Abstract
Background
Elephantorrhiza elephantina (Ee) and Pentanisia prunelloides (Pp) are two medicinal plants which are widely used to remedy various ailments including diarrhoea, dysentery, inflammation, fever, rheumatism, heartburn, tuberculosis, haemorrhoids, skin diseases, perforated peptic ulcers and sore joints in southern Africa (South Africa, Swaziland, Botswana and Zimbabwe). The following study was conducted to explore the in vitro cytotoxicity, antioxidant properties and phytochemical profile of the two medicinal plants.
Materials and Methods
The cytotoxicity of the aqueous and methanol extracts and fractions of both species was studied using the brine shrimp lethality tests (BST) for the first time.
Results
The results demonstrated that the lethality (LC50) for crude extracts for both plants ranged between 1.8 and 5.8 ppm and was relatively greater than that for the methanol, ethyl acetate and chloroform fractions of the extracts which ranged between 2.1 ppm and 27 ppm. This suggested that crude extracts were more potent than their respective fractions, further explaining that the different fractions of phytochemicals in these plant species work jointly (in synergy) to exert their therapeutic efficacy. Both aqueous and methanol extracts of the two medicinal plants demonstrated a high degree of antioxidant capacity against the DPPH radical with the Duh and Yen inhibition percentage ranging between 4.5% and 72%. Phytochemical studies of the rhizome extracts showed that the major compounds present include flavonoids, tannins, anthocyanidins, anthraquinones, triterpenoids (oleanolic acid), the steroidal saponin Diosgenin, the sugars, rhamnose, glucuronic acid, Arabinose and hexoses.
Conclusion
This is the first report of the detection and isolation of diosgenin and oleanolic acid from the rhizome extracts of Ee and Pp. All structures were determined using spectroscopic/spectrometric techniques (1H NMR and 13C and LC-ESI-MS) and by comparison with literature data.
Keywords: E. elephantina, P. prunelloides, Diosgenin, Oleanolic acid, cytotoxicity
Introduction
Elephantorrhiza elephantina (Ee) and Pentanisia prunelloides (Pp), are two medicinal plants which are widely used to remedy various ailments in southern Africa. E. elephantina is also known as elandsbean, or mupangara (in Shona), intolwane (in Xhosa and Zulu) and mositsane (in Sotho and Tswana) (Phillips, 1917; Jacot Guillarmod, 1971). The root of this plant is known in southern Africa for many traditional uses such as the treatment of hypertension, syphilis (Jacot Guillarmod, 1971), infertility in women, wasting in infants, dysmenorrhea and haemorrhoids amongst others (Gelfand et al., 1985; Hutchings et al., 1996). Other traditional uses of the root extracts of Ee include aphrodisiac or emetic (to mitigate the anger of the ancestors or for fevers - Hutchings et al., 1996). However, Ee is particularly known to be effective against stomach ailments such as abdominal pains, perforated peptic ulcers, (bloody) diarrhea and dysentery (Watt and Breyer-Brandwijk, 1962; Jacot Guillarmod, 1971; Gelfand et al., 1985; Hutchings, 1989a; Pujol, 1990). E. elephantina has a reddish root and in the Sotho culture, “red medicines” are associated with blood and good health. Furthermore, the San people use red plant parts to treat anaemia, weakness (i.e. to strengthen the blood) and fevers (Laidler, 1928). On the other hand, P. prunelloides [syn. P. variabilis Harv. var. intermedia Sond Adeniji et al. (2000); common name: wild verbena - Van Wyk et al. (2009)] is a multi-purpose plant used for the treatment of several internal and external ailments including boils, burns, snakebite, swelling, rheumatism, fever, chest pains, toothache, blood impurities, haemorrhoids, internal tumors, ulcers, venereal diseases, influenza and tuberculosis (Phillips, 1917; Watt and Breyer-Brandwijk, 1962; Jacot Guillarmod, 1971; Hutchings, 1989a,; Pujol, 1990; Hutchings et al., 1996; Maliehe, 1997; Grierson and Afolayan, 1999; Neuwinger, 2000). With stomach ailments in particular, the fresh root may be chewed and swallowed for the treatment of heartburn (Watt and Breyer-Brandwijk, 1962; Adeniji et al., 2000). Its vernacular names, i.e. setima-mollo (Sotho) translated as “fire extinguisher” (Moteetee and Van Wyk, 2011), icimamlilo (Zulu) which means “putting out the fire” and sooibrandbossie (Afrikaans) translated as “little heartburn bush” (Van Wyk et al., 2009), emphasizes this longstanding traditional use. Root decoctions of P. prunelloides may also be taken orally as an emetic and for diarrhoea, dysentery, indigestion (Moteetee and Van Wyk, 2011), vomiting, and for swelling of the stomach [decoctions of bruised and boiled root is mixed with sour milk and taken orally - Smith (1895)].
Studies have shown that, compounds known from E. elephantinacontains 5.8–22.3% tannins (Watt and Breyer-Brandwijk, 1962), which explains the redness of the root. Moreover, several phenolic compounds, including flavonoids such as kaempferol, dihydrokaempferol, ethyl β-D-galactopyranoside, quercetin 3-O-β-D-glucoside, trans-3-O-galloyl-3,3′,5,5′,7-pentahydroxyflavan and taxifolin 3′-O-glucoside (Mthembu, 2007), as well as (+)-catechin, ethyl gallate, methyl gallate and gallic acid form part of the chemical composition of this plant (Mthembu, 2007). Other compounds identified in the roots of this pant include sugars (16.8%); Watt and Breyer-Brandwijk, 1962) and β-sitosterol (Mthembu, 2007). The roots of P. prunelloides are reported to contain high levels of alcohol precipitable solids (0.7–7.0%, and these include polysaccharides and glycoproteins), amino acids (α-aminobutyric acid, valine, allo-isoleucine, serine, aspartic acid, asparagine and alanine, of which the latter is most abundant) as well as several unidentified terpenes of medium and low polarity (Ndlovu, 2007). Palmitic acid was further identified as a major non-polar compound in P. prunelloides by Yff et al., 2002.
In this work we report the results from a study on the cytotoxicity of the extracts of the two medicinal plants (Ee and Pp) on the brine shrimp lethality tests (Meyer, 1982), a technique useful for the screening of the isolates from plant extracts (Sam, 1993). Generally, the BST lethality bioassay provides a front line screen that can be backed up by more specific and expensive bioassays once the active compound has been isolated. Since E.elephantina and P. prunelloides are reputed for their folkloric uses in various diseases, these drew our attention for their pharmacological screening in applying the BST bioassay technique. In this very same study, the antioxidant activity of the extracts of E. elephantina and P. prunelloides was also evaluated using the DPPH radical.
Materials and Methods
Apparatus and Reagents
The standards; ascorbic acid, DPPH radical, Digitonin (AR grade), Quillaja saponin-21, anhydrous sodium chloride, potassium bromide, acetonitrile (AR grade), concentrated sulphuric acid, butanol, acetone (HPLC grade) and vanillin were purchased from Sigma Aldrich South Africa. Methanol (HPLC grade) was purchased from Merck, South Africa. Ultrapure water with resistivity > 18.4 MΩ/cm was used for all aqueous preparations. Silica gel type 60 (Merck, 230–400 mesh) was used for column chromatography. Thin layer chromatography was carried on a 0.25 mm thick silica gel plate, Merck silica gel 60 GF254) and elution was carried out using a solvent system comprising of butanol/acetone/water (4:1:2), v/v). Compounds were detected either under UV light (at 254 or by spraying with vanillin/Sulphuric acid (1:1, v/v).
Plant material and collection
Fresh plant rhizomes of E. elephantina and P. prunelloides were collected in June 2010 from seven different southern African regions in Swaziland, South Africa and Zimbabwe and identified by Dr Anna Moteetee, University of Johannesburg. Voucher specimens (SJM-0, SJM-01 to SJM-07) were deposited in JRAU Herbarium, Department of Botany and Plant Biotechnology (Kingsway Campus, University of Johannesburg. Fresh plant rhizomes were washed with water, dried, marcerated and kept in the fumehood at room temperature. The dried plant materials were then ground into fine powders, extracted using appropriate solvent and water, evaporated under reduced pressure and then stored in sample bottles at room temperature.
The sequential exraction of polar and non-polar compounds from E. elephantina and P. prunelloides
Samples of both E. elephantina and P. prunelloides were sequentially extracted with different solvent systems starting with the least polar, hexane ending with the most polar, water. The different crude extracts that were obtained were subjected to the BST test to probe for the fractions exhibiting toxicity potency.
Brine shrimp lethality tests for the sequential extracts of E. elephantina and P. prunelloides
Stock solutions of the extracts (1000 mg/L) were prepared from the fractions of the above referred sequential extraction of Elephantorrhiza elephantina from Zimbabwe (Zim) and KwaZulu Natal (KZN)-South Africa and Pentanisia prunelloides (Pp) from KZN and Orange. The respective stock solutions were sequentially diluted to come up with a series of triplicate concentrations ranging from 0.001 to 1000 µg/ml. Results obtained from the toxicity tests are shown on (Table 1)
Table 1.
Larvicidal effect of aqueous extracts of Pentanisia prunelloides on Artemisia Salina.
| Expt. | Conc. Of extract µg/mL |
Initial No. of larvae |
Total Death | Total survival | % Mortality |
| 1 | 1000 | 3×10 | 30 | 0 | 100% |
| 2 | 100 | 3×10 | 27 | 3 | 93% |
| 3 | 50 | 3×10 | 27 | 3 | 93% |
| 4 | 10 | 3×10 | 18 | 12 | 60% |
| 5 | 5 | 3×10 | 16 | 14 | 53% |
| 6 | 1 | 3×10 | 11 | 19 | 37% |
| (−ve) g/dm−3 | 3×10 | 0.0 | 30 | 0.0% | |
| 10–1000 | 3×10 | 30 | 0 | 100% | |
| 5 | 10 | 29 | 1 | 97% | |
| (+ve) g/dm−3 | 1 | 10 | 25 | 5 | 83% |
Brine Shrimp Lethality tests for crude extracts of E. elephantina and P. prunelloides
The Brine Shrimp eggs were bought from Roodeport, 229 Ondekkas Road in Johannesburg, South Africa. The standard solution of 6.2 g/L of Brine solution was prepared by dissolving 9.3 g of sodium chloride in enough distilled water to make 1.5 litres solution. Brine Shrimp eggs (0.3 g) were added to 300 mL of brine in a rectangular lunch box with the top half covered to facilitate the hatching of eggs. Aeration was facilitated as described above on the uncovered section of the rectangular tank. Shrimp eggs were allowed to hatch and mature as nauphlii in two days in the hatching tank. The free swimming nauphlii were attracted by a light to an open compartment of the hatching tank from which they could be collected for the assay. Methanol and aqueous extracts of both E. elephantina and P. prunelloides were dissolved in 1.0 ml dimethyl sulfoxide (DMSO) to come up with six different triplicate concentrations (1000, 100, 50, 10, 5 and 1 µg/mL). Ten brine shrimp larvae (10 nauphlii) were introduced into each of the triplicate segments of the segmented petri dishes. Brine was used as a negative control and potassium dichromate as a positive control. A parallel series of tests with the standard potassium dichromate solution (LC50 = 20–40 mg/L) were always set up. After 24 hours the nauphlii were examined against a lighted background with a magnifying glass and the number of survival larvae out of 30 shrimps per dose was recorded. The mean percentage mortality was plotted against the logarithm of concentrations and the concentration killing fifty percent so called the LC50 of the larvae was determined from the graph.
