Abstract
Background
Gladiolus dalenii Van Geel (Iridaceae) has been used for the treatment of depression and psychotic disorders in African traditional medicine. The aim of this study was to assess the effect of the aqueous extract from the corm of Gladiolus dalenii and its possible mechanisms of action.
Materials and Methods
We assessed the antidepressant properties of G. dalenii corm aqueous extract in mice, using the open field, forced swimming, and tail suspension tests. Spontaneous locomotor activity of mice given various doses of G. dalenii extract (per os) was determined in the open field, whereas immobility was evaluated in the other two tests.
Results
Extract maximal effect was observed at 15 mg/kg, as mice displayed a marked reduction in immobility time in both the forced swimming test (80%) and the tail suspension test (66%). In further studies aimed at investigating the mechanism of action of G. dalenii extract, the latter significantly antagonized the effect of N-methyl-D-aspartate (NMDA, 75 mg/kg) at both the doses 15 mg/kg (p<0.001) and 150 mg/kg (p=0.004). A significant reduction in immobility time was also observed following treatment with combinations of a sub-effective dose of extract (7.5 mg/kg) with either the NMDA receptor antagonist D-(−)-2-amino-7-phosphonoheptanoic acid (D-AP7, 50 mg/kg, P< 0.001), the serotonin reuptake inhibitor fluoxetine (5 and 10 mg/kg, P< 0.001and P< 0.001 respectively), and the multi-target antidepressant imipramine (5 and 10 mg/kg, P< 0.001 and P< 0.001 respectively). Moreover, neither G. dalenii extract alone nor its combinations with NMDA ligands imipramine and fluoxetine enhanced mouse spontaneous locomotor activity.
Conclusion
Altogether, these results suggest that G. dalenii has antidepressant properties, probably mediated through interactions with NMDA, serotonin and/ or noradrenergic systems, and may justify its use in traditional medicine.
Keywords: Gladiolus dalenii, depression, forced swimming test, tail suspension test, open field test, therapy
Introduction
Depression is the most common mood and psychiatric disorder and the fourth leading cause of disability worldwide (Murray and Lopez, 1996; Bromet et al., 2011). Depression would affect over 120 million people, and according to a recent epidemiological survey, its lifetime prevalence would be 10% to 15% of people (Lépine and Briley, 2011). This disorder, which negatively affects the quality of life and productivity of people affected, is expected to become the major causative agent of disease by 2030 (WHO 2004; Lépine and Briley, 2011). Depression is frequently chronic and unresponsive to drug treatment, and not surprisingly, has been associated with heavy economic costs. Various studies have revealed that on an annual basis health costs related to that disorder exceed 105 billion euros (Andlin-Sobocki & H. U. Wittchen, 2005; Woode et al., 2009). It appears, therefore, that more effective and cheaper drugs are needed, especially for low-income countries.
Gladiolus dalenii Van Geel (Iridaceae), also known as “Mantsap Letoupuh” (wild onion) in the Babadjou language (local language in the western region of Cameroon), is a robust herb that grows virtually everywhere in the grasslands, savannas and woodlands of sub-Saharan and southern Africa (Burkill, 1985). G. dalenii grows from a woody corm (2.5–3.5 cm diameter) covered by a coriaceous tunics fragmented irregularly. The corm is used in African traditional medicine to treat a wide range of conditions, including headaches, epilepsy, convulsions, intestinal spasms, venomous stings and bites, arthritis nasopharyngeal affections and diarrhoea (Burkill, 1985; Hutchings & Van Staden, 1994; Bandeira et al., 2001). In Cameroon, aqueous macerates of G. dalenii corms are used to treat epilepsy, depression, but also schizophrenia and other psychotic disorders.
Early studies have revealed the presence of alkaloids in Gladiolus spp. (Burkill, 1985), and G. dalenii corm crude extract was reported antifungal activity (Odhiambo et al., 2010). However, to our knowledge no scientific evidence for the neuropharmacological properties of G. dalenii has been reported to date. The present study, aimed at addressing this question, investigated the effect of G. dalenii corm aqueous macerate on two experimental models of depression, namely the forced swimming test (FST) and the tail suspension test (TST) (Porsolt et al., 1977; Steru et al., 1985; Cryan et al., 2005). The possible mechanisms of action of this extract were also investigated.
Material and methods
Plant Material and Preparation of Extracts
The corms of Gladiolus dalenii used in this study were harvested during the dry season (December 2009) from Babadjou (West Cameroon). Voucher specimen N° 25742/SRF/Cam has been deposited at the Yaoundé Herbarium. The corms were selected and crushed at room temperature. The paste (100 g) was macerated in 100 ml of distilled water for 5 h. The supernatant (macerate) was then collected and filtered with a Wattman N° 1 filter paper. After filtration, water was evaporated in a dry oven at 35°C, and 15 g of a brown solid extract was obtained. The yield of the extraction was 0.15%. The aqueous macerate from the corm of G. dalenii (GD) was prepared and then administered orally to mice 24, 6 and 1 h before each pharmacological test. After screening according to advice from the traditional healer, the following doses were used: 7.5, 15, 30, 75 and 150 mg/kg.
Drugs and Treatments
The following drugs were used as standards in the study: imipramine (5, 10 and 30 mg/kg, Sigma, St. Louis, USA), fluoxetine (5, 10 mg/kg, Sigma, St. Louis, USA ), caffeine (CAF, 7.5 mg/kg, Sigma, St. Louis, USA ), diazepam (DZP, 1 and 3 mg/kg, Roche), N-methyl-D-aspartate (NMDA, 75 mg/kg, Sigma, St. Louis, USA ), D-2-amino-7-phosphonoheptanoate (D-AP7, 50, 100 and 200 mg/kg, Sigma, St. Louis, USA). All drugs were dissolved in distilled water and administered 24, 6 and 1 h before the test by intraperitoneal (i.p.) route in a constant volume of 10 ml/kg body weight, except for G. dalenii and the vehicle (distilled water) which were given by oral route. The control group (CON) received distilled water.
Possible interactions between G. dalenii and NMDA receptors were assessed through the FST according to Sousa et al., (2004); Poleszark et al., (2007), and Skolnick et al., (1991). Three separate sets of experiments were performed. In the first set, the aqueous macerate of G. dalenii (7.5 mg/kg), D-AP7 (50, 100 and 200mg/kg) were given alone and the aqueous macerate of G. dalenii (7.5 mg/kg) was coadministered with D-AP7 (50 mg/kg). In the second set, the aqueous macerate of G. dalenii (15 and 150 mg/kg) and D-AP7 (200 mg/kg) were given alone, and then co-administered with NMDA (75 mg/kg). In the third set, fluoxetine a selective inhibitor of the recapture of serotonine (5 and 10 mg/kg) and/or (imipramine) an inhibitor of the recapture of serotonin and noradrenaline (5 and 10 mg/kg) were given alone and then coadministered with the aqueous macerate of G. dalenii (7.5 mg/kg).
