Abstract
Background
Leucaena leucocephala is native to Southern Mexico and Northern Central America, but is now naturalized throughout the tropics. The phyto-chemical data of L. leucocephala revealed the presence of terpenes, flavonoids, coumarins and sterols. Various parts of L. leucocephala have been reported to have medicinal properties.
Materials and Methods
Flavonoids were isolated from the aerial parts of L. leucocephala. Antioxidant activity of the extracts and the isolated compounds was evaluated using (DPPH), as well as their cytotoxic activity using a single tumor [Ehrlish ascites carcinoma cells].
Results
The flavonoidal constituents isolated from chloroform, ethyl acetate and n-butanol fractions of the aqueous alcoholic extract of aerial parts of Leucaena leucocephala were identified as Caffeic acid, Isorhamnetin, Chrysoeriol, Isorhamnetin 3-O-galactoside, Kaempferol-3-O-rubinoside, Quercetin-3-O-rhamnoside and Luteolin-7-glucoside. Chemical structures of the isolated compounds were identified by TLC, PC and spectral techniques (UV, 1H-NMR and MS). The ethyl acetate fraction and the isolated flavonoidal compounds showed high antioxidant activity compared to Trolox (standard antioxidant compound). The different fractions and isolated compounds of Leucaena leucocephala exhibited no cytotoxic activity against Ehrlich-ascitis carcinoma cell line at the tested concentrations.
Conclusion
This is the first record of the flavonoids in the aerial parts of Leucaena leucocephala (L.) except Quercetin-3-O-rhamnoside.
Keywords: Leucaena leucocephala., Flavonoids, Antioxidant, cytotoxic activity
Introduction
Leucaena leucocephala is a small, fast-growing mimosoid tree native to Southern Mexico and Northern Central America but is now naturalized throughout the tropics (Hill, 1971).
Leucaena is a genus of about 24 species of leguminous trees and shrubs (Mabberley, 1997). It belongs to the family Mimosaceae. Various parts of L. leucocephala have been reported to have medicinal properties ranging from control of stomach diseases to contraception and abortion and the seed gum has been reported to be useful as a binder in tablet formulation (Deodhar et al., 1998; Verma & Balkishen, 2007). Sulfated glycosylated form of polysaccharides from the seeds was reported to possess significant cancer chemo-preventive and anti-proliferative activities (Gamal-Eldeen et al., 2007). Also, mimosine, an amino acid from the seeds was reported to possess anticancer activity and to inhibit the growth of hair (Chang HC, et al., 1999). Other studies on the extracts of the seeds had shown varying activities including central nervous system depressant, anthelmintic and antidiabetic activities (Ademola et al., 2005; Syamsudin & Partomuan, 2010).
The genus leucaena is reported to contain hydrocynamic acid, leucaenine, epicatechin-3-O-gallate, quercetin-3-O-arabinofuranoside, quercetin-3-O-rhamnoside, apigenin (Aderogba et al., 2010) and tannic acid, also the seeds of L. leucocephala., contain a large amount of galactomannan mucilage located in endosperm (Hylin, & Sawait, 1984). The unsaponifiable, fatty alcohol, fatty acids as well as the volatile oil compounds of the plant were studied and identified (Hassan & Radwan, 2010).
The phytochemical data of L. leucocephala revealed the presence of terpenes, flavonoids, coumarins and sterols.
Recently, much attention has been focused on natural antioxidants. Although many natural antioxidants have been found in numerous plant materials (Dugan, 1979), tocopherols only are now widely used as the safe natural antioxidant. However, they have the limitation that they are not effective as synthetic antioxidants when used alone (El-Hossary et al., 2000), beside their high manufacturing costs. Therefore, search of natural antioxidant from plant sources would have many industrial outcomes.
The present work aims at studying the flavonoid constituents of the aerial parts of Leucaena leucocephala growing in Egypt, also to evaluate their anti-oxidant activity, of extracts and the isolated compounds, as well as evaluation of their cytotoxic activity using a single tumor [Ehrlish ascites carcinoma cells].
Materials and Methods
Plant Material
Leucaena leucocephala was collected from Orman Garden, Giza, Egypt during April 2009. The plant was identified by Dr. Ebrahim Elgarf, Taxonomist, Faculty of Science, Cairo University Egypt, to whom the authors are deeply indebted. The aerial parts of the plant were air dried and ground into fine powder. A voucher specimen was kept in the herbarium of NRC, Cairo.
Anti-oxidant Activity
Reagent and solvent
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, Aldrich Chemical Co.), 1,1 Diphenyl-2-picrylhydrazyl (DPPH, Sigma Chemical Co.) and Methanol HPLC.
Anti-tumor Activity
The anti-tumor activity of the extracts and the isolated compounds were tested against Ehrlich ascites carcinoma in vitro in the National Institute of Cancer, Cairo.
