CKD), end-stage renal disease (ESRD), or on hemodialysisdknown to exhibit accelerated calcificationdinverse associations have been reported between serum magnesium and calcification in various vascular beds (27) and with related measures of atherosclerosis or arteriosclerosis, such as carotid intima-medial thickness (IMT) and pulse-wave velocity (PWV) (17). In healthy populations, observational studies have also found serum magnesium to be inversely associated with IMT, presence of atherosclerotic plaque, and progression of atherosclerosis (28,29).
However, serum magnesium is a poorly correlated biomarker of magnesium intake (30,31). Only one observational study has examined dietary magnesium in association with CAC in a generally healthy population, observing no association (32). No study has examined the association between magnesium intake and AAC. Therefore, we tested the hypothesis that higher magnesium intake is associated with lower levels of calcification of the coronary arteries and abdominal aorta in a generally healthy population, by assessing the cross-sectional association between self-reported total (dietary and supplemental) magnesium intake with CAC and AAC in community-dwelling participants free of clinically apparent CVD.
METHODS
Study population
The National Heart, Lung, and Blood Institute's Framingham Heart Study is a longitudinal, community-based, observational study that began in 1948 in Framingham, Massachusetts. The children, and their spouses (“Offspring,” enrolled 1971–1975), of the original cohort participants have returned for follow-up examination following standardized protocols approximately every four years (33). The third-generation cohort (enrolled 2002 to 2005) includes 4,095 children of the Offspring (34). The present study includes dietary and risk factor data collected from participants who attended Offspring exam 7 (1998 to 2001; n = 3,539) or Third Generation exam 1 (2002 to 2005; n = 4,095), and who participated in exam 1 (2002 to 2005) of the ongoing Multi-Detector Computed Tomography (MDCT) substudy. Although previously described (35), in brief, 3,529 Offspring or Third Generation participants located in the greater New England area underwent MDCT scanning. Men were ≥35 years of age, women were ≥40 years of age and not pregnant, and all participants weighed ≤350 lbs (35).
We excluded participants from this analysis if they had missing or uninterpretable CT scans (n = 278); had clinically apparent CVD (n = 136), defined as CABG, valve replacement, percutaneous coronary stent placement, pacemaker, stroke, CHF, MI, or coronary insufficiency identified or occurring prior to the date of the clinic exam (35); had missing or invalid dietary information (n = 172, reporting <600 or ≥4,000 kcal/day for women, <600 or ≥4,200 kcal/day for men, or with ≥12 blank items); self-reported extreme values of magnesium or calcium intake (n = 48, with intake values in the 0.5th or 99.5th percentile); or were missing complete covariate information (n = 200, as defined subsequently). After exclusions, 2,695 participants remained in the present analyses.
The original data collection protocols were approved by the institutional review boards at Boston University and Massachusetts General Hospital, Boston, Massachusetts, and written informed consent was obtained from all participants. The present study protocol was reviewed by the Tufts University institutional review board.
Dietary assessment
The Harvard semi-quantitative, 126-item Food Frequency Questionnaire (FFQ) was used to assess dietary intake (36). The FFQ was mailed to participants prior to each exam, and participants were instructed to bring the completed FFQ with them to their exam appointment. The relative validity of the FFQ has been demonstrated in similar populations, with correlations of 0.69 to 0.72 between self-reported total magnesium intake estimated from FFQ and dietary records (36). Serum magnesium, a biomarker of magnesium status, was available only at Offspring exam 2 (1979–1982), approximately 20 years prior to the MDCT substudy, and no biomarker measures are available in the Third Generation. Therefore, serum magnesium was not assessed as an exposure in the associations studied here.
Outcome measures
CAC and AAC were quantified from CT scans using a modified Agatston score (AS), as previously described (35,37). In brief, each participant underwent 8-slice MDCT scanning consisting of 2 chest scans and 1 abdominal scan (Lightspeed Ultra, General Electric Medical Systems, Milwaukee, Wisconsin) during a single end-inspiratory breath-hold. For CAC, 48 contiguous 2.5-mm-thick slices were acquired in each scan. For AAC, the top of the S1 vertebral body selected as the most caudal extent of the abdominal volume to be imaged, and 30 contiguous 5-mm-thick slices were acquired to 15 cm above S1. A calcified lesion was defined as an area of ≥3 connected pixels with CT attenuation of >130 Hounsfield units. AS was calculated by multiplying the area of each lesion with a weighted attenuation score dependent on the maximal attenuation within the lesion. We defined prevalent CAC or AAC as AS >0, and high CAC (35) and high AAC (37) according to previously defined age- and sexspecific 90th-percentile cut-points relative to a healthy reference sample of the Framingham Heart Study.
Covariates
From interviews, information was obtained related to each participant's age, smoking status, menopausal status, physical activity, education, aspirin use, treatment for hyperlipidemia (e.g., niacin, fibrates, statins), osteoporosis (e.g., calcitonin preparations, selective estrogen receptor modulators, and other drugs affecting bone structure and mineralization including bisphosphonates, bisphosphonate combinations, and bone morphogenetic proteins), hypertension or CVD prevention (e.g., ACE inhibitors, nitroglycerin, calcium-channel blockers, beta-blockers), or diabetes (oral hypoglycemics or insulin), menopausal status, and use of estrogen or other hormone replacement therapy (HRT) in women. In women, menopausal status was defined as >1-year cessation of menses. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure was measured twice and averaged to calculate the systolic and diastolic blood pressures (SBP and DBP, respectively). Total cholesterol was measured enzymatically and the HDL-C fraction was measured after the precipitation of low-density lipoprotein cholesterol and very-low-density lipoprotein cholesterol. Fasting plasma glucose was measured in fresh specimens with hexokinase reagent. Fasting plasma insulin was measured using human-specific radio-immunoassay in the Offspring and enzyme-linked immunosorbent assay in the Third Generation (standardized to the Offspring for analysis). Type 2 diabetes was defined as fasting glucose ≥126 mg/dl or use of oral hypoglycemics or insulin. Serum C-reactive protein (CRP) was measured by particle enhanced immunonephelometry using high-sensitivity CRP reagent. Glomerular filtration rate (GFR) was calculated using the simplified Modification of Diet in Renal Disease study equation from serum creatinine, measured using the modified Jaffe method (38).