Animals
Adult male mice, Mus musculus Swiss, 23 ± 2 g and 2 months old, were obtained from the animal laboratory of the University of Ngaoundéré. Animals were housed in standard cages at 25°C, in a 12 h light cycle with food and water freely available. Animals were transported from the housing room to the testing area in their own cages and allowed to adapt to the new environment for 72 hours before testing. For the test, the animals were divided into 6, 8, 9, 10 and 12 groups with 6 animals per group depending on the test. The study was conducted in accordance with the Cameroon National Ethics Committee (Reg. No. FWA-IRB00001954) and in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimise animal suffering and to reduce the number of animals used in the experiments.
Qualitative phytochemical screening test
The tests were done to find the presence of the active chemical constituents such as alkaloids, flavonoids, saponins, anthraquinons, polyphenols and tannin by the methods described in Odebeyi & Sofowora and Jigna et al.(Odebiyi & Sofowora, 1978; Jigna et al., 2007).
Alkaloids
To the 2 ml of aqueous macerate of G. dalenii, 10 ml of 2% H2SO4 was added. The solution was mixed for 2 min and filtered. Next, 5 drops of Mayors reagents were added to 1 ml of the previous solution. Formation of white precipitate indicated the presence of alkaloids (Odebiyi & Sofowora, 1978).
Flavonoids
To 2 ml of the aqueous macerate of G. dalenii, a few drops of concentrated HCl were added followed by 0.5 g of zinc or magnesium turnings. After 3 minutes, a colour change to magenta red or pink indicated the presence of flavonoids (Jigna et al., 2007).
Saponins
Aqueous macerate of G. dalenii 2 ml was subjected to a frothing test. Briefly, a test tube containing 2ml of aqueous macerate of G. dalenii was shaken vigorously for 1–2 minutes. The presence of saponins was indicated by a characteristic honeycomb froth forming ≥1 cm in height, which persisted for 30 min (Odebiyi & Sofowora, 1978).
Tanins
To 0.5 ml of aqueous macerate of G. dalenii, 5 ml of 1.5% DMSO was added. The solution was warmed in a water bath at 70°C for 3 min and filtered. To 3 ml of the filtrate, 2 ml of 3% ferric chloride solution was added. Blue colour was observed for gallic tannins and green black for catecholic tannins (Odebiyi & Sofowora, 1978).
PolyPhenols
To 2 ml of aqueous macerate of G. dalenii, 5 ml of 1.5% DMSO was added and warmed in a water bath at 37–40°C for 15 min. The solution was filtered and 3 drops of 5% KF3(CN)6 were added to the filtrate. Purple colour indicated the presence of polyphenols (Odebiyi & Sofowora, 1978).
Anthraquinones
To 2 ml of aqueous macerate of G. dalenii, 5 ml of ether was added. Next, 5 ml of ammonia was added and the mixture was shaken vigorously. Red or purple coloration indicated the presence of anthraquinones (Odebiyi & Sofowora, 1978).
Forced swimming test (FST)
The FST is the most widely used pharmacological assay for assessing antidepressant activity. The test was carried out as described in Herrera-Ruiz et al. (2006). Mice were placed individually into glass cylinders (height 25 cm, diameter 10 cm) containing 15 cm of water at 23 — 25°C. Mice were left in the cylinder for 15 min to acclimatise and then removed. 24 h later, mice were placed in the cylinder for 6 min. The total duration of immobility was timed during the last 4 min of the 6 min test. The animal was judged to be immobile when it remained floating passively in the water, making only those movements necessary to keep its head above water.
Tail suspension test (TST)
The TST was performed as described by Steru et al. (1985). One hour following administration of G. dalenii, mice were suspended by the tail on the edge of a shelf, 58 cm above a table top using an adhesive tape placed approximately 1 cm from the tip of the tail. The animal was suspended for a period of 6 min, and the duration of immobility was scored during the last 4 min of the 6 min test period. Mice were considered immobile only when they hung passively and were completely motionless (Steru et al., 1985).
Exploratory activity in the open-field test (OFT)
The spontaneous locomotor and exploratory activities were assessed in an OFT as described previously by Rodrigues et al. (1996). The apparatus consisted of a wooden square box (40 cm × 40 cm × 45 cm), with lines dividing the floor into 16 smaller squares of equal dimensions (10 cm × 10 cm). Each animal was placed individually at the centre of the apparatus and observed for 5 min to record the locomotor (number of segments crossed with all four paws) and exploratory activities (number of times rearing on the hind limbs) (Ngo Bum et al., 2009).
Statistical analysis
All data are presented as mean ± SEM per group. Comparisons between experimental and control groups were performed by one-way analysis of variance (ANOVA) followed by Newman Keuls test for post-hoc comparison when appropriate. P<0.05 was considered statistically significant.
Results
Phytochemical characterisation
The results of phytochemical tests indicate the presence of alkaloids, flavonoids, saponins, tannins, polyphenols, and the absence of phlobotanins and anthraquinons in G. dalenii (data not shown).
Effect of GD in the FST
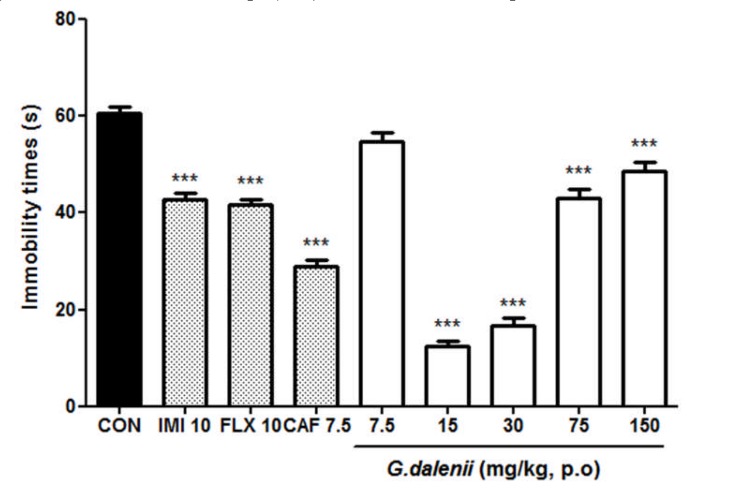
The administration of GD (15, 30 mg/kg) resulted in significant reduction of immobility time by 80 and 73% respectively relative to the untreated control [F (7, 39) = 160.820, P< 0.001] (Fig.1). The antidepressants, imipramine (10 mg/kg) and fluoxetine (10 mg/kg), showed a reduction of 29% [F (1, 9) = 83. 273, P<0.001] and 31% [F (1, 9) = 116.015, P<0.001] respectively. The caffeine (7.5 mg/kg) which is a psychostimulant compound showed a significant reduction of 52 % [F (1, 9) = 318. 594, P<0.001].