Apparatus and Techniques
The following equipment and techniques were used: Shimadzu UV.Pc. 2401 Spectrophotometer, Mass spectrophotometer Jeol-JMS-Ax 500, Double focusing mass spectrophotometer, The analysis was performed in Pharmaco. Central Lab., National Research Centre. 1H-NMR spectra were recorded in (DMSO-d6) on a Jeol-EX-270 MHz spectrometer (in Cairo University). Preparative, centrifugally, accelerated, radial, thin layer chromatography by using Chromatotron apparatus and Preparative paper chromatography (PPC).
Isolation of Flavonoids (Radwan et al., 1997; Harborne, 1984)
About 950g, of the defatted dried powder aerial parts of L. leucocephala were extracted with 70% ethanol (3x250ml). The combined alcoholic extracts were evaporated in vacuo at 40 °C. The residue was dissolved in hot distilled water (500ml), kept in the refrigerator overnight, filtered, cooled and extracted with successive portions of chloroform (3 x 250ml), followed by ethyl acetate (3x 250 ml) and finally with n-butanol (3 x 250ml). The chloroform fraction (0.9g), was subjected to preparative paper chromatography (3MM, n-butanol: acetic acid: water, 4: 1: 5, upper layer).
The ethyl acetate fraction (2.3g), which contains the main flavonoidal compounds, was subjected to preparative PC (3MM, 15% acetic acid). The main flavonoidal bands (Rf 0.09, 0.13 and 0.53), were cut and eluted separately by 90% methanol. The n-butanol fraction (1.73g), was also subjected to preparative PC (3MM, 15% acetic acid). The main flavonoidal bands (Rf 0.71, 0.56 and 0.31), were cut and eluted separately by 90%, methanol. Also, the eluted fractions were subjected for further purification using Sephadex LH-20 column using 90%, methanol or subjected to centrifugally accelerate rotatory TLC using (silica gel 60 PF254) discs, and elution was carried out with 95% chloroform in methanol.
Determination of scavenging effect on DPPH free radicals
The effect of the plant extracts on DPPH radical was studied employing the modified method described earlier by (Yamaguchi et al 1998). The decrease of the absorbance at 516nm, of the DPPH solution after addition of the sample (plant materials) was measured in a glass cuvette. An aliquot of 0.1ml M. methanol solution of DPPH was mixed with the methanolic solution of the sample, so that the relative concentration of plant materials versus the stable radical in the cuvette was 0.13, then the solution with tested sample was shaken vigorously. The absorbance was measured at the start and at 30 min. after being kept in the dark against a blank of methanol without DPPH. All tests were run in duplicate and averages were calculated (Nicolaos & Maria, 2002). The antioxidant activities of these samples were compared with Trolox.
Where:
% RSA = 100 [Abs of blank (516nm) − Abs of sample (516nm)] / Abs of blank (516 nm)
The results are expressed as radical scavenging activity (% RSA), as shown in (Table 1).
Table 1.
The Radical scavenging effect of samples on DPPH radical
| Tested compounds | *Absorbance 516/reaction period (min) | RSA % | |
| 10 mins | 20 mins | ||
| Trolox | 0.025 | 0.026 | 95.06 |
| Compound 1 | 0.052 | 0.057 | 89.43 |
| Compound 2 | 0.042 | 0.044 | 86.33 |
| Compound 3 | 0.056 | 0.057 | 89.0 |
| Compound 4 | 0.049 | 0.052 | 84.69 |
| Compound 5 | 0.061 | 0.064 | 87.88 |
| Compound 6 | 0.068 | 0.071 | 86.53 |
| Compound 7 | 0.048 | 0.052 | 90.31 |
| Ethyl acetate extract | 0.061 | 0.068 | 86.78 |
| Chloroform ext. | 0.75 | 0.78 | 85.29 |
| Butanol extract | 0.075 | 0.081 | 84.18 |
The absorbance reading at each reaction period is means of two measurements
Antitumor activity (MCLimans et al., 1957)
The anti-tumor activity of the extracts and the compounds isolated from both the aerial parts of the plant and from the seeds were tested against Ehrlich ascites carcinoma in vitro in the National Institute of Cancer, Cairo, Egypt.
Preparation of samples for cytotoxic study
One mg of each extract (70% ethanol, chloroform, ethyl acetate and n-butanol), of aerial parts of Leucaena leucocephala plant were dissolved separately in 0.1ml, of dimethyl sulfoxide (DMSO), and the volume completed to 1 ml with distilled water.
Screening for cytotoxic activity (MCLimans et al., 1957)
Both the plant extracts and the tested isolated compounds were screened in vitro using a single tumor (Ehrlich ascites carcinoma cells). The tumor was maintained in the laboratory by weekly intra-peritoneal transplantation in female albino mice from the animal house of Cairo Cancer Institute. A set of sterile test tubes was used for each test solution, where 2.5x106 tumor cells per ml were suspended in phosphate buffer. 0.1ml, of different dilutions of each test solution was added separately to the suspension, kept at 37 °C for 2 hours. Trypan blue dye exclusion test was then carried out to calculate the percentage of non-viable cells. Using a dose of 100µg/ml, 50µg/ml, and 25µg/ml of each extract. Concentrations causing less than 30% non-viable cells in the suspension were considered inactive, while those producing more than 70% non-viable cells were considered active. Results are recorded in (Table 2).