Statistical analyses
All self-reported nutrient intake values derived from the FFQ were adjusted for total energy using the residual method (39). Quartile categories of energy-adjusted, self-reported total (dietary and supplemental) magnesium intake were created to present participant characteristics and for use in regression analyses. Linear trends in the means or percentages of age- and sex-adjusted (age-, sex-, and energy-adjusted, for nutrients) participant characteristics across quartile categories were assessed using the median intake value in each category.
We used natural logarithmic (ln)-transformed values of CAC and AAC, after adding 1 to each score, due to a large number of zero values and to reduce skew. Tobit regression is an appropriate model for calcification data (40) and was applied for our primary tests of association between ln(CAC + 1) or ln(AAC + 1) and continuous self-reported total (dietary and supplemental) magnesium intake as the exposure (PROC LIFEREG, with a censored threshold of zero CAC or AAC). We present beta coefficients and SE per 50 mg/day of total (dietary and supplemental) magnesium intake. For analyses of quartile categories, we present adjusted means and SEs of ln(CAC + 1) or ln(AAC + 1) from least squares regression (PROC GLM), and p values for linear trend across quartile categories of self-reported total (dietary and supplemental) magnesium intake.
Regression models included known or potential confounders as follows: model 1 was adjusted for age at MDCT exam (in years), sex, exam cycle, energy intake (kcal/day), and calcium intake (mg/day). Model 2 was adjusted as for model 1, plus known CVD risk factors, which may also be mediators of the diet–calcification relationship, including BMI (kg/m2), smoking status (never/former/current), total–to–HDL cholesterol ratio, fasting insulin (ln-pmol/l), SBP (mm Hg), use of estrogen or HRT and menopausal status (in women, both yes/no), and treatment for hypertension or CVD prevention, hyperlipidemia, or diabetes (all yes/no), and alcohol intake (g/day). Model 3, the fully adjusted model, was adjusted as for model 2, plus dietary factors associated with CVD or implicated in calcification, including intakes of fiber (g/day), saturated fat (g/day), vitamin D (IU/day), and vitamin K (mcg/d). Further adjustment of model 3 for CRP (mg/l), regular aspirin use (yes/no), GFR (ml/min/1.73 m2), physical activity (h/day), treatment for osteoporosis (yes/no), highest completed education (no schooling; grades 1 to 8; grades 9 to 11; high school or technical school; some college; 2-year degree; 4-year degree; graduate or professional degree), or AAC (as ln[AS + 1], in CAC analysis only), did not substantively alter results (data not shown). In addition, analyses to account for familial correlations did not materially alter the results; we therefore present results unadjusted for these relationships. We assessed potential effect modification between total magnesium intake and total calcium intake, sex, BMI, and age by testing for statistical interaction using cross-product terms in Tobit regression analyses. As there were no statistically significant interactions (all, p > 0.05), interaction terms were removed but covariates were retained in the models. However, because of differences in CAC distributions between sexes, we repeated analyses in men and women separately using sex-specific quartile categories of energy-adjusted self-reported total (dietary and supplemental) magnesium intake, and present these exploratory results in the Online Appendix.
In secondary analyses, for comparison with published data, we estimated odds of having any CAC or AAC (AS 0 vs. >0), and high CAC or AAC (AS < vs. ≥90th percentile for age and sex relative to a healthy reference population [35,37]). We present odds ratios (OR) and 95% confidence intervals (CI) in each quartile category of energy-adjusted self-reported total (dietary and supplemental) magnesium intake, and p values for linear trend across categories. The lowest category of intake was used as the reference category.
All analyses were conducted in SAS 9.3 (SAS Institute Inc., Cary, North Carolina). A 2-sided p value <0.05 was considered statistically significant, because our primary outcomes—the continuous measures of CAC and AAC—are correlated.
RESULTS
Clinical and dietary characteristics of participants across quartile categories of energy-adjusted self-reported total (dietary and supplemental) magnesium intake are presented in Table 1. Mean adjusted self-reported total (dietary and supplemental) magnesium intake was 338 mg/day. On average, supplemental sources of self-reported magnesium intake only contributed 6.4% and 4.6% of total self-reported magnesium intake in women and men, respectively. In analyses of trend from lowest to highest category of self-reported total (dietary and supplemental) magnesium intake, those in the highest category were more likely to be female, older, more educated, have lower BMI, DBP, total cholesterol, total–to–HDL cholesterol ratio, fasting insulin, CRP, and an overall healthier diet. They were also more likely to use lipid-lowering treatment and aspirin, and less likely to have smoked regularly in the prior year.
Table 1.