Figure 1.
Effect of the aqueous macerate of G. dalenii on immobility time in FST in mice The aqueous macerate of G. dalenii was administered 24, 6 and 1 h before the test. Note the significant decreases in immobility time following administration of the extract (15, 30 mg/kg) and of the antidepressants IMI and FLX. The values are expressed as means ± SEM (n = 6 mice per group. ***p<0.001 compare to the negative control group (one way ANOVA followed by Newman-Keuls test). IMI: imipramine; FLX: fluoxetine; CAF: caffeine
Effect of the aqueous macerate of G. dalenii in the TST
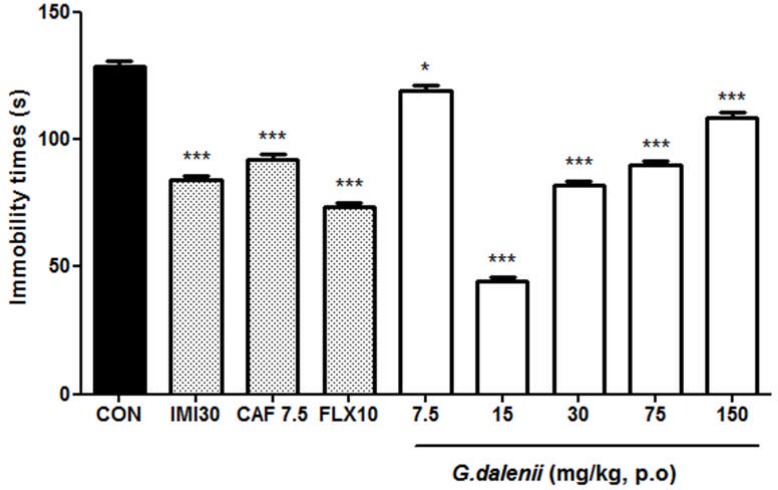
All doses of the aqueous macerate of G. dalenii produced a significant reduction in immobility time. The aqueous macerate of G. dalenii (15 and 30 mg/kg) strongly decreased the immobility time by 66%, 36%, respectively [F (7, 39) = 260. 266, P < 0.0001]. Whereas imipramine (30 mg/kg) and fluoxetine (10 mg/kg) correspondingly resulted in a reduction of 35 [F (1, 9) = 238.964, P<0.001] and 29% [F (1, 9) = 388.721, P<0.001] respectively (Fig. 2). Caffeine (7.5 mg/kg) also significantly reduced the immobility time by 48% [F (1, 9) = 144. 263, P<0.001].
Figure 2.
Effect of the aqueous macerate of G. dalenii on immobility time in TST in mice The aqueous macerate of G. dalenii was administered 24, 6 and 1 h before the test. Note the significant decreases in immobility time following administration of the extract (15, 30 mg/kg) and of the antidepressants IMI and FLX. The values represent means ± SEM (n= 6 mice per group).*p<0.05, ***p<0.001 compare to the negative control group (one way ANOVA followed by Newman-Keuls test). IMI: imipramine; FLX: fluoxetine; CAF: caffeine.
Effect of co-administration of the aqueous macerate of G. dalenii with imipramine in the FST
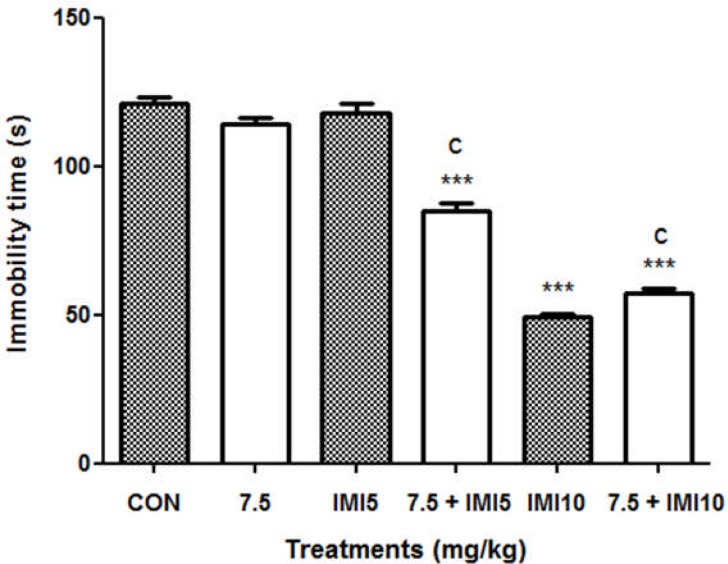
Administration of mice with the aqueous macerate of G. dalenii (7.5 mg/kg) or imipramine (5 mg/kg) alone had no effect on immobility time [F (1, 9) = 2.471, P = 0.150 and F (1, 9) = 0.078, P= 0.796, respectively]. However, the aqueous macerate of G. dalenii (7.5 mg/kg) combined with imipramine (5 mg/kg) significantly reduced the negative control immobility time in FST by 30% (Fig. 3). Imipramine (10 mg/kg) had significantly reduced the immobility time in mice by 53% relative to the negative control [F (1, 9) = 806.993, P< 0.001]. This effect was also observed following co-administration of the aqueous macerate of G. dalenii (7.5 mg/kg) with imipramine (5mg/kg) [F (1, 9) = 586.696, P<0.001].
Figure 3.
Effect of co-treatment of the aqueous macerate of G. dalenii with imipramine on immobility time in FST in mice
The aqueous macerate of the aqueous macerate of G. dalenii (7.5 mg/kg) and, IMI (5 and 10 mg/kg) were administered 24, 6 and 1 h before the test. Note the significant decreases in immobility time following co-administration of the extract (7.5 mg/kg) with IMI (5 mg/kg). The values represent means ± SEM (n= 6 mice per group). ***p<0.001 when compared to the negative control group and cp<0.001 as compared to the aqueous macerate of G. dalenii (7.5 mg/kg) (one way ANOVA followed by Newman-Keuls test). IMI: imipramine.