Determination of antioxidant activity of Ee and Pp by the DPPH radical
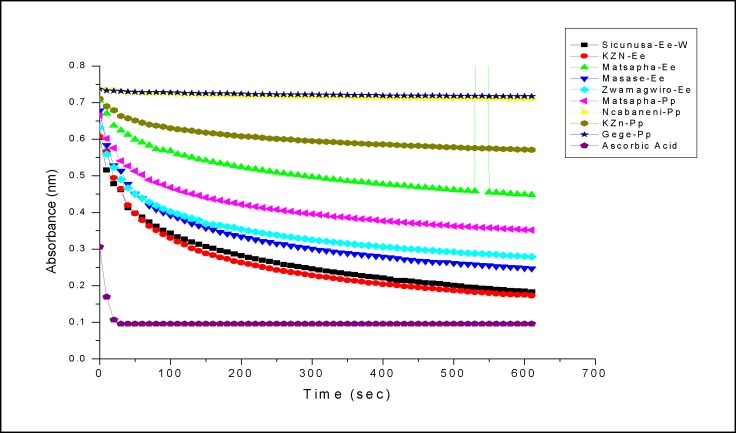
The antioxidant activity of extracts of the two species was measured in terms of hydrogen donating or radical scavenging ability, using the stable radical DPPH against ten aqueous and nine methanolic extracts. The instrument used was the UV-2450 UV-Vis spectrophotometer PC-Series at the University of Johannesburg in the Department of Applied Chemistry Doornforntain Campus. This instrument was operated at a wavelength of 515.5 nm taking 620 readings for DPPH radical kinetics both aqueous and methanol extracts of E. elephantina and P. prunelloides. 100µL stock solution (75µL) of substrate (concentration of stock solutions were 0.0005g/ml) was put into a cuvette and 3900µl of 6× 10−5 mol dm−3methanolic solution of DPPH was added. Absorbance measurements commenced immediately. The decrease in absorbance at 515.5 nm was determined at 10 sec intervals until the attainment of the steady state. Methanol was used to zero the spectrometer. Absorbance of the DPPH radical without antioxidant, i.e. the control was measured prior to undertaking the experiment. Special care was taken to minimize the loss of DPPH stock solution by covering the volumetric flask with aluminum foil. The percentage inhibition of the DPPH radical by the samples was calculated according to the formula of Yen and Duh % Inhibition = [(Ac (0)−(Ac (t)] × 100 where Ac (0) is the absorbance of the control at t= 0 sec and Ac (t)] is the absorbance of the antioxidant at the steady state. The results were recorded on Table 5.
Table 5.
Yen and Duh percentage (%) inhibition
| Methanol extracts | (%) | Aqueous extracts | (%) | |
| Origin of samples | E. elephantina | P. Prunelloides | E. elephantina | P. prunelloides |
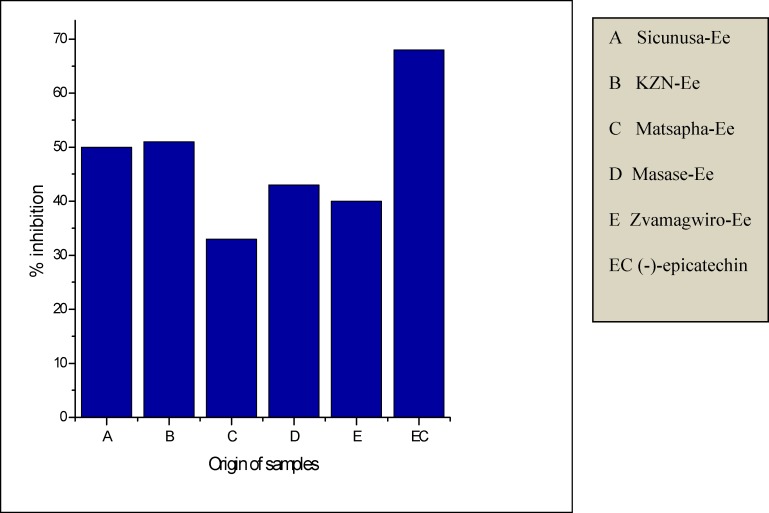
| Sicunusa | 72.0 | _ | 50.0 | _ |
| KZN | 55.0 | 30.0 | 51.0 | 13.0 |
| Matsapa | 57.0 | 33.0 | 33.0 | 23.0 |
| Masase | 60.0 | _ | 43.0 | _ |
| Zvamagwiro | 59.0 | _ | 40.0 | _ |
| Ncabanani | _ | 4.8 | _ | 4.5 |
| Gege | 69.0 | 5.0 | _ | 5.0 |
| Ascorbic acid | 67.5 | _ | ||
| (−)-epicatechin | _ | 68.0 |
_ No samples tested from that location
Quantitative determination of saponins
A mass of 20g of dry sample was ground and 100 mL of 20% aqueous ethanol was added. The mixture was heated in a water bath for 4hours at 55°C with constant stirring then filtered. The residue was re-extracted further with another 100 mL 20% ethanol. The combined extracts were reduced to 40 mL over a water bath at 90°C. The concentrate was transferred to a 250 mL separatory funnel partitioned with 3×20 mL diethyl ether shaking vigorously. The aqueous layer was recovered while the ether layer was discarded. Partitioning of the solution was done three times with 60 mL portions of n-butanol. The combined n-butanol fractions were washed twice with 10 mL of 5% aqueous sodium chloride. The resultant solution was heated in a water bath for the vaporization of n-butanol. The final dried fraction was further dried to constant mass in an oven to constant mass. The saponin content was calculated as a percentage of the starting material.
Thin layer chromatography of extracts
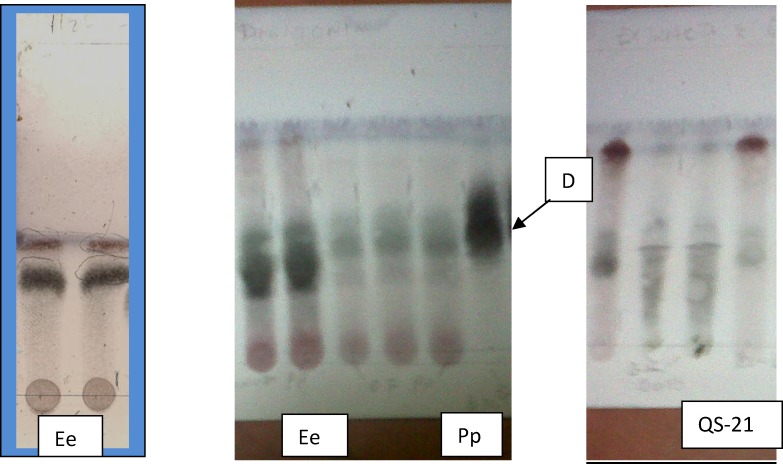
Portions of both E. elephantina and P. prunelloides were spotted on separate tlc plates and eluted with Butanol/Acetone/water (4:1:2). The developed tlc plates were sprayed with vanillin sulphuric acid and then heated in the oven at 110°C for 5 minutes. Portions of both species were subjected to column chromatography using the same eluent applied for ptlc. The resultant fractions of the two species were spotted on the same tlc plate against standard epicatechin for flavonoids and Digitonin for saponins. (see Fig 5) This was done for comparison purposes. Prep. tlc was then used to isolate the major compounds with TLC3 (MeOH/H2O/Acetone/Ethyl acetate/Chloroform) 10:8:30:40:12 as eluent. Standard digitonin and Quillaja-21 were also spotted against the isolates for comparison purposes (Fig 7).
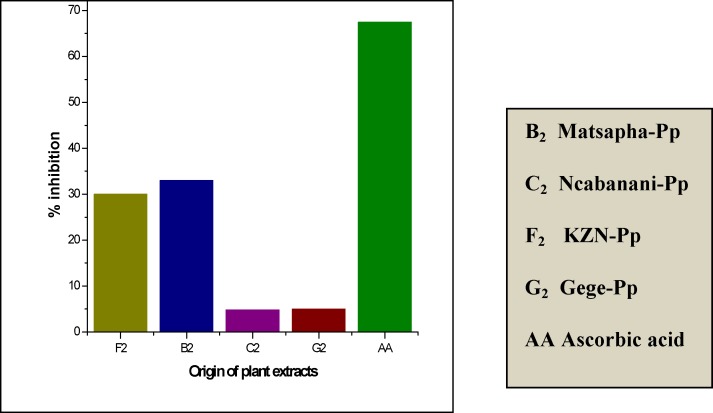
Figure 5.
%inhibition of methanol extracts of P. prunelloides samples from different location.
Figure 7a.
TLC for E. elephantina (Ee) and P. prunelloides (Pp) saponin fractions after baking at 110°C against Digitonin (D) and Quillaja saponin (QS-21) standards.
Column chromatography and spectroscopy performance of extracts
The dried residue (10.0 g) was suspended in minimum amount of methanol, immobilised on silica gel (8 g), and subjected to column chromatography (CC), using a 38 × 4.5 cm glass column filled with Merck silica gel 60 F254 in chloroform to a level about 8 cm from the top. The immobilized extract was added to the free volume at the head of the column. Fractionation was done by successive applications of chloroform, chloroform/methanol (2:1), and methanol. Three major fractions were collected and the solvent was evaporated in the fume hood overnight. One of the fractions was non-polar (NP), another was of medium polarity (MP) and the last was a polar (HP) fraction. The fraction with medium polarity and the polar one were used to isolate two of the major compounds.
FT-IR spectroscopic analysis extracts
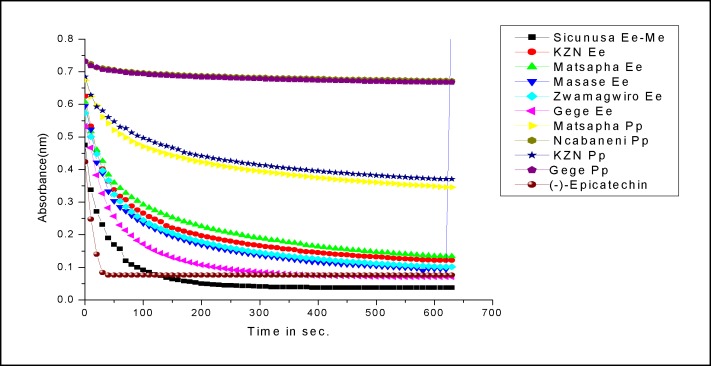
A little powder of plant specimen was mixed with Kbr salt using a mortar and pestle, and compressed into thin pellets. Infrared spectra were recorded between 4000 cm−1-500 cm−1 as Kbr pellets on the TL 8000 Balanced Flow FT-IR EGA (Perkin Elmer Spectrum series) instrument at the University of Johannesburg in the Department of Applied Chemistry, Doornforntain Campus. Infrared absorptions were recorded and are depicted on Figure. 4 to 5.
Figure (4a).
Kinetic profiles of the methanol extracts E. elephantina and P. prunelloides.