Participant Characteristics Across Quartile Categories of Energy-Adjusted Self-Reported Total (Dietary and Supplemental) Magnesium Intake in the Framingham Heart Study
| Quartile 1 (n = 673) | Quartile 2 (n = 674) | Quartile 3 (n = 674) | Quartile 4 (n = 674) | p Value | |
|---|---|---|---|---|---|
| Magnesium intake, mg/day | p Linear trend | ||||
| Median | 258.8 | 303.6 | 351.1 | 427.4 | |
| Range, mg/day | 159.8–283.9 | 284.0–325.4 | 325.5–383.6 | 383.9–669.4 | |
| General characteristics | |||||
| Age at CT exam, yrs | 51.4 (0.4) | 52.9 (0.4) | 52.4 (0.4) | 54.1 (0.4) | <0.001 |
| Female, % | 35.0 (2.0) | 49.0 (2.0) | 55.0 (2.0) | 59.0 (2.0) | <0.001 |
| BMI, kg/m2 | 28.6 (0.2) | 28.0 (0.2) | 27.7 (0.2) | 27.3 (0.2) | <0.001 |
| Physical activity, h/day | 4.9 (0.1) | 4.5 (0.1) | 4.7 (0.1) | 4.8 (0.1) | 0.19 |
| Current smoker, % | 17.0 (1.0) | 13.0 (1.0) | 11.0 (1.0) | 7.0 (1.0) | <0.001 |
| Completed at least high school, % | 98.0 (0.0) | 99.0 (0.0) | 99.0 (0.0) | 99.0 (0.0) | 0.48 |
| College degree or higher, % | 35.0 (2.0) | 47.0 (2.0) | 54.0 (2.0) | 56.0 (2.0) | <0.001 |
| Current HRT use, % of women | 18.0 (2.0) | 21.0 (2.0) | 20.0 (2.0) | 23.0 (2.0) | 0.50 |
| Post-menopausal, % of women | 49.0 (2.0) | 52.0 (2.0) | 49.0 (2.0) | 55.0 (2.0) | 0.09 |
| Clinical and laboratory characteristics | |||||
| SBP, mm Hg | 122.9 (0.6) | 122.4 (0.6) | 121.3 (0.6) | 120.8 (0.6) | 0.05 |
| DBP, mm Hg | 77.2 (0.4) | 76.5 (0.3) | 76.0 (0.3) | 75.7 (0.4) | 0.02 |
| Antihypertensive Rx, % | 18.0 (1.0) | 16.0 (1.0) | 16.0 (1.0) | 17.0 (1.0) | 0.88 |
| Total cholesterol, mg/dl | 201.1 (1.4) | 198.3 (1.3) | 196.8 (1.3) | 193.8 (1.3) | 0.002 |
| HDL cholesterol, mg/dl | 52.1 (0.6) | 53.7 (0.5) | 53.2 (0.5) | 53.9 (0.6) | 0.09 |
| Total-to-HDL cholesterol ratio | 4.2 (0.1) | 4.0 (0.1) | 4.0 (0.1) | 3.9 (0.1) | <0.001 |
| Lipid-lowering Rx, % | 9.0 (1.0) | 14.0 (1.0) | 12.0 (1.0) | 15.0 (1.0) | 0.008 |
| Fasting glucose, mg/dl | 99.0 (0.8) | 98.8 (0.8) | 98.9 (0.8) | 97.8 (0.8) | 0.64 |
| Fasting insulin, pmol/l* | 85.6 (1.0) | 82.3 (1.0) | 79.8 (1.0) | 79.0 (1.0) | 0.001 |
| Diabetes Rx, % | 1.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | 3.0 (1.0) | 0.45 |
| CVD prevention Rx, % | 13.0 (1.0) | 13.0 (1.0) | 13.0 (1.0) | 13.0 (1.0) | 0.97 |
| Aspirin use, % | 13.0 (1.0) | 16.0 (1.0) | 20.0 (1.0) | 22.0 (1.0) | <0.001 |
| Osteoporosis Rx, % | 4.0 (1.0) | 3.0 (1.0) | 3.0 (1.0) | 4.0 (1.0) | 0.64 |
| CRP, mg/l | 3.3 (0.2) | 3.1 (0.2) | 2.7 (0.2) | 2.6 (0.2) | 0.01 |
| GFR, ml/min/1.73 m2 | 93.9 (0.7) | 94.0 (0.7) | 94.1 (0.7) | 94.1 (0.7) | 0.99 |
| CAC, AS* | 5.93 (1.07) | 6.49 (1.07) | 5.75 (1.07) | 4.53 (1.07) | 0.002 |
| AS 0, % | 53.0 (2.0) | 54.0 (2.0) | 57.0 (2.0) | 61.0 (2.0) | 0.01 |
| AS >0–<10, % | 14.0 (1.0) | 11.0 (1.0) | 10.0 (1.0) | 10.0 (1.0) | |
| AS 10–<100, % | 15.0 (1.0) | 15.0 (1.0) | 15.0 (1.0) | 14.0 (1.0) | |
| AS 100–<400, % | 12.0 (1.0) | 12.0 (1.0) | 10.0 (1.0) | 8.0 (1.0) | |
| AS >400, % | 6.0 (1.0) | 7.0 (1.0) | 7.0 (1.0) | 7.0 (1.0) | |
| AAC, AS* | 24.53 (1.09) | 21.33 (1.09) | 15.80 (1.09) | 15.96 (1.09) | 0.001 |
| AS >0, % | 56.0 (2.0) | 55.0 (2.0) | 50.0 (2.0) | 51.0 (2.0) | 0.02 |
| Dietary characteristics | |||||
| Magnesium, total, mg/day | 250.9 (1.3) | 302.4 (1.3) | 350.3 (1.3) | 442.7 (1.3) | <0.001 |
| From diet, mg/day | 250.8 (1.6) | 300.5 (1.6) | 334.4 (1.5) | 380.0 (1.6) | <0.001 |
| From supplements, mg/day | 1.8 (1.3) | 3.6 (1.3) | 17.6 (1.3) | 64.4 (1.3) | <0.001 |
| Calcium, total, mg/day | 803.7 (15.2) | 947.1 (15.1) | 1078.5 (15.0) | 1279.0 (15.2) | <0.001 |
| From diet, mg/day | 696.6 (10.9) | 813.5 (10.8) | 886.0 (10.7) | 929.1 (10.8) | <0.001 |
| From supplements, mg/day | 112.1 (11.9) | 138.5 (11.8) | 197.4 (11.7) | 354.8 (11.9) | <0.001 |
| Energy, kcal/day | 2036 (24) | 1830 (24) | 1949 (24) | 2092 (24) | <0.001 |
| Vitamin K, mcg/day | 109.2 (4.7) | 145.9 (4.6) | 185.4 (4.6) | 236.2 (4.7) | <0.001 |
| Vitamin D, IU/day | 246.5 (10.0) | 345.4 (9.9) | 427.7 (9.9) | 599.7 (10.0) | <0.001 |
| Saturated fat, g/day | 27.3 (0.2) | 25.9 (0.2) | 24.1 (0.2) | 21.8 (0.2) | <0.001 |
| Fiber, g/day | 14.2 (0.2) | 17.2 (0.2) | 20.0 (0.2) | 23.7 (0.2) | <0.001 |
| Alcohol, g/day | 11.8 (0.6) | 11.2 (0.5) | 10.4 (0.5) | 9.5 (0.6) | 0.01 |
| Multivitamin use, % | 27.0 (2.0) | 39.0 (2.0) | 52.0 (2.0) | 79.0 (2.0) | <0.001 |
Values are mean (SE), unless otherwise indicated. All characteristics are age- and sex-adjusted, except for age and sex, which are mutually adjusted. Dietary characteristics are also energy-adjusted.