Effect of co-administration of the aqueous macerate of G. dalenii with fluoxetine in the FST
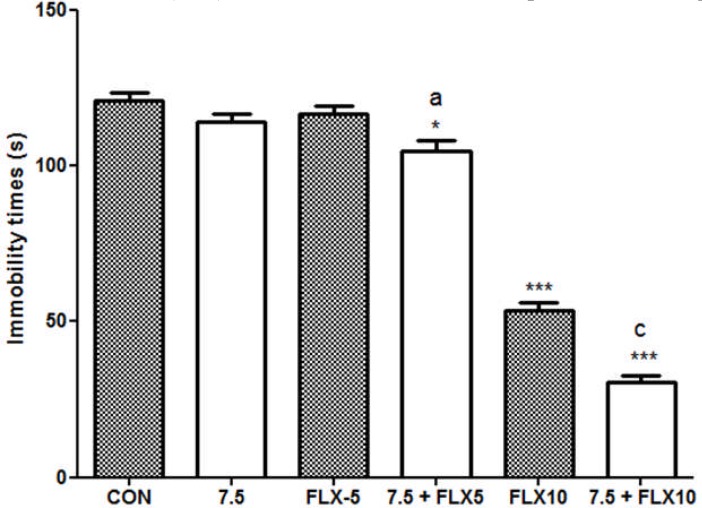
Administration of mice with the aqueous macerate of G. dalenii (7.5 mg/kg) [F (1, 9) = 2.471, P = 0.150] or fluoxetine (5 kmg/kg) [F (1, 9) = 0.658, P = 0.438] alone had no effect on mouse immobility time during the FST. The co-administration of the aqueous macerate of G. dalenii (7.5 mg/kg) and fluoxetine (5 mg/kg) significantly reduced by 13 % the negative control immobility time in the FST (Fig. 4). Likewise, mouse immobility time decreased from 56% after treatment with fluoxetine (10 mg/kg) alone [F (1, 9) = 366.688, P<0.0001] to 75% following co-treatment with the aqueous macerate of G. dalenii [F (1, 9) = 826. 535, P<0.001] compared to the negative control.
Figure 4.
Effect of co-treatment of the aqueous macerate of G. dalenii with fluoxetine on immobility time in FST in mice
The aqueous macerate of G.dalenii (7.5 mg/kg) and, fluoxetine (5 and 10 mg/kg) were administered 24, 6 and 1 h before the test. Note the significant decreases in immobility time following co-administration of the extract (7.5 mg/kg) with fluoxetine (5 mg/kg). The values represent means ± SEM (n= 6 mice per group). ***p<0.001 when compared to the negative control group and ap<0.05; cp<0.001 as compared to the aqueous macerate of G. dalenii (7.5 mg/kg) (one way ANOVA followed by Newman-Keuls test). FLX: fluoxetine.
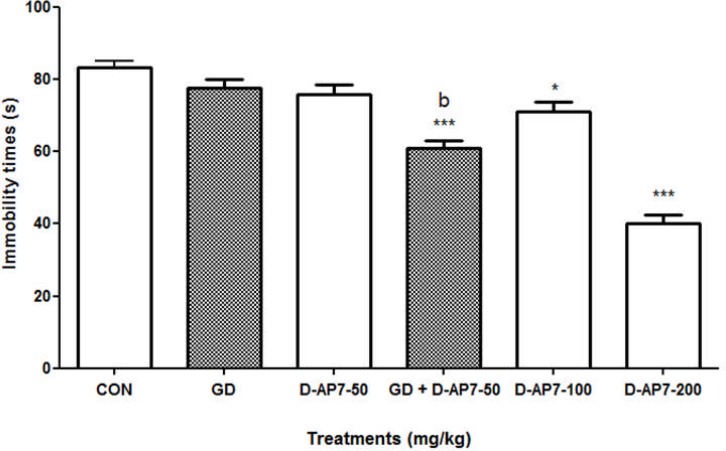
Effect of the administration of D-AP7 and the co-administration of the aqueous macerate of G. dalenii in presence of D-AP7 in the FST
Mouse immobility time did not change following treatment with the aqueous macerate of G. dalenii (7.5 mg/kg) [F (1, 9) = 2.334, P = 0.181] or D-AP7 (50 mg/kg) alone [F (1, 9) = 3.984, P = 0.77] in the FST (Fig. 5). In contrast, there was a 27% reduction in mouse immobility time after co-administration of the aqueous macerate of G. dalenii (7.5 mg/kg) and D-AP7 (50 mg/kg) [F (1, 9) = 63.84, P< 0.001] compared to the negative control. D-AP7 administered alone only produced significant effects in mice at the doses of 100 and 200 mg/kg, which reduced the immobility time by 13% and 51% [F (1, 9) = 7.483, P< 0.023 and F (1, 9) = 162.44, P<0.001, respectively] compared to the negative control.
Figure 5.
Effect of the administration of D-AP7 and the co-administration of the aqueous macerate of G. dalenii in presence of D-AP7 in the FST The aqueous macerate of G. dalenii (7.5 mg/kg) and D-AP7 (50, 100 and 200 mg/kg) was administered 24, 6 and 1 h before the test. Note the significant decreases in immobility time following co-administration of the extract (7.5 mg/kg) with D-AP7 (50 mg/kg). The values represent means ± SEM (n= 6 mice per group).*p< 0.05; ***p<0.001 compared to the negative control group and bp<0.01 as compared to the aqueous macerate of G. dalenii (7.5 mg/kg) (one way ANOVA followed by Newman-Keuls test). GD: the aqueous macerate of G. dalenii.