LC-ESI-MS analysis of the saponin isolates of E. elephantina and P. prunelloides
The LC-ESI-MS Waters E2695 instrument was used for the analysis and characterization of saponins. The instrument had a 3100 mass selective detector equipped with the electron spray ionization source (ESI) (positive and negative) with quadrupole mass analyzer. The quadrupole temperature of the MS was 120°C, while the dessolution temperature was 350°C, the capillary voltage was at 3500 volts and the spectra were acquired in both the selected ion mode (SIM) and fill scan mode with polarity switching (+ve and −ve modes). The temperature of the drying gas, nitrogen (N2) was 300°C and the flow rate of the nebulising gas, N2 was 500 mL/hr. The eluent system comprised acidified aqueous solvent (FA), Acetonitrile and methanol. Acidification provided a better retention and separation in the C18 column. The LC-MS spectra and chromatogram are shown in (Fig 9–15).
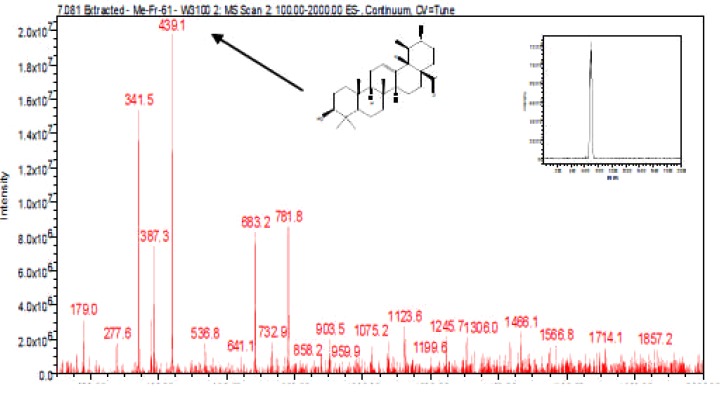
Figure 9(a).
LC-ESI-MS spectra and chromatogram of Oleanolic moieties
Figure 15(a).
LC-ESI-MS spectra of Diosgenin saponin from E. elephantina fraction 59-1a.
NMR spectroscopy
The structures of the isolated compounds were elucidated using ID and 2D NMR spectroscopy. The 1D (1H and 13C) were performed on a Bruker Avance DRX 400, operating at 400.132 MHz for 1H spectra and 100.625 MHz for 13C spectra. The 2D experiments (COSY, HSQC, HMBC and DEPT) were done at 300 MHz for 1H and 75 MHz for 13C spectra using a Bruker Avance 300 spectrometer. Deuterated methanol (d4-CD3OD) was used to dissolve the solvent. The chemical shifts are reported in parts per million (ppm) referenced to the solvent shifts or a small amount of tetramethylsilane (TS).
Results and discussion
The lethality of crude extracts of E. elephantina and P. prunelloide
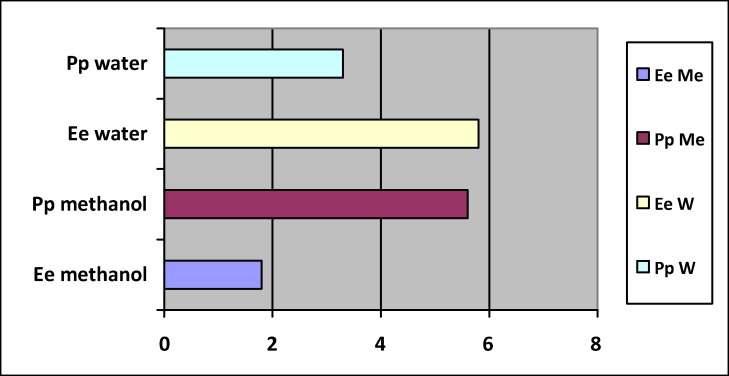
The degree of lethality against the brine shrimp was found to be directly proportional to the concentration of the crude extracts studied in this work. The LC50 results for the two plant species evaluated in this screening procedure are listed in (Tables 1–2). The respective LC50 values for the aqueous and methanol extracts of E. elephantina and P. prunelloides are shown on Fig 2. The graphs from which the LC50 values were obtained are shown in Fig 1 (a and b) for methanol extracts only). Generally, all extracts from both P. prunelloides and E. elephantina demonstrated high biological activity with the methanol extract of E. elephantina exhibiting a greater activity compared to all extracts tested presenting an LC50 of 1.8. This value for the methanolic extract of E. elephantina methanol extract would be considered toxic whereas all the other extracts fall under the moderately toxic and toxic range of LC50 >1<10. It is also noteworthy to highlight that both the aqueous extracts of the two species are not highly toxic especially as water is a solvent of choice for traditional healers. While the methanol extract of E. elephantina could be considered moderately toxic, traditional healers do not use solvents for the prescription and administration of their phytomedicines particularly for these two medicinal plants. These extracts can be regarded as promising candidates for plant-derived bioactive compounds based on the BSTs performed.
Table 2.
Larvicidal effect of methanol extracts of Pentanisia prunelloides on Artemisia Salina.
| Expt. | Conc. of extract µg/mL |
Initial larvae |
No. of Total Death | Total survival in |
% Mortality |
| 1 | 1000 | 3×10 | 30 | 0 | 100% |
| 2 | 100 | 3×10 | 28 | 2 | 93% |
| 3 | 50 | 3×10 | 23 | 7 | 77% |
| 4 | 10 | 3×10 | 22 | 8 | 73% |
| 5 | 5 | 3×10 | 14 | 16 | 47% |
| 6 | 1 | 3×10 | 6 | 24 | 20% |
| (−ve) g/dm−3 | 3×10 | 0 | 30 | 0 |
Figure 2.
LC50 values for mixtures of methanol and aqueous extracts against Brine Shrimp.
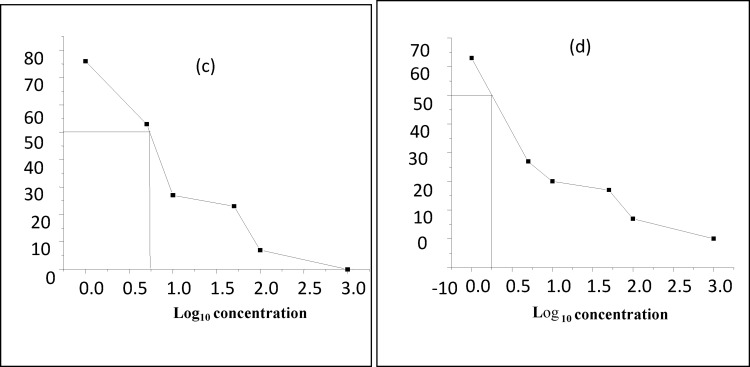
Figure 1.
(a): crude P. pentanisia methanol extract LC50 = 5.6 (b) crude E. elephantina methanol extract LC50 = 1.8.
The BST lethality tests for extracts of polar and nonpolar solvents
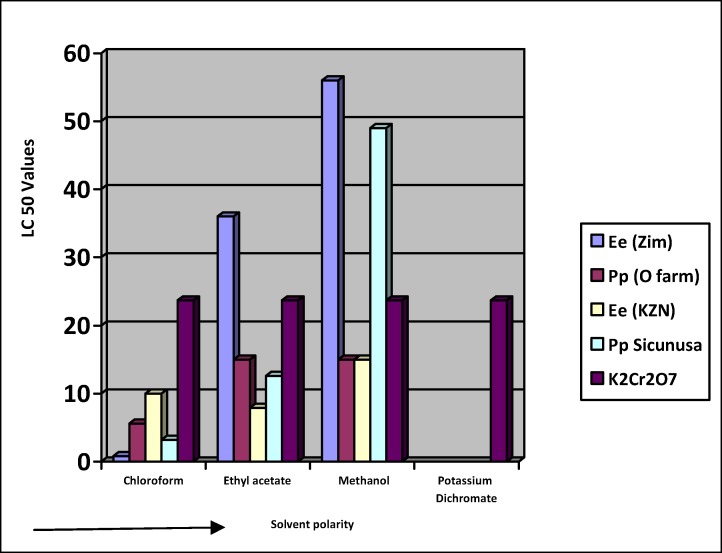
The results for the BST tests for extracts from the sequential extraction are reflected in Tables 3 below. The corresponding LC50 values were summarized on Fig 3. Generally, the fractions of both E. elephantina and P. prunelloides exhibited higher LC50 values against the Brine shrimp relative to both the crude and mixtures of crude extracts apart from the chloroform E. elephantina from Zimbabwe fraction with an LC50 value of 0.8. The higher LC50 were an indication of less lethality. Considering the LC50 value of 1.8 for the E. elephantina from KZN crude extract, (Fig 3) the corresponding LC50 values for the chloroform, ethyl acetate and methanol fractions exhibited higher LC50 values (10, 7.9, 15) respectively. All these fractions were obtained from the same crude extracts of E. elephantina but their individual effect on the brine shrimp exhibited lower toxicities i.e. higher LC50 values compared to their combined effect in the crude extract. This observation could be attributed to the synergistic interactions of the components of the crude extract. It should also be highlighted that saponins generally detected in the polar solvent (methanol) constitute a greater proportion of the combined fractions as demonstrated by their high percentage yields. But their LC50 values are comparatively high indicating that their abundance in medicinal plants may not on its own be a justification for the observed bioactivities. It is generally the trace phytochemicals found in medicinal plants that play a major role in bioactivity. A typical observation in the case of chloroform extracts was that extracts had inexplicably low percentage yields for both species across all the geographical locations which demonstrated a high level of lethality against the brine shrimps compared to both the medium and polar fractions. While the polar fractions are relatively less lethal compared to the other fractions, the LC50 values are far above the levels of some of the well known potent triterpenoids like Oleanolic acid with an LC50 value of 72 mg/L. The results of this study revealed that the toxicity of the two plants extracts decreases with increase in polarity of solvent used for the extraction. This could be perceived in terms of the polarity of compounds extracted by each solvent in addition to their intrinsic bioactivity and by their ability to dissolve or diffuse in different solvent media used in the assay. The presence of these phytochemicals is of paramount importance in the pharmaceutical industry.
Table 3.
Percentage yields of P. prunelloides and E. elephantina for different solvents
| Sample origin and percentage yields (% ) | ||||
| Extraction solvent | Zim. (Ee) | KZN(Ee) | O. Farm(Pp) | KZN(Pp) |
| n-Hexane | 0.066 | 0.043 | 0.103 | 0.69 |
| Chloroform | 0.13 | 0,17 | 0.46 | 0.28 |
| Ethyl acetate | 0.13 | 0.15 | 0.10 | 0.17 |
| Methanol | 21.78 | 25.2 | 19.02 | 8.0 |
Figure 3.
LC50 values of Chloroform, Ethyl acetate and Methanol fractions of E. elephantina and P. prunelloides from the sequential extraction.