Analyzed in the natural logarithm scale and back-transformed. Geometric mean (geometric SE) are presented.
AAC = abdominal aortic calcification; AS = Agatston score; BMI = body mass index; CAC = coronary artery calcification; CRP = C-reactive protein; CT = computed tomography; CVD = cardiovascular disease; DBP = diastolic blood pressure; GFR = estimated glomerular filtration rate; HDL = high-density lipoprotein cholesterol; HRT = hormone replacement therapy; Rx = use of medication/treatment; SBP = systolic blood pressure; SE = standard error.
Primary analyses
CAC was present (AS >0) in 43.7% of participants (33.7% of women and 53.7% of men). AAC was more prevalent: 52.9% of participants had some detectable AAC (AS >0), and prevalence was similar between the sexes (50.9% of women and 55.3% of men). Of those with prevalent AAC, 65.3% had detectable CAC (55.3% of women and 74.3% of men with prevalent AAC, had detectable CAC).
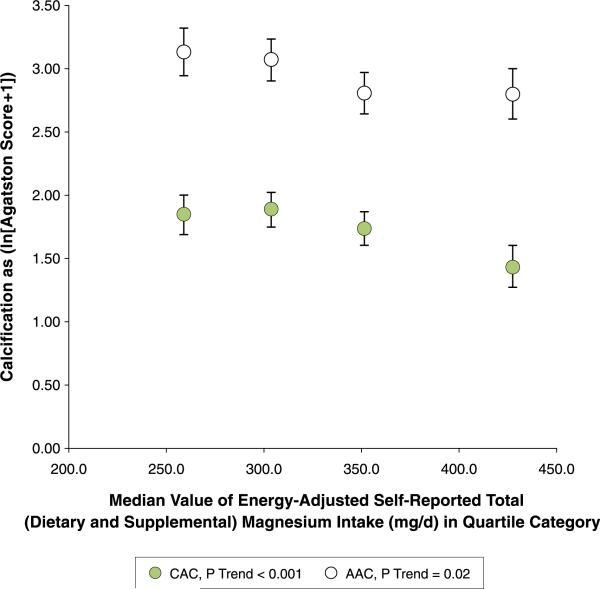
In the fully adjusted model (model 3), higher self-reported total (dietary and supplemental) magnesium intake was associated with 22% lower CAC per 50 mg/day increment (Table 2). Self-reported total magnesium intake was significantly associated with 17% lower AAC in the basic model (model 1, p = 0.001), but was attenuated after adjusting for risk factors (model 2, 9% lower; p = 0.09), and vitamins D and K, saturated fat, and fiber (model 3, 12% lower; p = 0.07). In sex-specific exploratory analyses, although tests of interaction with sex were not statistically significant, the inverse associations appeared to be stronger in women than in men for both continuous outcomes (Online Table 1). The trends of mean CAC and AAC across quartile categories of self-reported total (dietary and supplemental) magnesium intake are consistent with the Tobit regression results (Table 3, Fig. 1 for pooled analyses, and Online Table 2 and Online Fig. 1 for sex-specific analyses).
Table 2.
Associations and SEs of 50-mg/day Increments in Energy-Adjusted Self-Reported Total (Dietary and Supplemental) Magnesium Intake with CAC and AAC
| Model* | n | β † | SE | p Value |
|---|---|---|---|---|
| CAC as ln(AS + 1) | ||||
| Model 1 | 2,695 | –0.18 | 0.06 | 0.001 |
| Model 2 | –0.13 | 0.05 | 0.011 | |
| Model 3 | –0.25 | 0.07 | <0.001 | |
| AAC as ln(AS + 1) | ||||
| Model 1 | 2,681 | –0.19 | 0.06 | 0.001 |
| Model 2 | –0.09 | 0.06 | 0.09 | |
| Model 3 | –0.13 | 0.08 | 0.07 |
Tobit regression analyses were adjusted as follows: model 1 adjusted for calcium and energy intake, age, sex, and exam cycle. Model 2 adjusted as for model 1, plus BMI, smoking status, SBP, fasting insulin, total-to-high-density lipoprotein cholesterol ratio, use of hormone replacement therapy (women only), menopausal status (women only), treatment for hyperlipidemia, hypertension or cardiovascular disease prevention, or diabetes, and alcohol intake. Model 3 adjusted as for model 2, plus intake of vitamins K and D, saturated fat, and fiber.
β Coefficients of Tobit regression can be interpreted as most linear regression coefficients on the natural log scale, that is, as percent changes per 50-mg/day increments in magnesium intake, obtained by exponentiating the coefficient and subtracting 1. For example, in model 3 of the CAC regression, the –0.25 β coefficient can be thought of as [e–0.25 – 1] = –22%, or 22% lower CAC per 50-mg/day increment in intake.
Abbreviations as in Table 1.
Table 3.
Adjusted Means and SEs of CAC and AAC across Quartile Categories of Energy-Adjusted Self-Reported Total (Dietary and Supplemental) Magnesium Intake*
| Quartile 1 (n = 673) | Quartile 2 (n = 674) | Quartile 3 (n = 674) | Quartile 4 (n = 674) | p Value | |
|---|---|---|---|---|---|
| Magnesium intake, mg/day | p Linear trend | ||||
| Median | 258.8 | 303.6 | 351.1 | 427.4 | |
| Range | 159.8–283.9 | 284.0–325.4 | 325.5–383.6 | 383.9–669.4 | |
| CAC as ln(AS + 1) | |||||
| Model 1 | 1.78 (0.07) | 1.86 (0.07) | 1.74 (0.07) | 1.52 (0.07) | 0.004 |
| Model 2 | 1.77 (0.07) | 1.85 (0.07) | 1.75 (0.07) | 1.52 (0.07) | 0.006 |
| Model 3 | 1.85 (0.08) | 1.88 (0.07) | 1.74 (0.07) | 1.43 (0.08) | 0.0005 |
| AAC as ln(AS + 1) | |||||
| Model 1 | 3.21 (0.09) | 3.04 (0.09) | 2.77 (0.09) | 2.77 (0.09) | 0.001 |
| Model 2 | 3.10 (0.09) | 3.06 (0.08) | 2.81 (0.08) | 2.83 (0.09) | 0.01 |
| Model 3 | 3.13 (0.10) | 3.07 (0.08) | 2.80 (0.08) | 2.80 (0.10) | 0.02 |
For AAC, n = 2,681. Differences between intake categories, when the outcome is presented on the natural log scale as done here, can be interpreted as percent differences between highest and lowest categories by exponentiating the mean in the highest and lowest categories, and taking the ratio of the exponentiated means. For example, in model 3 of the AAC regression, e2.80/e3.13 = 0.72, or 28% lower AAC in the highest compared to the lowest intake category. Models adjusted for as in Table 2.