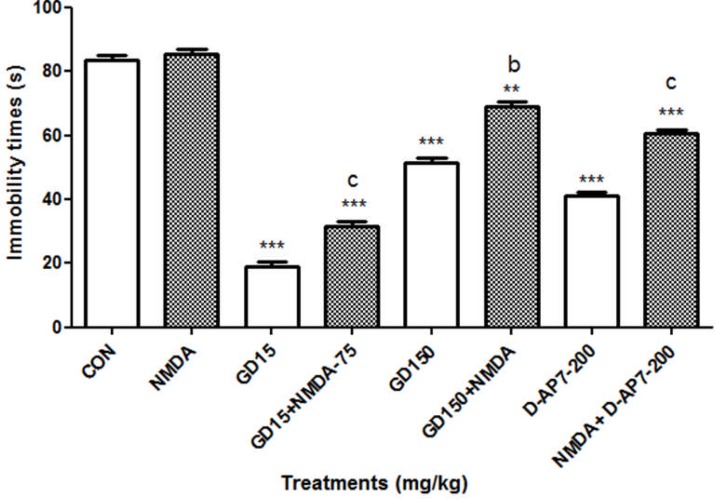
Effect of the aqueous macerate of G. dalenii and D-AP7 in presence of the NMDA on immobility time in FST
D-AP7 (200 mg/kg) (a competitive NMDA receptor antagonist) and the aqueous macerate of G. dalenii (15 mg/kg) significantly decreased the immobility time of mice by 45 and 77% (P< 0.001) respectively in the FST compared to the negative control. In contrast NMDA (75 mg/kg) (specific agonist of the NMDA receptor) had no effect on immobility time in mice when it was administered alone [F (1, 9) = 3.202, P = 0.107] (Fig. 6.). However, the effect of NMDA was antagonised by 200 mg/kg of D-AP7 as indicated by a 30 % reduction in immobility time [F (1, 9) = 118.391, P< 0.001] relative to NMDA group. A similar antagonism was observed when NMDA (75 mg/kg) was co-administered with the aqueous macerate of G. dalenii (15 mg/kg), which significantly antagonised the effect of the NMDA by 62% [F (1, 9) = 364.594, P< 0.001] in FST.
Figure 6.
Effect of the aqueous macerate of G. dalenii and D-AP7 in presence of the NMDA on immobility time in FST
The aqueous macerate of G. dalenii (15 and 150 mg/kg), D-AP7 (200 mg/kg) and NMDA (75 mg/kg) were administered 24, 6 and 1 h before the test. Note the significant decreases in immobility time following co-administration of the aqueous macerate of G. dalenii (15 and 150 mg/kg) or D-AP7 (200 mg/kg) with NMDA (75 mg/kg). The values represent means ± SEM (n= 6 mice per group). ***p<0.001 compare to the negative control group and c p<0.001 compare to NMDA (75 mg/kg) (one way ANOVA followed by Newman-Keuls test). GD: the aqueous macerate of G. dalenii.
Effect of the aqueous macerate of G. dalenii, imipramine, fluoxetine, and NMDA ligands on mouse (open field test)
The aqueous macerate of G. dalenii did not enhance the spontaneous locomotor (crossing) and exploratory (rearing) activities at doses which had strongly decreased the immobility time. Administration of mice with diazepam (1 mg/kg) or imipramine (10 mg/kg) also had no affect on either spontaneous locomotor (crossing) or exploratory (rearing) activities. However, administration of mice with diazepam (3 mg/kg), imipramine (30 mg/kg), fluoxetine (10 mg/kg) or D-AP7 (100 and 200 mg/kg) decreased the spontaneous locomotor activity relative to the negative control. Mice administered with caffeine (7.5 mg/kg) had significantly increased spontaneous locomotor activity [F (1, 9) = 53.450, P< 0.001] relative to the negative control (Table 1). Neither the administration of the aqueous macerate of G. dalenii alone nor the administer of the aqueous macerate of G. dalenii together either with fluoxetine or imipramine significantly enhanced the spontaneous locomotor activity in mice relative to the negative control (Table 2). Table 3 showed that neither treatment of mice with D-AP7 alone nor D-AP7 co-administered with the aqueous macerate of G. dalenii had any effect on the spontaneous locomotor activity in mice. Table 4 showed that NMDA treatment did not affect the spontaneous locomotor activity in mice, even when it was co-administered with D-AP7 or the aqueous macerate of G. dalenii.
Table 1.
Effect of administration of the aqueous macerate of G. dalenii on spontaneous locomotor activity in mice assessed on open field test
| Treatments | Doses (mg/kg) | Crossings | Rearings |
| CON | - | 78.6 ± 3.1 | 18.3 ± 2.3 |
| Imipramine | 10 | 75.5 ± 2.8 | 17.5 ± 1.5 |
| Imipramine | 30 | 59 ± 3*** | 13.5 ± 2.2* |
| Fluoxetine | 10 | 63.5 ± 2.3*** | 19.3 ± 1.7 |
| Cafeine | 7.5 | 103.8 ± 3.2*** | 23.6 ± 3* |
| Diazepam | 1 | 86.5 ± 2.5 | 17 ± 2.2 |
| Diazepam | 3 | 66.5 ± 3.3*** | 15.1 ± 1.7 |
| The aqueous macerate of G. dalenii | 7.5 | 81.1 ± 3.2 | 17.6 ± 2.1 |
| The aqueous macerate of G. dalenii | 15 | 84.1 ± 3.2 | 21.3 ± 2 |
| The aqueous macerate of G. dalenii | 30 | 79.3± 3 | 18.8 ± 3.2 |
| The aqueous macerate of G. dalenii | 75 | 68.6 ± 3** | 15.6 ± 2 |
| The aqueous macerate of G. dalenii | 150 | 27.1 ± 2.6*** | 7.8 ± 1.6*** |
The aqueous macerate of G. dalenii, fluoxetine, imipramine, caffeine, and diazepam were administrated 24, 6 and 1 hour before the test. The values represent means ± SEM (n= 6 mice per group).
p<0.05;
p<0.01;
p<0.001 compare to the negative control group (one way ANOVA followed by Newman-Keuls test).
Table 2.
Effect of administration of the aqueous macerate of G. dalenii, imipramine, and fluoxetine on spontaneous locomotor activity in mice assessed on open field test
| Treatments | Doses (mg/kg) | Crossings | Rearings |
| CON | - | 100.3 ± 2.3 | 27.3 ± 5.3 |
| The aqueous macerate of G. dalenii | 7.5 | 80.6 ± 2.8*** | 17.1 ± 3.2* |
| Imipramine | 5 | 79.6 ± 2.2** | 13.8 ± 3.2*** |
| Imipramine | 10 | 68.3 ± 2.5*** | 7 ± 2.7*** |
| Fluoxetine | 5 | 74.1 ± 3.2*** | 12.6 ± 3.2*** |
| Fluoxetine | 10 | 61.6 ± 2.7*** | 20.8 ± 3.2 |
| The aqueous macerate of G. dalenii + imipramine | 7.5 + 5 | 90.8± 2.8 | 21.6 ± 3.7 |
| The aqueous macerate of G. dalenii + imipramine | 7.5 + 10 | 72.1 ± 2.6*** | 17.5 ± 2.3* |
| The aqueous macerate of G. dalenii + fluoxetine | 7.5 + 5 | 82.6 ± 2.4** | 8 ± 2*** |
| The aqueous macerate of G. dalenii + fluoxetine | 7.5 + 10 | 69.3 ± 2.4*** | 17.8 ± 1.2*** |
The aqueous macerate of G. dalenii, imipramine and fluoxetine were administrated 24, 6 and 1 hour before the test. The values represent means ± SEM (n= 6 mice per group).
p<0.05;
p<0.01;
p<0.001 compare to the negative control group (one way ANOVA followed by Newman-Keuls test).