Qualitative phytochemical profiles
Phytochemical analysis tests were carried out on the crude extracts (Table 4) as well as different fractions from the sequential fractionation samples (Results not shown). Both species exhibited all the six different secondary metabolites tested although in varying degrees. Saponins and flavonoids tended to be more pronounced for both crude extracts and fractions as depicted by the intensity of colours across all samples from different geographical locations (Table 4). A further analysis showed E. elephantina to have a relatively greater content of the two classes of phytochemicals as exhibited by the intensity of the colour changes for the prescribed standard tests. These two classes of compounds are generally polar and this observation tentatively corroborated the findings on the quantitative determinations (Table 4) that exhibited more polar compounds from the two species. The qualitative tests demonstrated the abundance of the major secondary metabolites that could lend support to the extensive use of the two medicinal plants in phytotherapy.
Table 4.
The phytochemical profile of crude extracts of E. elephantina and P. prunelloides from different locations.
| Sample origin and estimation of major phytochemicals | ||||
| Major phytochemicals | Zim.(Ee) | KZN(Ee) | O. Farm(Pp) | KZN(Pp) |
| Alkaloids | + | + | + | + |
| Saponins | +++ | +++ | ++ | ++ |
| Anthraquinones | +++ | +++ | ++ | ++ |
| Tannins | +++ | +++ | ++ | ++ |
| Flavonoids | +++ | +++ | ++ | ++ |
| Anthocyanidins | +++ | +++ | ++ | ++ |
+ = trace amount ++ = detectable +++ = substantially detectable - not detectable
The DPPH antioxidant kinetics against extracts of E. elephantina and P. Prunelloides
Considering both Fig. 4(a–b), the absorbance of the DPPH/Substrate mixture decreases with time and comes to a plateau indicating the end point of the reduction process. This confirms the presence of antioxidants in the two species. This trend was observed irrespective of the origin of the two species or type of crude extract under consideration. Some of the crude extracts exhibited an equivalent or greater antioxidant capacity than standard (-)-Epicatechin as demonstrated by the Yen and Duh percentage inhibition values especially Gege (69%), Sicunusa (72%) and Masase (60%) E. elephantina methanol extracts (Table 5). For both aqueous and methanol extracts all E. elephantina extracts exhibited greater DPPH radical bleaching capacity, hence greater antioxidant capacity than all their corresponding P. prunelloides extracts across all the geographical locations studied. Typical methanol extract examples are [Gege (Ee 69%, Pp 5%)], Matsapa (Ee 57% Pp33%)] and for aqueous extracts [KZN Ee (51% Pp13%)], [Matsapa (Ee33% Pp23%)] Table 5. Furthermore, all E. elephantina extracts irrespective of extracting solvent or geographical location showed greater antioxidant capacity than all the P. prunelloides extracts. The Yen and Duh percentage inhibition values for E. elephantina ranged from 33 to 72% while those for P. Prunelloides ranged from 4.5 to 33% across both methanol and aqueous extracts. (Table 5) It can therefore be rationalized from this data that E. elephantina renders a greater proportion of antioxidants than P. prunelloides in both aqueous and methanol solutions. It can further be inferred that there are more extractable antioxidants using methanol than using water as the solvent. The ability of the above extracts in scavenging DPPH through hydrogen (H) transferring reactions is strong evidence of their ability in intercepting reactive oxygen species (ROS). As mentioned previously, the plant extracts tested in this study are widely used in folklore medicine to treat various medical disorders, such as atherosclerosis, diabetes mellitus, rheumatoid arthritis, fevers. However, the pathogenesis of such diseases involves oxidative stress which releases free radicals in infected cells of the human body. Such free radicals could be detoxified by the intake of medicinal plant extracts such as those investigated in this study as (Murdaca, et al., 2012).
Figure 4(b).
Kinetic profiles of aqueous extracts of E. elephantina and P. prunelloides.
Quantitative composition of saponins in E. elephantina and P. prunelloides
The relative percentage composition of saponins in the two species are shown in (Table 6) below. E. elephantina samples exhibited a higher quantity of saponins (1.56%, 1.44%) compared to P. prunelloides samples (0.24%, 0.71% (Table 6). Despite the fact that the samples were drawn from different geographical locations, the difference in the saponin content between the two species was conspicuous. The intensity of the precipitate formed was more pronounced for both E. elephantina extracts compared to both aqueous and methanol P. prunelloides extracts. This again suggested a relatively higher saponin content of this class of phytochemicals in E. elephantina. This group of phytochemicals is very important in phytotherapy and may have a significant role in the treatment of ailments for which these two medicinal plants are prescribed by traditional healers.
Table 6.
The relative saponin percentage composition of E. elephantina and P. Prunelloides.
| Sample | Origin of sample | Saponin percentage (%) |
| E. elephantine | Zimbabwe | 1.56 |
| KZN | 1.44 | |
| P. prunelloides | Orange Farm | 0.24 |
| Sicunusa | 0.71 |
The health benefits of saponins include the reduction of cholesterol levels in the intestinal tract hence mitigating obesity and antimutagenicity thus preventing cancer cells from growing. The non sugar parts of saponins have a direct antioxidant activity, hence, reducing the risk of cardiovascular disorders. It is most likely that the antioxidant capacities exhibited by extracts of these two species emanated partly from this group of phytochemicals apart from the flavonoids and others yet to be discovered. The relatively high content of saponins suggests that both E. elephantina and P. prunelloides have cytotoxic effects such as intestinal cell membrane permeabilization. Saponins are reported to mimic the sex hormone involved in the controlling the onset of labour in women and the subsequent release of milk (Okwu, et al; 2004). It is most likely that the efficacy of concoctions of P. prunelloides administered to expectorant women and those that deliver without the release of the placenta for the enhancement of ease delivery (Okwu, et al; 2003 and 2004) emanates partly from this class of compounds.
TLC for the fractions of E. elephantina and P. prunelloides against saponin standards
The green colour after spraying with vanillin sulphuric acid and baking confirmed the presence of saponins in the methanol extracts of E. elephantina (Ee) and P. prunelloides (Pp) (Fig 6.8). Furthermore, TLC analysis of saponin fraction against Digitonin afforded sub-fraction 61 of E. elephantina which exhibited the presence of saponins and triterpenoids as depicted by the green and purple colours respectively (Fig 6.8). The prepapratory TLC for fraction 59 afforded five sub-fractions that exhibited the purple colour for triterpenoid after spraying with vanillin sulphuric acid and baking (Appendix 13). Both fractions 61 and 59 of E. elephantina gave positive results from Liebermann-Burchard and Molish reactions which suggested that they were trietepenoid saponins.
FT-IR spectra of saponins from E. elephantina
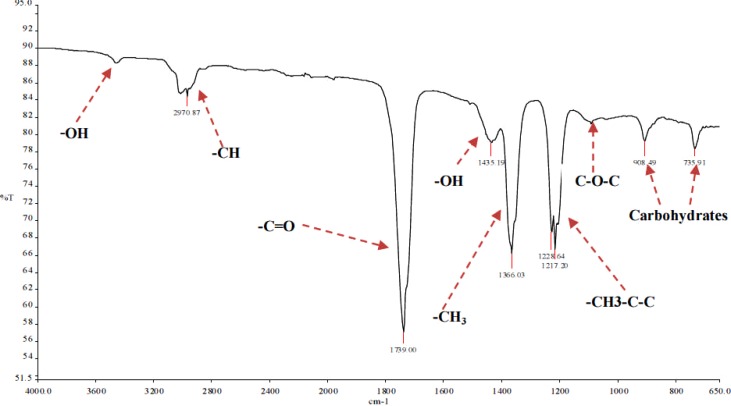
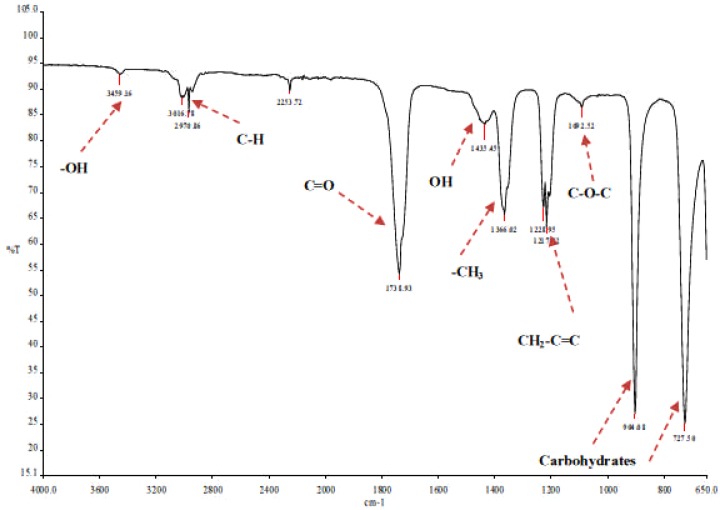
The phytochemistry analysis gave positive results for saponins. These were confirmed by infrared absorption bands (Fig 7–8) of the hydroxyl (-OH) ranging around 3459 cm−1; C-H ranging from 3016 cm−1 to 2925 cm−1; and C=O absorption bands ranging from 1739 cm−1 to 1738.93 cm−1. Oligosaccharide linkage to sapogenins absorption bands were evidenced by the C-O-C bands, between 1052.75 cm−1 and 1092.63 cm−1. The peaks 904 and 727 were assigned as characteristic absorption bands of carbohydrates according to (Nakanishi, et al., 1977). The stronger the relative intensity of the absorption bands between 1150 and 700 cm−1 the higher the starch content (Nakanishi, et al., 1977; Li, et al., 2004; Hua, et al., 2003; Woo, et al., 1999). The difference in sugar chain of fraction 59-2 and 59-4 could therefore be explained by the fact that the former fraction eluted earlier than the latter due to stearic reasons.
Figure 8a.
FT-IR spectra of E. elephantina isolate 59-2.