Abbreviations as in Table 1.
Figure 1. Adjusted Means of CAC and AAC According to Self-Reported Total (Dietary and Supplemental) Magnesium Intake.
Adjusted means ± SE of CAC (green circles) and AAC (white circles) (as ln [AS + 1]) according to median values of energy-adjusted self-reported total (dietary and supplemental) magnesium intake in quartile categories in 2,695 participants of the Framingham Heart Study. Highest versus lowest intake was associated with 34% lower CAC (p linear trend: <0.001), and 28% lower AAC (p linear trend: 0.02). Values are adjusted for age, sex, exam cycle, body mass index, smoking status, systolic blood pressure, fasting insulin, total–to–high-density lipoprotein cholesterol ratio, use of hormone replacement therapy (women only), menopausal status (women only), treatment for hyperlipidemia, hypertension or cardiovascular disease prevention, or diabetes, and intake of energy, calcium, alcohol, vitamins K and D, saturated fat, and fiber. AAC = abdominal aortic calcification; AS = Agatston score; CAC = coronary artery calcification.
Secondary analyses
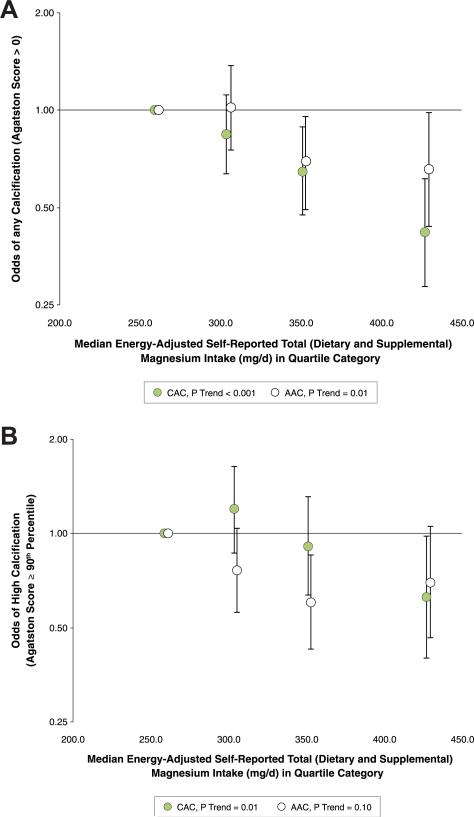
We examined the associations of self-reported total (dietary and supplemental) magnesium intake with odds of having any calcification (AS >0) and odds of having high calcification (AS ≥90th percentile for age and sex) at either vascular site (Table 4, Fig. 2). In fully adjusted models, compared to those in the lowest quartile category of self-reported total (dietary and supplemental) magnesium intake, those in the highest category had 58% lower odds of any CAC, 37% lower odds of high CAC, and 34% lower odds of any AAC. There was a nonsignificant inverse association between high intake and odds of having high AAC. Sex-specific exploratory analyses resulted in similar, statistically significant associations for odds of any CAC between men and women. For odds of high CAC, any AAC, and high AAC, linear trends were not statistically significant in either sex (Online Table 3, Online Figs. 2 and 3).
Table 4.
Adjusted Odds Ratios and 95% CIs of Prevalent or High CAC or AAC across Quartile Categories of Energy-Adjusted Self-Reported Total (Dietary and Supplemental) Magnesium Intake
| Quartile 1 (n = 673) | Quartile 2 (n = 674) | Quartile 3 (n = 674) | Quartile 4 (n = 674) | p Value | |
|---|---|---|---|---|---|
| Magnesium intake, mg/day | p Linear trend | ||||
| Median | 258.8 | 303.6 | 351.1 | 427.4 | |
| Range | 159.8–283.9 | 284.0–325.4 | 325.5–383.6 | 383.9–669.4 | |
| CAC >0 | |||||
| Model 1 | 1 (Ref) | 0.95 (0.73–1.23) | 0.79 (0.60–1.03) | 0.60 (0.45–0.81) | <0.001 |
| Model 2 | 1 (Ref) | 0.93 (0.71–1.23) | 0.79 (0.60–1.05) | 0.58 (0.43–0.79) | <0.001 |
| Model 3 | 1 (Ref) | 0.84 (0.63–1.11) | 0.65 (0.47–0.89) | 0.42 (0.29–0.62) | <0.001 |
| CAC ≥90th | |||||
| Model 1 | 1 (Ref) | 1.20 (0.89–1.62) | 0.94 (0.68–1.29) | 0.71 (0.50–1.02) | 0.02 |
| Model 2 | 1 (Ref) | 1.26 (0.92–1.72) | 0.99 (0.71–1.38) | 0.71 (0.49–1.03) | 0.02 |
| Model 3 | 1 (Ref) | 1.20 (0.87–1.64) | 0.92 (0.64–1.32) | 0.63 (0.40–0.98) | 0.01 |
| AAC >0 | |||||
| Model 1 | 1 (Ref) | 0.99 (0.75–1.30) | 0.71 (0.54–0.94) | 0.70 (0.51–0.95) | 0.01 |
| Model 2 | 1 (Ref) | 1.07 (0.80–1.44) | 0.76 (0.56–1.02) | 0.77 (0.55–1.06) | 0.03 |
| Model 3 | 1 (Ref) | 1.02 (0.75–1.37) | 0.69 (0.49–0.95) | 0.66 (0.44–0.98) | 0.01 |
| AAC ≥90th | |||||
| Model 1 | 1 (Ref) | 0.75 (0.57–0.99) | 0.62 (0.46–0.83) | 0.73 (0.54–0.99) | 0.05 |
| Model 2 | 1 (Ref) | 0.80 (0.59–1.08) | 0.65 (0.48–0.90) | 0.79 (0.57–1.10) | 0.17 |
| Model 3 | 1 (Ref) | 0.76 (0.56–1.04) | 0.61 (0.43–0.86) | 0.70 (0.47–1.05) | 0.10 |
Figure 2. Adjusted Odds of Prevalent or High CAC and AAC According to Self-Reported Total (Dietary and Supplemental) Magnesium Intake.