Table 3.
Effect of administration of the aqueous macerate of G. dalenii, and D-AP7 on spontaneous locomotor activity in mice assessed on open field test
| Treatments | Doses (mg/kg) |
Crossings | Rearings |
| CON | - | 115.3 ± 2.8 | 39.3 ± 2 |
| D-AP7 | 50 | 111 ± 5 | 27.3 ± 1.9*** |
| The aqueous macerate of G. dalenii | 7.5 | 93.3 ± 3.3*** | 16.6 ± 2.2*** |
| The aqueous macerate of G. dalenii + D-AP7 | 7.5 + 50 | 105.3 ± 3.7** | 26 ± 3*** |
| D-AP7 | 100 | 87.3 ± 3.1*** | 24 ± 2.7*** |
| D-AP7 | 200 | 84.6 ± 3.4*** | 20.8 ± 2.1*** |
The aqueous macerate of G. dalenii and D-AP7 were administrated 24, 6 and 1 hour before the test. The values represent means ± SEM (n= 6 mice per group).
p<0.01;
p<0.001 compare to the negative control group (one way ANOVA followed by Newman-Keuls test).
Table 4.
Effect of administration of the aqueous macerate of G. dalenii, NMDA and D-AP7 on spontaneous locomotor activity in mice assessed on open field test
| Treatments | Doses (mg/kg) | Crossings | Rearings |
| CON | - | 117.1± 3.6 | 37.8 ± 1.8 |
| NMDA | 75 | 129 ± 3.3 | 32.6 ± 4.6 |
| D-AP7 | 200 | 83.8 ± 2.9*** | 20.8 ± 1.8*** |
| D-AP7 + NMDA | 200 + 75 | 111.1 ± 2.6 | 28.1 ± 1.9*** |
| The aqueous macerate of G. dalenii | 15 | 97.1 ± 2.5*** | 21.3 ± 2*** |
| The aqueous macerate of G. dalenii + NMDA | 15 + 75 | 113 ± 2.7 | 27.3 ± 1.1 |
| The aqueous macerate of G. dalenii | 150 | 58.5.1 ± 2.1*** | 16 ± 2*** |
| The aqueous macerate of G. dalenii + NMDA | 150 + 75 | 111 ± 2.1 | 29.3 ± 1.9 |
The aqueous macerate of G. dalenii, NMDA and D-AP7 were administered 24, 5 and 1 hour before the test. The values represent means ± SEM (n= 6 mice per group);
p<0.001 compare to the negative control group (one way ANOVA followed by Newman-Keuls test).
Discussion
Our results indicate the potential antidepressant properties of the aqueous macerate of G. dalenii. Administration of mice with the aqueous macerate of G. dalenii significantly reduced the immobility time of mice in both the FST and the TST relative to the negative control. This immobility, referred to as behavioral despair in animals, is believed to reproduce a condition similar to human depression (Steru et al., 1985). Similar finding have been reported after treatment of mice with classical antidepressant drugs such asimipramine andfluoxetine in the FST and TST (Porsolt et al., 1977; Cryan et al., 2005; Borsini and Meli, 1988). The role of the aqueous macerate of G. dalenii in the FST and TST is significant and could indicate the antidepressant properties of the aqueous macerate of G. dalenii (Porsolt et al., 1977; Borsini and Meli, 1988). During the TST, mice are suspended by their tail, struggle and then become immobile. The time taken for mice to become immobile is indicative of depression (Steru et al., 1985).
The aqueous macerate of G. dalenii also showed a stronger antidepressant-like effect than IMI (10 and 30 mg/kg) and fluoxetine (10 mg/kg) in mice during the FST. In addition, the aqueous macerate of G. dalenii showed a similar effect on mice in the TST at a lower dose than in the FST. In the FST, mice forced to swim in a narrow space with no escape will stop swimming after an initial period of vigorous activity and move only when necessary to keep their heads above water. Immobility time is considered to be a measure of depression-like behaviors in mice, as the mice have stopped swimming and given up on finding an escape route (Crawley et al., 1997 Woode et al., 2009). The FST is generally considered to be useful for detecting acute antidepressant effects of SSRI and other type of antidepressants including tricyclics, monoamine oxidase inhibitors, and atypicals (Porsolt et al., 1977; Steru et al., 1985). Moreover it has predictive validity for detecting SSRI effects. Indeed, Yu et al., 2002 and Szewczyk et al., 2009 showed that fluoxetine, an SSRI, significantly reduced the immobility in mice during the FST after acute and chronic administration. So the fact that the aqueous macerate of G. dalenii have showed a stronger antidepressant-like effect than imipramine (10 and 30 mg/kg) and fluoxetine (10 mg/kg) in mice during the FST, showed that the aqueous macerate of G. dalenii may have an antidepressant-like effect.
TST and FST are commonly used techniques to measure depression in mice and to screen potential antidepressant drugs. Many antidepressant drug families have been assessed using the TST and FST, including tricyclics, serotonin-specific reuptake inhibitor, monoamine oxidase inhibitors, and atypicals (Porsolt et al., 1977; Steru et al., 1985). Several reports have demonstrated that increased mobility time in mice during the FST and TST correlates with increased clinical effects of antidepressant drugs (Willer, 1984; Rosa et al., 2003). In the data presented here, mice treated with the aqueous macerate of G. dalenii performed significantly better compared to the negative control in both the TST and FST. These results strongly indicate the potential antidepressant properties of the aqueous macerate of G. dalenii.
However, FST and TST have the limitation of, on occasion, producing false positive or negative results. In some studies, drugs known to enhance locomotor activity were found to produce a false positive effect in the FST and TST, whereas drugs that decreased locomotor activity could produce a false negative outcome (Borsini, & Meli, 1988). To overcome the limitations associated with the FST and TST, we investigated the effect of the aqueous macerate of G. dalenii treatment in mice in Open Field Test (OFT). The OFT is a classic technique used to evaluate locomotor activity and willingness to explore in rodents (Novas et al., 1988; Sousa et al., 2004).