The -OH, C-H, C=C, C=O and C-O-C absorption bands found in P. prunelloides and E. elephantina are suggestive of Oleonane-type triterpenoid saponins. These Oleonane-type triterpenoid saponins are characterized by the C=O infrared absorbance due to acid/ester. Such triterpenoid saponins are also likely to be bidesmosides since they have two attachments for glycones (i.e. glycosidic and ester groups) to the sapogenin. The above referred infrared functional group absorptions characteristic of saponins are cited in literature (Kirmizigul, et al., 2002; Natori, et al., 1981; Da Silver Bernadete, et al., 2002)
LC-ESI-MS for saponins from E. elephantina and P. prunelloides
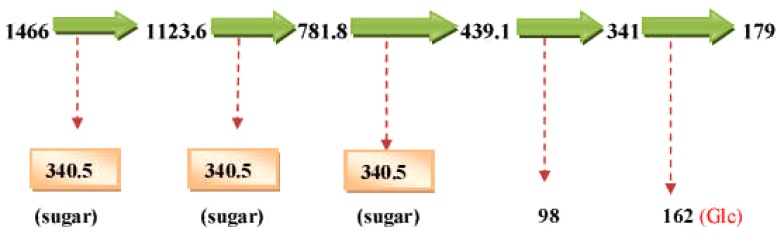
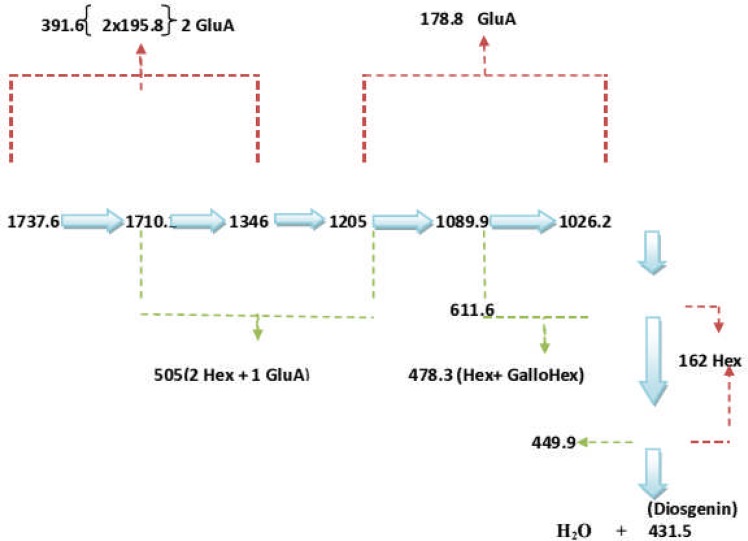
The LC-ESI-MS of E. elephantina fraction 61 afforded a pseudomolecular ion at 1466.1. The LC-ESI mass spectra and chromatogram of the isomer Rt 7.081 (peak no. 3) is shown in Figure 9a. The fragmentation pattern at m/z 439 was characteristic of oleanolic acid skeleton (Fig 9b). The mass ion at m/z 1466.1 was [M]−because the LC-ESI-MS instrument was operated in the negative mode. A loss of 342.5 amu (m/z 1123.6) indicated that a diglycoside sugar moiety [M-H-GluA-Glc] was cleaved resulting in the formation of the ion at m/z 781.8 [M-H-GluA-Glc]. The successive loss of 340.5 amu from the ion at m/z 781.8 (439.1) indicated a diglycoside sugar moiety [M-H-GluA-Glc] had been further cleaved. The loss of a total of six or four sugar moieties taking 1466 or 1123.6 amu (Figure9b) as pseudomolecular ions respectively, would indicate the aglycone to be Oleanolic acid (M.W. = 439). The fragmentation pattern (Fig 9b) indicated the presence of glucose (MW = 162), glucuronic acid (MW = 178), rhamnose [m/z 146, (683.2-536.8)]. The fragmentation pattern also showed loss of fragments of m/z = 342, which may suggest the loss of disaccharide units (Fig 9b), although the specific rotation of the aglycone could not be accurately determined.
Figure 9(b).
Proposed schematic fragmentation E. elephantina isolate.
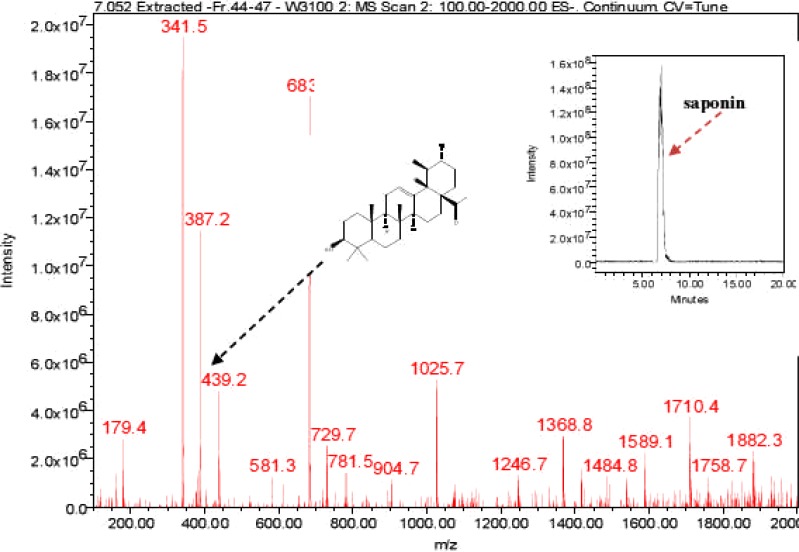
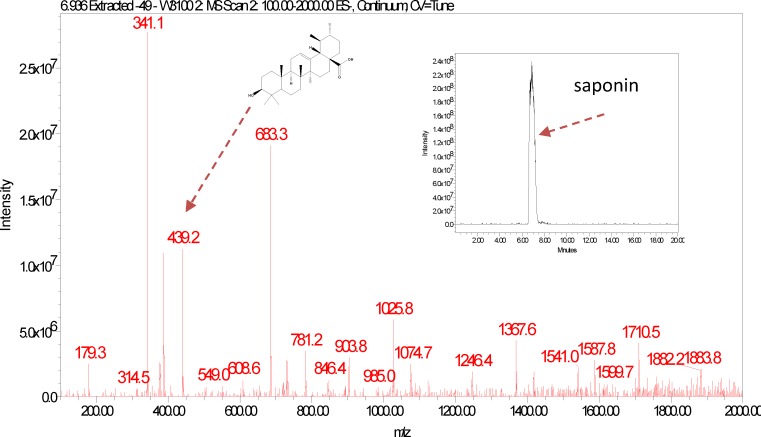
P. prunelloides fraction 44–47 at Rt 7.052 and fraction 49 Rt 6.935
The LC-ESI-MS of P. prunelloides fractions 44–47 and 49 exhibited a pseudomolecular ion at 1882 (Fig. 10–11). The fragmentation pattern as discussed above for the E. elephantina fraction indicated the aglycone as oleanolic acid, (Fig 10b) although exhibiting different pseudomolecular ions at higher atomic mass units. The fragmentation pattern also indicated the presence of glucose, glucuronic acid and oleanolic acid. The fragment at m/z 439 was characteristic of the presence of the oleanolic acid moiety. Although the LC-ESI-MS data cannot differentiate between the possible isomeric glycoside residues or their linkage, it is useful in determining the molecular weight of some major saponin fractions as well as the fragmentation pattern from which useful structural information can be deduced (Self, et al., 1986; Porter, et al., 1991; Rice-Evans & Miller, 1996). The fragmentation pattern of P. prunelloides saponin fractions exhibited loss of a relatively greater number of sugar moieties compared to the corresponding fractions for E. elephantina, considering m/z 1882 to be the pseudomolecular mass of P. prunelloides fraction (Fig 10b) and m/z 1466 for E. elephantina (Fig. 9b).
Figure 10(a).
LC-ESI-MS spectra and chromatogram of P. prunelloides saponin isolate.
Figure 11.
LC-ESI-MS spectra and Chromatogram of P. runelloides fraction 49
Figure 10(b).
Proposed schematic fragmentation of P. prunelloides saponin isolate.
Initially, the mere presence of oleanolic acid as an aglycone in the two species would justify the medicinal applications of the two species independently in phytotherapy. This triterpenoid is implicated in providing several health benefits. Oleanolic acid and its isomer ursolic acid are both triterpenoid compounds found in plants used in the human diet and in medicinal herbs. They are found in the form of the free acid or as aglycones of triterpenoid saponins. They have also been reported to have hepatoprotective properties (Liu, 1995), antiallergic (Banno, et al., 2004), antiulcer (Oversna, et al., 2004), cardioprotective (Senthil, et al., 2007), antimicrobial (Ngouela, et al., 2006), activities. They are also reported to exhibit anti-inflammatory and analgesic (Vasconcelos, et al., 2006) activities. It is most likely that the anti-inflammatory properties of these two medicinal plants reported from ethnobotany are associated with this class of phytochemicals. The two isomers have also been shown to exhibit antiparasitic activity against Leishmania species (Torres-Santos, et al., 2004) and Trypanosoma, and an antiprotozoal against plasmodium falciparum species (Van Baren, et al., 2006). They have also been used to treat kidney diseases (Yoshimura, et al., 2003) and hypertension (Somova, et al., 2004). The two compounds have been demonstrated to confer protective effects against periodontal pathogens, antiviral activity against HIV (Ovesna, et al., 2004), and antitubercular potential against Myobacterium tuberculosis (Gua, et al., 2004) apart from immunomodulatory hypoglycaemic (Zhang, et al., 2006) and antifertility activities (Chattopadhyay, et al., 2005). It can, therefore, be postulated that the use of both E. elephantina and P. prunelloides to remedy chest problems could be linked to this class of compounds and their glycosides.
LC-ESI-MS spectra and chromatogram of proposed sugar moieties from both E. elephantina and P. prunelloides
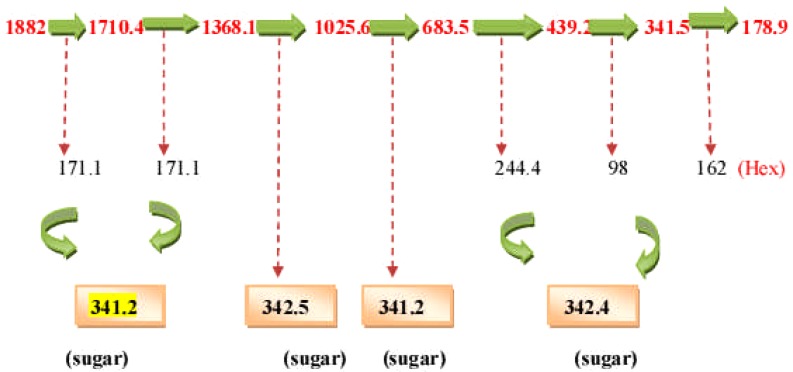
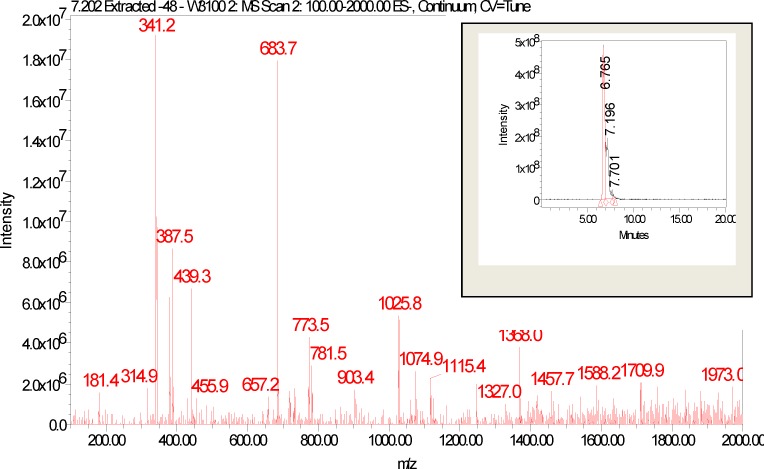
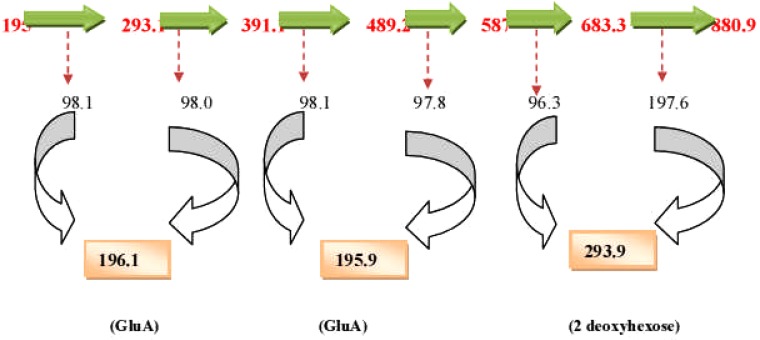
The spectra and chromatogram at Rt 9.508 was observed to be common to both E. elephantina and P. prunelloides (Fig 12). The fragmentation pattern of the observed pseudomolecular ions and fragments were proposed to be characteristic for the sugar moieties (Fig 12). The proposed fragmentation pattern is shown in (Fig 13). The fragmentation pattern exhibited the presence of Glucuronic acid (m/z =177/195) three hexose (m/z) = 486), a tetramer of two hexose and two pentose sugars (m/z = 587), a dimer of two rhamnose, (m/z = 293), a dimer of two glucuronic acids [m/z = 2 × 195 = 390.8)], a tetramer of two rhamnose (293) and two glucuronic acid (390.9), i.e. m/z 684.6. The corresponding LC-ESI-MS spectra and chromatogram of P. prunelloides at Rt 9.394 was recorded in Fig 6.15(a).and the fragmentation pattern showed similar fragment ions as for E. elephantina explained above but the major difference being a longer sugar chain for P. prunelloides. The difference in the sugar chain was even more pronounced in the fragment ion for P. prunelloides fraction 48 at Rt 7.202 (Fig 12a). The corresponding proposed fragmentation pattern (Fig 12b) suggested the linkages of disaccharides with m/z 342 and the presence of Glucuronic acid m/z 179.1. The proposed fragmentation pattern further suggested about five dimers with m/z 342 each, which implied about ten monomers either a Hexose and GluA(163 + 179) or GluA and Rhamnose i. e. m/z (195 + 146) respectively Fig. 12b. The presence of the sugar chains in these compounds could also account for the presence of saponins observed in these two medicinal plants. The length and sequence of glycosides sugar moieties are reported to have a bearing on the chemical as well as pharmacological properties of saponins although there are conflicting views on trend of the effect. In one report the bioactivity of saponins is alleged to increase as the sugar moieties are eliminated from the saponin structure (Shiriwa, et al., 1991). In other reports (Lee, et al., 2000; Huang, et al., 2008) it is reported that the aglycone with four sugar units exhibited greater available cytotoxicity than that with three sugars. Nonetheless, all reports concur that saponins have pharmacological effects and their presence may act as an indicator for therapeutic potency of medicinal plants. While determination of the linkages and the exact sugar length were beyond the scope of this study, these observations could act as leads to further research on the phytochemistry of these two plants.