Adjusted ORs (95% CI) of any (AS >0) (A) or high (AS ≥90th percentile for age and sex relative to a healthy referent population) (B) CAC (green circles) or AAC (white circles) according to median values of energy-adjusted self-reported total (dietary and supplemental) magnesium intake (mg/day) in quartile categories in 2,695 participants of the Framingham Heart Study. CI = confidence interval; OR = odds ratio; other abbreviations as in Figure 1.
DISCUSSION
The main finding of this study is that in individuals free of clinically apparent CVD, higher self-reported total (dietary and supplemental) magnesium intake, estimated by food frequency questionnaire, is associated with lower levels of CAC, a sensitive, discriminating measure of subclinical CVD and overall burden of atherosclerosis. Those with the highest self-reported total magnesium intake had approximately one-half the odds of having any detectable CAC, compared to those with the lowest intake, which suggests magnesium intake may have a protective role in inhibiting calcification initiation. The observed associations with CAC were significant after adjusting for a range of cardiometabolic risk factors and potential mediators, as well as after further adjusting for AAC levels, suggesting that magnesium may be acting specifically in the coronary arteries over and above its other known anti-inflammatory, antihypertensive, and antidyslipidemic functions to affect calcification (7–9).
To date, only 1 cross-sectional analysis, conducted in MESA (the Multi-Ethnic Study of Atherosclerosis), has examined self-reported magnesium intake in relation to CAC. Although no significant association was observed between self-reported magnesium intake and CAC in that study, the authors observed that higher magnesium intake was associated with lower odds of high common carotid IMT (32), an indicator of atherosclerotic disease moderately correlated with CAC. Some differences between our analysis and the MESA study that may explain the inconsistent observations include population differences (multiple races/ethnicities in MESA; predominantly white in Framingham), inclusion of calcium intake as a confounder in our analysis, and the application of cut-points of CAC (>0 or >100) in MESA, rather than also evaluating it as a continuous measure. Although we did not examine IMT or other measures of plaque in our analysis, and thus cannot comment on magnesium intake's associations with atherogenic plaque, we cannot rule out the possibility that magnesium has additional roles in plaque formation separate from calcified plaque, a relationship that has also been shown in some animal and cell studies (21,22).
Several studies have examined common sources of dietary magnesium—chocolate (41), coffee (42,43), fish (44), and whole grains (45)—in relation to CAC, and the observations of these studies have been consistent. In the Family Heart Study, chocolate consumption was inversely associated with odds of CAC (AS >100) in a dose-response manner (41). In the Rotterdam Study, higher coffee intake was inversely associated with severe CAC in older women, but not older men (43). In younger individuals (18 to 30 years of age), coffee showed no association with the presence or progression of CAC over 15 to 20 years (42). Whole-grain intake was not associated with CAC in another MESA study, despite significant inverse associations with other CVD risk factors (45). Finally, researchers in Rotterdam reported that those with higher fish intake had lower prevalence of moderate CAC (AS 11 to 400) and severe CAC (AS >400) compared to nonconsumers, associations that were not attributable to intake of either docosahexaenoic or eicosapentaenoic acid—the fatty acids to which fish consumption's cardiovascular benefits are often attributed (44). These inconsistencies may be attributed to variation in study populations, variation in the contributions of these foods to overall magnesium intake, and the complex interaction of foods in the diet. A magnesium supplementation trial has not yet been conducted in generally healthy adults with respect to CAC, nor are we aware of CAC as a secondary endpoint in magnesium supplementation trials with other primary endpoints. However, a small pilot study in patients with ESRD undergoing chronic hemodialysis—at particularly high risk for rapid calcification progression—reported a non-significant progression of CAC of just 8% (versus typical 50%) over 18 months using a magnesium/calcium carbonate binder (approximately 700 mg/day elemental magnesium and 1,200 mg/day elemental calcium) in lieu of the standard calcium-based phosphate binder (46).
To our knowledge, ours is the first study to examine self-reported total magnesium intake in relation to AAC, which like CAC is an independent predictor of CVD morbidity and mortality (3–5). Despite some pathological differences (47), CAC and AAC are thought to be similar phenomena, and presence of AAC is a good predictor of CAC. We are unable to explain the differing magnitudes of association we observed between self-reported total magnesium intake and calcification in the 2 vascular beds, apart from speculating on a potential primary role of magnesium in unknown processes more predominantly associated with, and therefore specific to, atherosclerotic calcification of the coronary arteries. We had hypothesized that magnesium intake would have similar associations with both CAC and AAC. One of magnesium's putative roles in preventing biomineralization of extraskeletal tissue is its inhibition of hydroxyapatite formation, in which magnesium destabilizes the crystal structure and inhibits precipitation (17,26,27). In addition, magnesium has been shown to inhibit osteogenic differentiation of vascular smooth muscle cells (15,16,48) and increase the expression of calcification-inhibiting proteins, while decreasing activity of bone-related proteins (16) and preventing cell apoptosis (15).
Because current CT imaging technology does not differentiate between medial and intimal calcification, we could not rule out the presence of medial calcification as a possible explanation for some of the differing associations between CAC and AAC. Medial calcification, which occurs in conditions of longstanding metabolic imbalance (e.g., diabetes, CKD), is thought to be rare in the coronary arteries (49), but may be more prevalent in the abdominal aorta in the presence of mild metabolic or mineral derangement. However, our results did not materially change after excluding participants with prevalent diabetes (5% of study sample) or impaired kidney function (2% of study sample), and we controlled for glycemic traits in our analysis. These discrepancies, and dietary magnesium's role in processes specific to atherosclerotic calcification in the coronary arteries, deserve further investigation.