Our data showed that the antidepressant-like effect of the aqueous macerate of G. dalenii was not due to psychostimulant action, as no alteration in the locomotor activity was observed in mice during the OFT given low doses of the aqueous macerate of G. dalenii (that showed strong antidepressant-like effect) or diazepam (1mg/kg). However, the pyschostimulant cafeine strongly reduced the immobility time of mice during the FST, while significantly increasing the spontaneous locomotor activity during the OFT, showing the difference between antidepressant drugs and psychostimulant drugs. On this basis we can rule out the possibility of a false positive antidepressant-like effect of the aqueous macerate of G. dalenii in FST and TST.
In this study an attempt was also made to investigate the mechanism of the potential antidepressant action of the aqueous macerate of G. dalenii. Several lines of evidence in human and animal studies indicate that glutamate homeostasis and glutamate neurotransmission are deregulated in depressive disorders (Hansen et al., 1983; Kugaya & Sanacora, 2005; Wu, 2009). Among glutamate receptors, the contribution of NMDA receptors to depression and treatment with antidepressants are particularly well documented (Nowak et al., 1995; Szewczyk et al., 2009). Both pre-clinical and clinical studies have shown that compounds that reduce the transmission of NMDA receptors exert antidepressant-like effects (Skolnick, 1999). In 1990, Trullas and Skolnick demonstrated the antidepressant-like activity of D-AP7 in mice in the FST and TST. Other studies have both confirmed and extended this initial result (Maj et al., 1992; Skolnick et al., 1996; Przegalinsky et al., 1997). We found that both the aqueous macerate of G. dalenii (15 and 150 mg/kg) and D-AP7 (100 and 200 mg/kg) significantly decreased the immobility time of mice during the FST. The mechanism behind this finding is not fully understood. However it has been hypothesized that the aqueous macerate of G. dalenii acts as an NMDA receptor antagonist (Maj et al., 1992a). Our results support this hypothesis in that the aqueous macerate of G. dalenii (15 and 150 mg/kg) significantly inhibited the immobility time of mice induced by the NMDA (75 mg/kg) when the two drugs were used concomitantly. In addition, the aqueous macerate of G. dalenii was twice as effective as D-AP7 (200 mg/kg) to reduce the immobility time. Moreover, neither the aqueous macerate of G. dalenii (7.5 mg/kg), nor D-AP7 (50 mg/kg) reduced the immobility time of mice during the FST when they were given alone. The co-administration of 50 mg/kg D-AP7 with a subeffective dose of the aqueous macerate of G. dalenii was more effective in reducing the immobility time in mice during the FST than D-AP7 (100 mg/kg) administered alone. Thus the combined treatment of mice with the aqueous macerate of G. dalenii and NMDA receptor antagonists produced a synergistic antidepressant-like effect in the FST. These data suggest that the effect of the aqueous macerate of G. dalenii could be mediated through the interaction with the NMDA system.
The co-administration of a subeffective dose of the aqueous macerate of G. dalenii with increasing doses of antidepressant drugs (5 and 10 mg/kg) such as imipramine (serotonin/noradrenaline uptake inhibitor) and fluoxetine (selective serotonin reuptake inhibitors) resulted in synergistic effect. These data suggest that the effect of the aqueous macerate of G. dalenii could be also mediated through the interaction of the aqueous macerate of G. dalenii with serotoninergic/ noradrenergic systems or NMDA system, as previously demonstrated (Trullas& Skolnick, 1990; Nowak et al., 1995; Rogoz et al., 2002; Poleszak et al., 2005).
The phytochemical characterization of the aqueous macerate of G. dalenii in this study revealed the presence of flavonoids, saponins, tannins and polyphenols. This finding, coupled with recent studies showing that extract containing flavonoids have antidepressant activity in mice in both the FST and TST (Zhang, 2004; Sakakibara et al., 2006), suggests that the antidepressant effects of the aqueous macerate of G. dalenii might be related to the presence of flavonoids.
In conclusion, the results of this study indicate that the aqueous macerate of G. dalenii possesses antidepressant-like effects in rodent models of depression. This finding is significant and may justify the use of G. dalenii, in traditional medicine. The effect of the aqueous macerate of G. dalenii treatment on mice appeared to be stronger than common antidepressants imipramine and fluoxetine, as well as D-AP7. Our results also indicate that the effect of the aqueous macerate of G. dalenii in FST involves NMDA, serotoninergic and/or noradrenergic systems. However, further studies are needed to confirm and extend these results.
Acknowledgements
The financial support by the University of Ngaoundéré and Pr Sharon Juliano are gratefully acknowledged.