Figure 12(a).
LC-ESI-MS spectra and chromatogram of proposed sugar moieties from prunelloides.
Figure 13(a).
LC-ESI-MS spectra and chromatogram of P. prunelloides fraction 48.
Figure 12(b).
Proposed fragmentation pattern of sugar moieties of E. elephantina fraction 61 at Rt = 9.336.
The spectra and chromatogram at Rt 9.508 was observed to be common to both E. elephantina and P. prunelloides (Fig 12b). The fragmentation pattern of the observed pseudomolecular ions and fragments were proposed to be characteristic for the sugar moieties (Fig 13b). The proposed fragmentation pattern is shown in (Fig 13b). The fragmentation pattern exhibited the presence of Glucuronic acid (m/z =177/195) three hexose (m/z) = 486), a tetramer of two hexose and two pentose sugars (m/z = 587), a dimer of two rhamnose, (m/z = 293), a dimer of two glucuronic acids [m/z = 2 × 195 = 390.8)], a tetramer of two rhamnose (293) and two glucuronic acid (390.9), i.e. m/z 684.6. The corresponding LC-ESI-MS spectra and chromatogram of P. prunelloides at Rt 9.394 was recorded in Fig12a) and the fragmentation pattern showed similar fragment ions as for E. elephantina explained above but the major difference being a longer sugar chain for P. prunelloides. The difference in the sugar chain was even more pronounced in the fragment ion for P. prunelloides fraction 48 at Rt 7.202 (Fig13a). The corresponding proposed fragmentation pattern (Fig 13b) suggested the linkages of disaccharides with m/z 342 and the presence of Glucuronic acid m/z 179.1. The proposed fragmentation pattern further suggested about five dimers with m/z 342 each, which implied about ten monomers either a Hexose and GluA(163 + 179) or GluA and Rhamnose i. e. m/z (195 + 146) respectively Fig. 13b. The presence of the sugar chains in these compounds could also account for the presence of saponins observed in these two medicinal plants. The length and sequence of glycosides sugar moieties are reported to have a bearing on the chemical as well as pharmacological properties of saponins although there are conflicting views on trend of the effect. In one report the bioactivity of saponins is alleged to increase as the sugar moieties are eliminated from the saponin structure (Shiriwa, et al., 1991). In other reports (Lee, et al., 2000; Huang, et al., 2008) it is reported that the aglycone with four sugar units exhibited greater available cytotoxicity than that with three sugars. Nonetheless, all reports concur that saponins have pharmacological effects and their presence may act as an indicator for therapeutic potency of medicinal plants. While determination of the linkages and the exact sugar length were beyond the scope of this study, these observations could act as leads to further research on the phytochemistry of these two plants.
Figure 13(b).
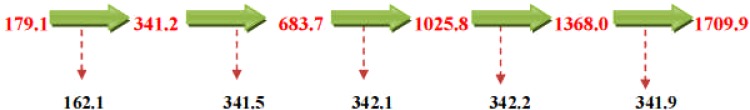
Proposed fragmentation pattern of sugar moieties of P. prunelloides fraction 48 at Rt = 7.202.
Analysis of the fragmentation patterns of saponins in this study suggested the presence of several Oligosaccharides. The presence of these phytochemicals is noteworthy since some of them have been implicated for having bacterial antiadhesive properties. A prominent example is the trisaccharide, Galβ-1-4-Glc-NAcβ-3Galβ, which has been reported to inhibit the adherence of S. aureus to baccal epithelial cells (Andersson, et al., 1984; Otnaess & Halvorsen, 1980). Fucosylated Oligosaccharides are also reported to inhibit the binding of Campylobacter jejuni, strains of E. coli and their heat-stable toxin, and enteropathogenic E. coli to enterocytes (Newburg, et al., 1990; Crane, et al., 1994; Cervantes, 1995; Cravitoto et al.,, 1995). On the other hand, sialylated oligosaccharides strongly inhibit the binding of Influenza A virus and S. fimbriated enteropathogenic E. coli to their respective target cells (Zopf and Roth, 1996). Oligosaccharides have been reported to be anti-infective agents (Lancet, 1996). Considering the high content of sugars in the two species and the effect of this class of compounds on Influenza A virus (Zolf and Roth, 1966), it may be justified why P. prunelloides was used in the treatment of influenza in 1918.
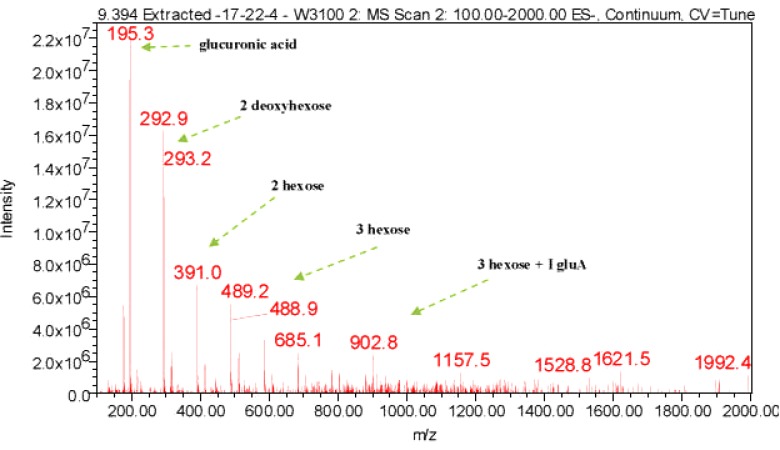
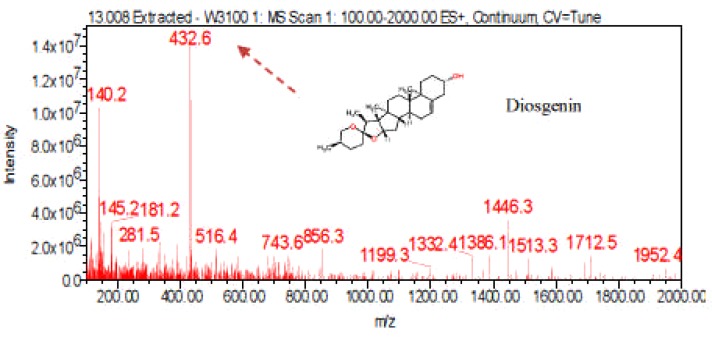
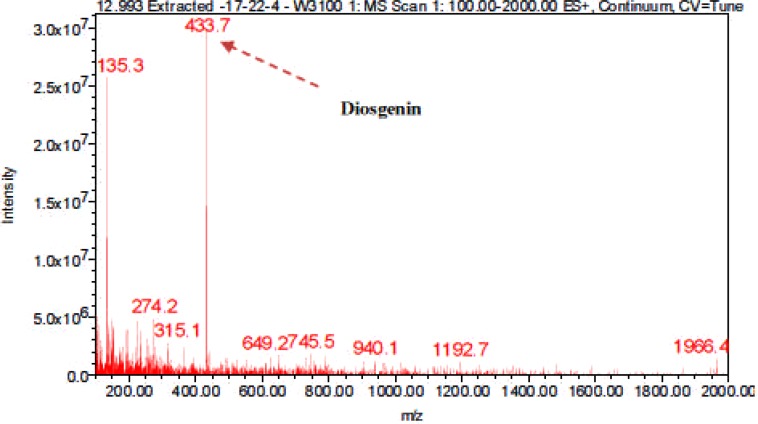
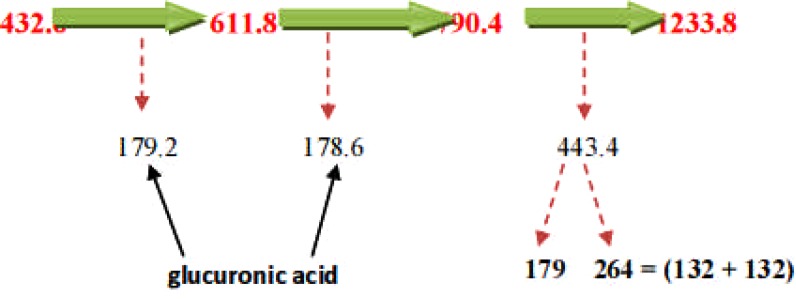
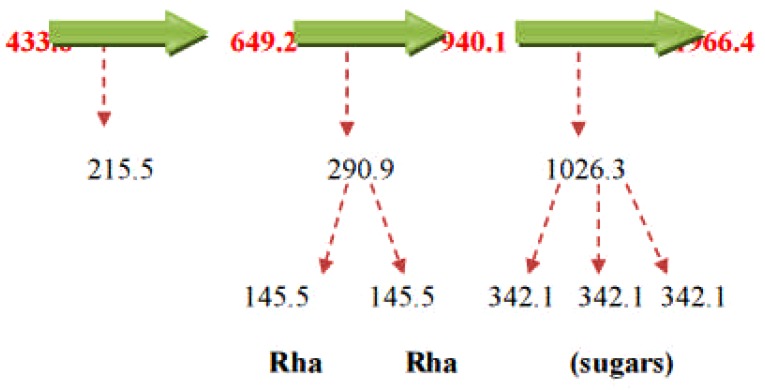
LC-ESI-MS spectra of proposed Diosgenin saponin glycosides from E. elephantina and P. prunelloide
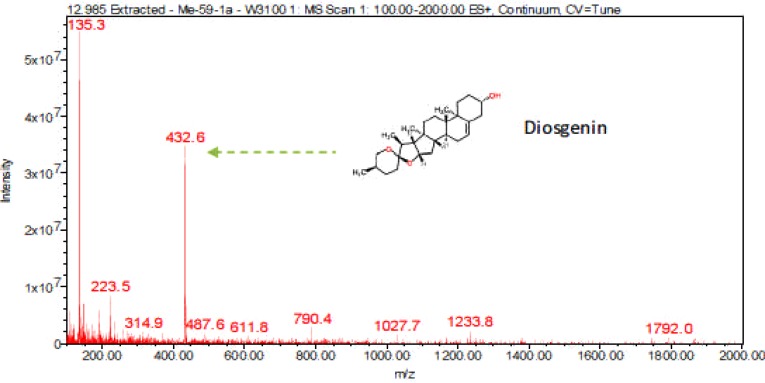
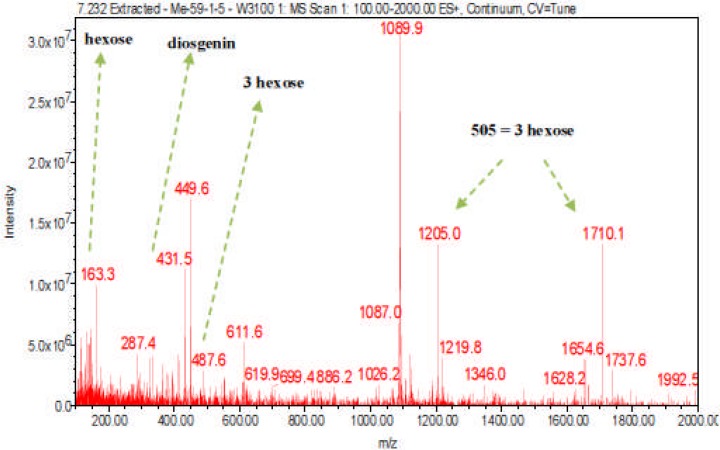
Digitonin standard, E. elephantina, and P. prunelloides saponin fractions exhibited a common peak at about Rt = 9.450 (Fig 14, 15a and 16a) respectively. The corresponding proposed fragmentation pattern of the respective samples were suggested on (Fig 15b) for E. elphantina saponin and (Fig 16b) for P. prunelloides saponin. Diosgenin or its isomers were proposed to be the aglycones in all the samples as depicted by the pseudomolecular mass m/z 432.6. The proposed saponin from E. elephantina as deduced from fragmentation pattern (Fig15b) exhibited the presence of Glucuronic acid (m/z 179.20, Arabinose, m/z 135 and gallohexose, m/z 314.9). P. prunelloides on the other hand exhibited the presence of Glucuronic acid and Rhamnose (Fig 16b). The sugar chains for P. prunelloides seemed to be relatively longer, in the range of seven to eight monomers (Fig16b) while those for E. elephantina were in the range of four to five monomers (Fig 15b). There was also one proposed Diosgenin saponin perculiar to E. elephantina (Fig 17a) as depicted by a very distinct base peak at m/z 1089. The corresponding fragmentation pattern also suggested the presence of GluA and hexose sugars as the major sugar residues (Fig 17b). Out of the fifteen most intense peaks, eleven of them showed typical loss of carbohydrate residues supporting their identity as saponins. The presence of Diosgenin as an aglycone for saponins in these two medicinal plants may also account for the alleged therapeutic values as well as the antimicrobial activities of these two medicinal plants. If these results are to go by, it will be for the first time this aglycone is reported in both plants.