Study limitations
As a cross-sectional analysis, we cannot infer a temporal relationship from our observations. Although our observations have plausible biological underpinnings, the mechanisms underlying these associations remain elusive. High self-reported magnesium intake may be a surrogate marker of a healthy lifestyle; for example, higher intake trended with less smoking and lower BMI, but more use of lipid-lowering medications and aspirin. Although we controlled for lifestyle characteristics implicated in CVD or calcification (e.g., smoking, calcium, fiber, saturated fat, physical activity, education), nevertheless, unknown confounding and residual confounding may yet be factors, as they are in any observational study, and their effects on the magnitude or significance of our observations are difficult to estimate. Longitudinal studies followed by randomized trials will be necessary to confirm the relationship between magnesium intake and calcification. The estimated means of calcium in categories of self-reported magnesium intake were derived from a linear regression approach; while estimates from Tobit and linear regressions were similar, the presented means may overestimate or underestimate the magnitude of the association. Finally, our participants were predominantly white of European descent; thus our observations may not be generalizable to other races/ethnicities.
CONCLUSIONS
We observed strong, favorable associations between higher self-reported total (dietary and supplemental) magnesium intake and lower calcification of the coronary arteries, an important, discriminating measure of subclinical atherosclerotic burden that has been shown to reclassify risk of CVD morbidity and mortality. Our observations suggest that future research may consider magnesium's effect on CAC to be a potential physiological mechanism through which dietary magnesium mitigates risk of stroke, non-fatal MI, and fatal CHD. In addition to further research on magnesium in relation to the number and density of calcified lesions, and calcified and noncalcified plaque burden, prospective research is also required to elucidate magnesium's relationships with these and other sites of vascular calcification, as well as the possible benefits of magnesium supplementation in inhibiting onset and progression of atherosclerosis and calcification, with the goals of identifying magnesium's mechanism of action in lowering the risk of future cardiovascular events, and ultimately lowering the burden of cardiovascular disease.
Supplementary Material
Acknowledgments
The authors thank Gail Rogers, MS, for her review of the statistical methods, and the participants of the Framingham Heart Study for their contributions and dedication.
At the time of writing, Dr. Hruby was supported by an American Heart Association Predoctoral Fellowship. This work was also supported by the National Heart, Lung, and Blood Institute's Framingham Heart Study (contract no. N01-HC-25195) and the United States Department of Agriculture (USDA agreement no. 58-1950-0-014). Dr. Jacques has been a member of the Bay State Milling Nutrition Science Advisory Council and of the Dannon Yogurt Advisory Board. Dr. Meigs is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K24 DK080140).
ABBREVIATIONS AND ACRONYMS
- AAC
abdominal aortic calcification
- AS
Agatston score
- CAC
coronary artery calcification
- CKD
chronic kidney disease
- CT
computed tomography
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- IMT
intima-medial thickness
- MDCT
Framingham Heart Study Multi-Detector Computed Tomography Sub-study
- PWV
pulse-wave velocity
Footnotes
APPENDIX
For supplemental tables and figures, please see the online version of this article.
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Alexopoulos D, Toulgaridis T, Davlouros P, et al. Prognostic significance of coronary artery calcium in asymptomatic subjects with usual cardiovascular risk. Am Heart J. 2003 Mar;145:542–8. doi: 10.1067/mhj.2003.169. [DOI] [PubMed] [Google Scholar]
- 2.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–65. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 3.Allison MA, Hsi S, Wassel CL, et al. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32:140–6. doi: 10.1161/ATVBAHA.111.235234. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, Kauppila LI, O'Donnell CJ, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–34. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 5.Levitzky YS, Cupples LA, Murabito JM, et al. Prediction of intermittent claudication, ischemic stroke, and other cardiovascular disease by detection of abdominal aortic calcific deposits by plain lumbar radiographs. Am J Cardiol. 2008;101:326–31. doi: 10.1016/j.amjcard.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–95. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bo S, Pisu E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr Opin Lipidol. 2008;19:50–6. doi: 10.1097/MOL.0b013e3282f33ccc. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem. 2002;238:163–79. doi: 10.1023/a:1019998702946. [DOI] [PubMed] [Google Scholar]
- 9.Geiger H, Wanner C. Magnesium in disease. Clin Kidney J. 2012;5(Suppl 1):i25–38. doi: 10.1093/ndtplus/sfr165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr. 2012;95:362–6. doi: 10.3945/ajcn.111.022376. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Iso H, Ohira T, Date C, Tamakoshi A. Associations of dietary magnesium intake with mortality from cardiovascular disease: the JACC study. Atherosclerosis. 2012;221:587–95. doi: 10.1016/j.atherosclerosis.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 12.Al-Delaimy WK, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Magnesium intake and risk of coronary heart disease among men. J Am Coll Nutr. 2004;23:63–70. doi: 10.1080/07315724.2004.10719344. [DOI] [PubMed] [Google Scholar]
- 13.Chiuve SE, Sun Q, Curhan GC, et al. Dietary and plasma magnesium and risk of coronary heart disease among women. [April 1, 2013];J Am Heart Assoc. doi: 10.1161/JAHA.113.000114. Available at: http://jaha.ahajournals.org/content/2/2/e000114.abstract. [DOI] [PMC free article] [PubMed]
- 14.Chiuve SE, Korngold EC, Januzzi JL, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr. 2011;93:253–60. doi: 10.3945/ajcn.110.002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kircelli F, Peter ME, Sevinc Ok E, et al. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant. 2011;27:514–21. doi: 10.1093/ndt/gfr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montezano AC, Zimmerman D, Yusuf H, et al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56:453–62. doi: 10.1161/HYPERTENSIONAHA.110.152058. [DOI] [PubMed] [Google Scholar]