References
- 1.Andlin-Sobocki P, Wittchen HU. Cost of affective disorders in Europe. Eur J Neurol. 2005;12:34–38. doi: 10.1111/j.1468-1331.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 2.Bandeira SO, Gaspar F, Pagula FP. Ethnobotany and Healthcare in Mozambique. Pharm Biol. 2001;39:70–73. doi: 10.1076/phbi.39.s1.70.0002. [DOI] [PubMed] [Google Scholar]
- 3.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacol. 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 4.Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, De Girolamo G, De Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lépine J-P, Levinson D, Matschinger H, Mora M EM, Browne MO, Posada-Villa J, Viana MC, Williams DR, Kessler RC. Cross-national epidemiology of DSM-IV major depressive episode. BMC Medicine. 2011;1741:9–90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkill HM. The Flora of West Tropical Africa. 2nd Edn. Vol. 4. Royal Botanic Gardens, London: Kew; 1985. [Google Scholar]
- 6.Crawley JN, Belknap JK, Collins A, Crabble JC, Frankel W, Henderson N, Hitzermann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacol. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 7.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Hansen CR, Jr, Malecha M, Mackenzie TB, Kroll J. Copper and zinc deficiencies in association with depression and neurological findings. Biol psychiatry. 1983;18:395–401. [PubMed] [Google Scholar]
- 9.Herrera-Ruiz M, Jimenez-Ferrer JE, Lima TCM, Aviles-Montes D, Perez-Garcia D, Gonzalez-Cortazar M, Tortoriello J. Anxiolytic and antidepressant-like activity of a standardized extract from Galphimia glauca. Phytomed. 2006;13:23–28. doi: 10.1016/j.phymed.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Hutchings A, Van Staden J. Plant used for stress-related ailments in traditional Zulu, Xhosa and Sotho medicine. Part 1 Plants used for headaches. J Ethnopharmacol. 1994;43:89–124. doi: 10.1016/0378-8741(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 11.Jigna P, Sumitra VC. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plant. Turk J Biol. 2007;31:53–58. [Google Scholar]
- 12.Kugaya A, Sanacora G. Beyond monoamines: Glutamatergic function in mood disorders. CNS Spectrum. 2005;10:808–819. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- 13.Lépine J-P, Briley M. The increasing burden of depression. Neuropsych Dis Treat. 2011;7:3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maj J, Rogoz Z, Skuza G, Sowinska H. The effect of CGP37849 and CGP39551, competitive NMDA receptor antagonists, in the forced swimming test. Pol J Pharmacol. 1992;44:337–346. [PubMed] [Google Scholar]
- 15.Ngo Bum E, Taiwe GS, Moto FCO, Ngoupaye GT, Nkantchoua GCN, Pelanken MM, Rakotonirina SV, Rakotonirina A. Anticonvulsant, anxiolytic, and sedative properties of the roots of Nauclea latifolia Smith in mice. Epilep Behav. 2009;15:434–440. doi: 10.1016/j.yebeh.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Novas M, Wolfman C, Medina JH, De Robertis E. Proconvulsant and anxiogenic effects of n-butyl-β-carboline-3-carboxylate, an endogenous benzodiazepine binding inhibitor from brain. Pharmacol Biochem Behav. 1988;30:331–336. doi: 10.1016/0091-3057(88)90463-7. [DOI] [PubMed] [Google Scholar]
- 17.Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptors complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 18.Odebiyi OO, Sofowora EA. Phytochemical screening of Nigerian medicinal plants 2. L loydia. 1978;41:234–235. [PubMed] [Google Scholar]
- 19.Odhiambo J, Sibo G, Lukhoba C, Dossaji S. Antifungal activity of crude extracts of Gladiolus dalenii Van Geel (Iridaceae) AJTCAM. 2010;7:0189–6016. doi: 10.4314/ajtcam.v7i1.57254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Inter Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 21.Poleszak E, Wlaz P, Szewczyk B, Kedzierska E, Wyska E, Librowski T, Szymura-Oleksiak J, Fidecka S, Pilc A, Nowak G. Enhancement of antidepressant-like activity by joint administration of imipramine and magnesium in the forced swim test: Behavioural and pharmacokinetic studies in mice. Pharmacol Biochem Behav. 2005;81:524–529. doi: 10.1016/j.pbb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Przegalinsky E, Tatarczynska A, Deren-Wesolek, Chojnacka-Wojcik Antidepressant-like effects of a partial agonist at strychnine-insensitive glycine receptors and a competitive NMDA receptor antagonist. Neuropharmacol. 1997;36:31–37. doi: 10.1016/s0028-3908(96)00157-8. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues ALS, Rocha JBT, Mello CF, Sousa DO. Effect of perinatal lead exposure on rat behaviour in open field and two-way avoidance tasks. Pharmacol Toxicol. 1996;79:150–156. doi: 10.1111/j.1600-0773.1996.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 24.Rogoz Z, Skuza G, Maj J, Danysz W. Synergistic effect of uncompetitive NMDA receptors and antidepressant drugs in the forced swimming test in rats. Neuropharmacol. 2002;42:1024–1030. doi: 10.1016/s0028-3908(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 25.Rosa AO, Lin J, Calixto JB, Santos AR, Rodrigues AL. Involvement of NMDA receptors and L-arginine-nitric oxide pathway in the antidepressant-like effects of zinc in mice. Behav Brain Res. 2003;144:87–93. doi: 10.1016/s0166-4328(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 26.Sakakibara H, Ishida K, Grundmann O, Nakajima J, Seo S, Butterweck V, Minami Y, Saito S, Kawai Y, Nakaya V, Terao J. Antidepressant effect of extracts from Ginkgo biloba leaves in behavioural models. Biol Pharm Bull. 2006;29:1767–1770. doi: 10.1248/bpb.29.1767. [DOI] [PubMed] [Google Scholar]
- 27.Skolnick P. Antidepressants for the new millennium. Eur J Pharmacol. 1999;375:31–42. doi: 10.1016/s0014-2999(99)00330-1. [DOI] [PubMed] [Google Scholar]
- 28.Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate receptors following antidepressant treatment: Implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 29.Skolnick P, Miller R, Young A, Boje K, Trullas R. Chronic treatment with 1-aminocyclopropanecarboxylic acid desensitizes behavioral responses to compounds acting at the N-methyl-D-aspartate receptor complex. Psychopharmacol. 1991;107:489–496. doi: 10.1007/BF02245261. [DOI] [PubMed] [Google Scholar]
- 30.Sousa FCF, Melo CTV, Monteiro AP, Lima VTM, Gutierrez S JC, Pereira BA, Barbosa-Filho JM, Vasconcelos SMM, Fonteles MF, Viana GSB. Antianxiety and antidepressant effects of Riparin III from Aniba riparia (Nees) Mez (Lauraceae) in mice. Pharmacol Biochem Behav. 2004;78:27–33. doi: 10.1016/j.pbb.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Steru L, Chermat R, Thierry J, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacol. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 32.Szewczyk B, Poleszak E, Wlaz P, Wróbel A, Blicharska E, Cichy A, Dybala M, Siwek A, Pomierny-chamiolo L, Piotrowska A, Branki P, Pilc A, Nowak G. The involvement of serotonergic system in antidepressant effect of zinc in the forced swim test. Prog Neuro-Physchopharmacol Biol Pshychiatry. 2009;33:323–329. doi: 10.1016/j.pnpbp.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization, author. The global burden of disease: 2004 update. 2004. http://www.who.int/entity/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf. 2004.
- 34.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant action. Eur J Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 35.Willer P. The validity of animal models of depression. Psychopharmacol. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- 36.Woode E, Amidu N, Owiredu WKBA, Boakye-Gyasi E, Ansah C, Duwiejua M. Antidepressants like effects of an ethanolic extract of Sphenocentrum jollyanum Pierre roots in mice. Inter J Pharmacol. 2009;5:22–29. [Google Scholar]
- 37.Wu G. Amino acids: Metabolism, function, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 38.Yu ZF, Kong LD, Chen Y. Antidepressant activity of aqueous extracts of Curcuma longa in mice. J Ethnopharmacol. 2002;83:161–165. doi: 10.1016/s0378-8741(02)00211-8. [DOI] [PubMed] [Google Scholar]
- 39.Zhang ZJ. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Scis. 2004;75:1659–1699. doi: 10.1016/j.lfs.2004.04.014. [DOI] [PubMed] [Google Scholar]