Figure 14.
LC-ESI-MS spectra of standard Digitonin
Figure 16(a).
LC-ESI-MS spectra and chromatogram of proposed Diosgenin saponin isolated from P. prunelloides fraction 17-22-4.
Figure 15(b).
Fragmentation pattern of proposed diosgenin saponin from E. elephantina.
Figure 16(b).
Fragmentation pattern of proposed Diosgenin saponin from P. prunelloides.
Figure 17(b).
Fragmentation pattern of proposed Diosgenin saponin from E. elephantina fraction 59-1
NMR spectra of Saponin isolate from E. elephantina (Fraction 61)
The 1H and the 13C NMR spectra were recorded (Fig 6.24 and 6.25) respectively. The 1H NMR spectrum (CDCl3) revealed the presence of 12 tertiary methyl groups at (ppm) 0.8473, 0.8591, 0.8706, 0.8865, 0.8991, 0.9115, 1.0349, 1.1393, 1.1909, 1.2291 and 1.2587 a doublet at σ1.628 assigned to the secondary methyl of deoxy sugar (Rhamnose). 1H NMR was also indicative in its downfield region of the presence of six anomeric proton signals at (ppm) 4.0096, 4.0164, 4.1375, 4.1456, 6.7897, and 6.8043. 13C NMR spectrum of E. elephantina fraction 61 confirmed the presence of oleanane skeleton due to the characteristic signals for the substance; (ppm) 143.48 (C13), 120.54 (C12) and 76.78 (C3). The 13C signal at 69.5 indicates that one of the C-6 hydroxyl group of glucose was esterified.
Limitations of the study
While toxicity tests were carried out applying the brine shrimp lethality tests (BST), more tests could be undertaken using other techniques in order to come up with a more comprehensive conclusion with regards to the toxicities of the two plant species. This is because determining pharmacological and toxicological effects of herbal medicine presents certain challenges for various reasons. Initially, theories and analyses of phytotherapeutic remedies are far from being unified especially as there is no black box called the “cytotoxicity meter” to measure this highly complex phenomenon. In short, results obtained from toxicological tests are relative and are not an actual reflection of the efficacy of drugs themselves. Actually, the LC50 and LD50 values upon which toxicities are based are themselves neither physical nor chemical measures but derived parameters. In short, these are connotative rather than denotative definitions of the concept cytotoxicity.
Part of the challenge stems from the fact that the dynamics of complex biological systems upon which cytotoxicity is based are themselves not absolutely understood. Hence, reducing complex biological processes to statistical data may result in other limitations. It is reasonable to assert that there is an unpredictable change in the absolute concentrations of drugs as they are determined in the test tubes to the actual concentrations in the patient's body after administration. This assertion is prompted by the fact that the methods in the derivations of LC50 values are based on static drug concentrations with interactions being determined at a single time point. Furthermore, the pharmacodynamic and pharmakinetic considerations cannot be absolutely ruled out. Hence, making direct correlations between in vitro and in vivo interactions is not always possible based on the data so far presented. Moreover, a single drug may have several pharmacological actions, yet it is only those concentrations reached by standard doses that are considered relevant. This presents another challenge because even if the plant may contain the appropriate therapeutic phytochemical constituents, these may be in insufficient amounts to account for the observed effects when isolated. This may be alleged due the reported observations (Unrich-Merzenich, et al., 2007) that some herbal medications are more efficacious when an extract is administered compared t o isolated single constituents. This may imply that a predominant mechanism could be potentiated by lesser mechanisms initiated by minor phytochemicals or drugs.
There is also a need to explore the toxicities of a wider range of concentrations of extracts of the two plants on a broader range of Gram negative and Gram positive pathogens with the view to propose the mechanisms associated with their efficacy both separately as well as in combination. The ability of the tested extracts to inhibit the growth of different clinical pathogens suggests that further study is needed to identify active compounds and their modes of action on pathogenic organisms
There is also a need to get more quantities of saponins with the view to fully characterise them especially as they constitute a significant amount of secondary metabolites in both species.
Conclusion
The results of this study show that the tested plant extracts have a wide microbial activity against five species of clinical pathogens, as well as significant antioxidant capability. Cytotoxicity and anti-inflammatory activities of these extracts may be explained due to presence of flavonoids and saponins that were identified. The use of these plants by traditional healers in the treatment of inflammation may have some scientific justification as the plants have been shown to have anti-inflammatory activity. Bioactivity guided fractionation from the more active fractions (chloroform and methanol fractions) is currently underway. The isolation of new and effective anti-inflammatory agents is important for potential drug development as natural products provide unlimited opportunities for new drugs because of the unmatched availability of chemical diversity. This will eventually go a long way to validate the use of southern Africa medicinal plants by traditional healers
Figure 6.
%inhibition of aqueous extracts of E. elephantina samples from different location.
Figure 8b.
FT-IR spectra of E. elephantina isolate 59.4.
Figure 17(a).
LC-ESI-MS spectra of proposed Diosgenin saponin from E. elephantina fraction 1
Acknowledgements
We wish to thank the National Research Foundation (South Africa) for financial support, UNISWA (University of Swaziland) for facilitating our sampling trips, and Dr A. Moteetee (UJ, Dept Botany and Plant Biotechnology) for the identification of plant species as well as assisting in the preparation of specimen vouchers for herbarium deposition.