- 17.Salem S, Bruck H, Bahlmann FH, et al. Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification. Am J Nephrol. 2012;35:31–9. doi: 10.1159/000334742. [DOI] [PubMed] [Google Scholar]
- 18.Ferrè S, Baldoli E, Leidi M, Maier JAM. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim Biophys Acta. 2010;1802:952–8. doi: 10.1016/j.bbadis.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Shah NC, Liu J-P, Iqbal J, et al. Mg deficiency results in modulation of serum lipids, glutathione, and NO synthase isozyme activation in cardiovascular tissues: relevance to de novo synthesis of ceramide, serum Mg and atherogenesis. Int J Clin Exp Med. 2011;4:103–18. [PMC free article] [PubMed] [Google Scholar]
- 20.Altura BT, Brust M, Bloom S, Barbour RL, Stempak JG, Altura BM. Magnesium dietary intake modulates blood lipid levels and atherogenesis. Proc Natl Acad Sci U S A. 1990;87:1840–4. doi: 10.1073/pnas.87.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King JL, Miller RJ, Blue JP, Jr., O'Brien WD, Jr., Erdman JW., Jr Inadequate dietary magnesium intake increases atherosclerotic plaque development in rabbits. Nutr Res New York N. 2009;29:343–9. doi: 10.1016/j.nutres.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouchi Y, Tabata RE, Stergiopoulos K, Sato F, Hattori A, Orimo H. Effect of dietary magnesium on development of atherosclerosis in cholesterol-fed rabbits. Arter Dallas Tex. 1990;10:732–7. doi: 10.1161/01.atv.10.5.732. [DOI] [PubMed] [Google Scholar]
- 23.Britton WM, Stokstad ELR. Aorta and other soft tissue calcification in the magnesium-deficient rat. J Nutr. 1970;100:1501–5. doi: 10.1093/jn/100.12.1501. [DOI] [PubMed] [Google Scholar]
- 24.Iseri LT, French JH. Magnesium: nature's physiologic calcium blocker. Am Heart J. 1984;108:188–93. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 25.Peters F, Epple M. Simulating arterial wall calcification in vitro: biomimetic crystallization of calcium phosphates under controlled conditions. Z Für Kardiologie. 2001;90(Suppl 3):81–5. doi: 10.1007/pl00022850. [DOI] [PubMed] [Google Scholar]
- 26.Laurencin D, Almora-Barrios N, de Leeuw NH, et al. Magnesium incorporation into hydroxyapatite. Biomaterials. 2011;32:1826–37. doi: 10.1016/j.biomaterials.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Massy ZA, Drüeke TB. Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis and survival. Clin Kidney J. 2012;5(Suppl 1):i52–61. doi: 10.1093/ndtplus/sfr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto T, Hara A, Ohkubo T, et al. Serum magnesium, ambulatory blood pressure, and carotid artery alteration: the Ohasama study. Am J Hypertens. 2010;23:1292–8. doi: 10.1038/ajh.2010.168. [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Folsom AR, Melnick SL, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol. 1995;48:927–40. doi: 10.1016/0895-4356(94)00200-a. [DOI] [PubMed] [Google Scholar]
- 30.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57:182–9. doi: 10.1093/ajcn/57.2.182. [DOI] [PubMed] [Google Scholar]
- 31.Peacock JM, Folsom AR, Arnett DK, Eckfeldt JH, Szklo M. Relationship of serum and dietary magnesium to incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 1999;9:159–65. doi: 10.1016/s1047-2797(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 32.De Oliveira Otto MCC, Alonso A, et al. Dietary Micronutrient Intakes Are Associated with Markers of Inflammation but Not with Markers of Subclinical Atherosclerosis. J Nutr. 2011;141:1508–15. doi: 10.3945/jn.111.138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 34.Splansky GL, Corey D, Yang Q, et al. The third generation cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–35. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann U, Massaro JM, Fox CS, Manders E, O'Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008;102:1136–1141.e1. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 37.Chuang ML, Massaro JM, Levitzky YS, et al. Prevalence and distribution of abdominal aortic calcium by gender and age group in a community-based cohort (from the Framingham Heart Study). Am J Cardiol. 2012;110:891–6. doi: 10.1016/j.amjcard.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox CS, Larson MG, Keyes MJ, et al. Kidney function is inversely associated with coronary artery calcification in men and women free of cardiovascular disease: the Framingham Heart Study. Kidney Int. 2004;66:2017–21. doi: 10.1111/j.1523-1755.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 39.Willett W. Nutritional Epidemiology. Oxford University Press; New York, NY: 1998. [Google Scholar]
- 40.Reilly MP, Wolfe ML, Localio AR, Rader DJ. Coronary artery calcification and cardiovascular risk factors: impact of the analytic approach. Atherosclerosis. 2004;173:69–78. doi: 10.1016/j.atherosclerosis.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Djoussé L, Hopkins PN, Arnett DK, et al. Chocolate consumption is inversely associated with calcified atherosclerotic plaque in the coronary arteries: the NHLBI Family Heart Study. Clin Nutr Edinb Scotl. 2011;30:38–43. doi: 10.1016/j.clnu.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reis JP, Loria CM, Steffen LM, et al. Coffee, decaffeinated coffee, caffeine, and tea consumption in young adulthood and atherosclerosis later in life: the CARDIA study. Arterioscler Thromb Vasc Biol. 2010;30:2059–66. doi: 10.1161/ATVBAHA.110.208280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Woudenbergh GJ, Vliegenthart R, van Rooij FJ, et al. Coffee consumption and coronary calcification: the Rotterdam Coronary Calcification Study. Arterioscler Thromb Vasc Biol. 2008;28:1018–23. doi: 10.1161/ATVBAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- 44.Heine-Bröring RC, Brouwer IA, Proença RV, et al. Intake of fish and marine n 3 fatty acids in relation to coronary calcification: the Rotterdam Study. Am J Clin Nutr. 2010;91:1317–23. doi: 10.3945/ajcn.2009.28416. [DOI] [PubMed] [Google Scholar]
- 45.Lutsey PL, Jacobs DR, Kori S, et al. Whole grain intake and its cross-sectional association with obesity, insulin resistance, inflammation, diabetes and subclinical CVD: the MESA study. Br J Nutr. 2007;98:397. doi: 10.1017/S0007114507700715. [DOI] [PubMed] [Google Scholar]
- 46.Spiegel DM, Farmer B. Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: a pilot study. Hemodial Int Int Symp Home Hemodial. 2009;13:453–9. doi: 10.1111/j.1542-4758.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 47.Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- 48.Leidi M, Dellera F, Mariotti M, Maier JAM. High magnesium inhibits human osteoblast differentiation in vitro. Magnes Res Off Organ Int Soc Dev Res Magnes. 2011;24:1–6. doi: 10.1684/mrh.2011.0271. [DOI] [PubMed] [Google Scholar]
- 49.Doherty TM, Fitzpatrick LA, Inoue D, et al. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–72. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.