References
- 1.Adeniji KO, Amusan OOG, Dlamini PS, Enow-Orock EG, Gamedze ST, Gbile ZO, Langa AD, Makhubu LP, Mahunnah RLA, Mshana RN, Sofowora A, Vilane MJ. Traditional medicine and pharmacopoeia - contribution to ethnobotanical and floristic studies in Swaziland. Swaziland: The Scientific, Technical and Research Commission of the Organization of African Unity (OAU/STRC); 2000. [Google Scholar]
- 2.Andersson B, Porras O, Hanson L A, Langergard T, Svanborg-Eden C. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenza by human milk and recent oligosaccharides. Journal of Infectious Diseases. 1984;153:232–237. doi: 10.1093/infdis/153.2.232. [DOI] [PubMed] [Google Scholar]
- 3.Banno N, Akihisa T, Tokuda H, Yosukawa K, Hikashihara H, Ukiya M, Watakabe K, Kimura Y, Hasegawa J, Nishino H. riterpene acids from the leaves of Perilla frutescens and their antiiflammatoty and anti-tumor-promoting effects. Biosci Biotechnol Biochem. 2004;68:85–90. doi: 10.1271/bbb.68.85. [DOI] [PubMed] [Google Scholar]
- 4.Cervantes L E, Newburg D S, Ruiz-Palacios G M. α1-2 Fucosylated chains (H-2 and Lewisb) are the main human receptor analogues for Camphylobacter (abstract) Pediatry Research. 1995;37:171A. [Google Scholar]
- 5.Crane J K, Azar S S, Stam A, Newburg D S. ligosaccharides from human milk block binding and activity of the Escherichia coli heat-stable enterotoxin (Sta) in T84 intestinal cells. Journal of Nutrition. 1994;124:2358–2364. doi: 10.1093/jn/124.12.358. [DOI] [PubMed] [Google Scholar]
- 6.Cravitoto A, Tello A, Villafan H, Ruiz J, Del vedoto S, Neeser J R. Inhibition of localized adhesion of enteropathologenic Escherichia coli to Hep-2 cells by immunoglobulin and oligosaccharide fractions of human colostrums and breast milk. Journal of Infectious Diseases. 1995;163:1247–1255. doi: 10.1093/infdis/163.6.1247. [DOI] [PubMed] [Google Scholar]
- 7.Gua L G, Wang Y, Franzblau S G, Montenegrio G, Timmermann B N. onstituents of Quinchamlium majus with potential antitubercular activity. Z Naturforsch. 2004;59:797–802. doi: 10.1515/znc-2004-11-1206. [DOI] [PubMed] [Google Scholar]
- 8.Da Silver Bernadete P, de Sousa Ac, Silver G M, Mendes T P, Parente J P. Bioactive steroidal saponin from Agave attenuate. Z natarforch. 2002;57C:423–428. doi: 10.1515/znc-2002-5-603. (Ger) [DOI] [PubMed] [Google Scholar]
- 9.Gelfand M, Mavi S, Drummond RB, Ndemera B. The traditional medical practitioner in Zimbabwe: his principles of practice and pharmacopoeia. Gweru: Mambo Press; 1985. [Google Scholar]
- 10.Grierson DS, Afolayan AJ. An ethnobotanical study of plants used for the treatment of wounds in the Eastern Cape, South Africa. Journal of Ethnopharmacology. 1999;67:327–332. doi: 10.1016/s0378-8741(99)00082-3. [DOI] [PubMed] [Google Scholar]
- 11.Hua R, Sun S Q, Zhou Q, Wang B Q, Noda I. Journal Pharm Biomed Anal. 2003;33:199–209. doi: 10.1016/s0731-7085(03)00253-x. [DOI] [PubMed] [Google Scholar]
- 12.Huang X-F, Lin Y-Y, Kong L-Y. Steroids from the roots of Asparagus officinalis and their cytotoxic activity. J Integr Plant Biol. 2008;50(6):717–722. doi: 10.1111/j.1744-7909.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 13.Hutchings A. A survey and analysis of traditional medicinal plants as used by the Zulu, Xhosa and Sotho. Bothalia. 1989a;19(1):111–123. [Google Scholar]
- 14.Hutchings A, Scott AH, Lewis G, Cunningham A. Zulu Medicinal Plants. Pietermaritzburg: Natal University Press; 1996. [Google Scholar]
- 15.Jacot Guillarmod A. Flora of Lesotho Cramer. Lehre: 1971. [Google Scholar]
- 16.Kirmizigul S, Anil H. New Triterpenic saponins from Celphalaria transsyvanica. Turk J Chem. 2002;26:947–954. [Google Scholar]
- 17.Laidler PW. The magic medicine of the Hottentots. South African Journal of Science. 1928;25:433–447. [Google Scholar]
- 18.Lee S J, Ko W G, Kim J H, Moon C K, Lee B H. Induction of apoptosis by a novel intestinal metabolite of ginseng saponin via cytochrome c-mediated activation of caspase-3 protease. Biochemical Pharmacology. 2000;60:677–685. doi: 10.1016/s0006-2952(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 19.Li Y M, Sun S Q, Zhou Q, Qin Z, Tao J X, Wang J, Fang Vibrational Spectroscopy. 2004;36:227–232. [Google Scholar]
- 20.Liu J. Pharmacology of oleanolic acid and Ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 21.Maliehe EB. Medicinal Plants and Herbs of Lesotho. Lesotho: Mafeteng Development Project; 1997. (in Sesotho) [Google Scholar]
- 22.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, Mclaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Medicine. 1982;45:31–34. [PubMed] [Google Scholar]
- 23.Moteetee A, Van Wyk B-E. The medical ethnobotany of Lesotho: a review. Bothalia. 2011;41:209–228. [Google Scholar]
- 24.Mthembu XS. A phytochemical study of Schefflera umbellifera and Elephantorrhiza elephantina. Pietermaritzburg: University of KwaZulu-Natal; 2007. M.Sc. dissertation. [Google Scholar]
- 25.Murdaca G, Colombo BM, Cagnati P, Gulli R, Spanò F, Puppo F. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis. 2012;224:309–317. doi: 10.1016/j.atherosclerosis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi K, Solomon P H. Infrared Absorption Spectroscopy. Holden-Day Inc; 1977. [Google Scholar]
- 27.Natori S, Ikekawa N, Sazuki M, editors. Advances in Natural Products Chemistry. Tokyo 112, Japan: Kodansha Ltd; 1981. Extraction and Isolation of Biologically Active Compounds; pp. 275–287. [Google Scholar]
- 28.Neuwinger HD. African Traditional Medicine - a dictionary of plant use and applications. Stuttgart: Medpharm Scientific Publishers; 2000. [Google Scholar]
- 29.Newburg D S, Pickering L K, McCluer R H, Cleary T G. ucosylated oligosaccharides of human milk protect sucking mice from heat-stable enterotoxin of Escherela, A preichia coli. Journal of Infectious Diseases. 1990;162:1075–1080. doi: 10.1093/infdis/162.5.1075. [DOI] [PubMed] [Google Scholar]
- 30.Okwu D E, Ekeke O. Phytochemical screening and mineral composition of chewing sticks in Southern Nigeria. Global J Pure Appl Sci. 2003;9:235–238. [Google Scholar]
- 31.Okwu DE, Okwu M E. hemical composition of Spondia Mombin plants. J Sustain Agri Environ. 2004;6:140–147. [Google Scholar]
- 32.Otnaess A B, Halvorsen S. Non-antibody components in human milk inhibit E coli heat labile enterotoxin measured by an enzyme-linked immunosorbent assay. Acta Pathol Scund. 1980;88:247–253. doi: 10.1111/j.1699-0463.1980.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 33.Ovesna Z, Vachalkova A, Horvathova K, Tothova D. Pentacyclic triterpenoic acids: new chemoprotective compounds [minireview] Neoplasma. 2004;51:327–333. [PubMed] [Google Scholar]
- 34.Porter L J, Ma Z, Chan B G. Cocoa Proanthocyanidins: Major Flavanoids and Identification of some Minor Metabolites. Phytochemistry. 1991;30:1657–1663. [Google Scholar]
- 35.Phillips EP. Annals of the South African Museum. Vol. 16. London: Adlard & West Newman; 1917. A contribution to the flora of the Leribe Plateau and environs: with a discussion on the relationships of the flora of Basutoland, the Kalahari, and the south-eastern regions; pp. 1–379. [Google Scholar]
- 36.Pujol J. Naturafrica - the Herbalist Handbook. Durban: Jean Pujol Natural Healers Foundation; 1990. [Google Scholar]
- 37.Rice-Evans C A, Miller N J. Antioxidant activities of flavonoids as bioactive components of food. BioChem, Soc Trans. 1996;24:790–795. doi: 10.1042/bst0240790. [DOI] [PubMed] [Google Scholar]
- 38.Sam TW. Toxicity testing using the brine shrimp: Artemia salina. In: Colegate SM, Molyneux RJ, editors. Bio-active natural products detection, isolation and structural determination. Boca Raton: CRC Press; 1993. pp. 442–456. [Google Scholar]
- 39.Self R D, Eagles J, Galletti G C, Mueller-Harvey I, Hartley R D, et al. FAB Mass Spectrometry of Phenols (syn. Vegetable Tannins) Biomed Environ Mass Spec. 1986;13:449–468. [Google Scholar]
- 40.Senthil S, Chandramohan G, Pungalendi K V. Isomers (oleanolic acid and Ursolic acid) differ in their protective effect against isoproterenol-induced myocardial ischemia in rats. Int Journal Cardiol. 2007;119:131–133. doi: 10.1016/j.ijcard.2006.07.108. [DOI] [PubMed] [Google Scholar]
- 41.Shiriwa M, Harada K, Okubo K. Composition and structure of group B saponin in soybean seed. Agric Biol Chem. 1991;55:911–917. [PubMed] [Google Scholar]
- 42.Smith A. A contribution to the South African materia medica. Juta, Cape Town. Dysfunction in rheumatic autoimmune diseases. Atherosclerosis. 1895;224:309–317. [Google Scholar]
- 43.Somova L I, Shode F O, Mipando M. Cardiotonic and antidysrhythmic effects of oleanolic and Ursolic acids, methyl maslinate and uvaol. Phytomedicine. 2004;11:121–129. doi: 10.1078/0944-7113-00329. [DOI] [PubMed] [Google Scholar]
- 44.Torres-Santos E C, Lopes D, Oliveira R R, Carauta J P, Falcao C A, Kaplan M A, Rossi-Bergamann B. Antileishmanial activity of isolated triterpenoids from Pourouma guianensis. Phytomedicine. 2004;11:114–120. doi: 10.1078/0944-7113-00381. [DOI] [PubMed] [Google Scholar]
- 45.Ulrich-Merzenich U, Zeitler H, jobst D, Panek D, Vetter H, Wagner H. Application of the “Omic” technologies in phytomedicine. Phytomedicine. 2007;14:70–82. doi: 10.1016/j.phymed.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Van Baren C, Anao I, Leo Di Lira P, Debenedetti S, Houghton P, Croft S, Martino V. Triterpenic acids and flavonods from Satureja parvifolia.Evaluation of their antiprotozoal activity. Z Naturforsch. 2006;611:89–92. doi: 10.1515/znc-2006-3-406. [DOI] [PubMed] [Google Scholar]
- 47.Van Wyk B-E, Van Oudtshoorn B, Gericke N. Medicinal Plants of South Africa. Pretoria: Briza publications; 2009. [Google Scholar]
- 48.Vasconcelos M A, Royo V A, Ferreira D S, Crotti A E, Silva M L A, Carvalho J C, Bastos J K, Cunha W R. In vivo analgesic and anti-inflammatory activities of oleanolic acid and Ursolic acid from Miconia albicans. Z Naturforsch. 2006;61:477–482. doi: 10.1515/znc-2006-7-803. [DOI] [PubMed] [Google Scholar]
- 49.Watt JM, Breyer-Brandwijk MG. The medicinal and poisonous plants of southern and eastern Africa. 2nd edition. London: Livingston; 1962. [Google Scholar]
- 50.Woo Y A, Kim H J, Chung H. Analyst. 1999;124:1223–1226. [Google Scholar]
- 51.Yff BTS, Lindsey KL, Taylor MB, Erasmus DG, Jäger AK. The pharmacological screening of Pentanisia prunelloides and the isolation of the antibacterial compound palmitic acid. Journal of Ethnopharmacology. 2002;79(1):101–107. doi: 10.1016/s0378-8741(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 52.Yoshimura H, Sugawara K, Saito M, Murakami S, Miyata N, Kawashima A, Morimoto S, Gao N, Zhang X, Yang J. In vitro TGF-beta 1 antagonistic activity of Ursolic and oleanolic acids isolated from Cleodendrathus spectus. Planta Med. 2003;69:673–675. doi: 10.1055/s-2003-41110. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Hong D, Zhou Y, Shen Q, Li J Y, Hu L H, Li j. Ursolic acid and its derivative inhibit protein tyrosine phosphatase uptake. Biochem Biophys Acta. 2006;1760:1505–1512. doi: 10.1016/j.bbagen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Zopf D, Roth S. Oligosaccharide anti-infective agents. Lancet. 1996;347:1017–1021. doi: 10.1016/s0140-6736(96)90150-6. [DOI] [PubMed] [Google Scholar]