Table 2.
Ultra-violet spectral data (nm) of the isolated flavonoides and their aglycones
| Flavonoides | UV.abs in MeOH |
NaOH | AlCl3 | AlCl3+Hcl | NaOAc | NaOAc+H3BO3 |
| Compound 1 | 325, 291, 245 | 346, 306, 252 | ----- | ----- | ----- | ----- |
| Compound 2 | 365, 269sh, 258 |
420, 331, 270(DEC.) |
420,387, 300sh, 268 |
413sh, 369, 280, 260 |
391(DEC.), 320, 274 |
359, 287sh, 264 |
| Compound 3 | 344, 269, 247sh, 240 |
403, 331 sh, 271sh, 260 |
391, 366 sh, 294, 271, 259 |
383, 353, 290, 274, 255 |
399, 326, 272 | 347, 298 sh, 267 |
| Compound 4 | 351, 306sh 266sh, 255 |
413, 273 | 406, 352, 293, 272 |
399, 345, 294sh, 274 |
382, 272 | 357, 307sh 273, 257 |
| Compound 5 | 355, 264, 251 |
396, 303sh, 266 |
431, 353, 263 |
422, 347, 302sh, 264 |
418sh, 383sh, 268 |
373, 324sh 265sh |
| Compound 6 | 367, 299sh, 268sh, 252 |
413, 325, 272 |
435, 329sh, 271 |
408, 363sh, 304sh, 264 |
381, 323, 274 |
383, 295sh, 265 |
| Compound 7 | 346, 288sh, 263, 251 |
401, 313sh, 267sh |
431, 329, 301sh, 271 |
386, 353, 278, 266sh |
400,386sh, 321sh., 266,253 |
431sh, 371, 258 |
Results
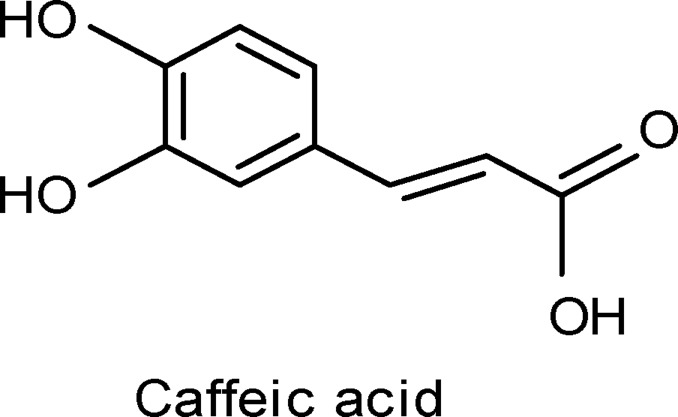
Compound 1 (Caffeic acid)
The sky blue band (0.65) isolated from the chloroform fraction by using preparative paper chromatography (3MM, 20%, acetic acid), and eluted by methanol, gave after purification on Sephadex LH-20 column (90% methanol), a single phenolic compound corresponding to that of caffeic acid (18.3mg), which identified by using authentic sample, PC, TLC, UV (Table 3), and EI-Ms [M+]180 and fragments at m/z 163 (M+-OH), 145 (M+-H2O, OH), 136. These fragment patterns are in agreement with that reported for caffeic acid (Harborne, 1973).
Table 3.
Antitumor activity of different fractions and isolated flavonoids of Leucaena leucocephala
| The components | Concentration in µg/ml | %Inhibition of Cell Viability |
| 70% Ethanolic Ext. | 25 50 100 |
5 5 10 |
| Chloroform | 25 50 100 |
5 10 20 |
| Ethyl acetate extract | 25 50 100 |
10 14 20 |
| Butanol extract | 25 50 100 |
10 10 25 |
| Compound 1 | 25 50 100 |
5 20 41 |
| Compound 2 | 25 50 100 |
5 16 36 |
| Compound 3 | 25 50 100 |
0 15 33 |
|
Compound 425 50 100 |
11 14 48 |
|
| Compound 5 | 25 50 100 |
0 15 33 |
| Compound 6 | 25 50 100 |
10 20 28 |
| Compound 7 | 25 50 100 |
5 25 43 |
Compound 2 (Isorhamnetin)
The flavonoidal band (Rf 0.09) isolated from the ethyl acetate fraction by preparative PC (3MM-15% acetic acid) and eluted by 90% methanol, gave after purification on Sephadex LH-20 column (90% methanol), a single flavonoidal compound corresponding to that of isorhamnetin (14mg), which identified by TLC, PC, UV (Mabry et al., 1970), (Table, 2) and EI-Ms: m/z 316 [M+ ] and fragment ions at m/e 301(M+-CH3], 285(M+-OCH3], 152 A1, 153 (A1+1), 164 B1 which are characteristic for that of isorhamnetin (Mabry & Markham, 1975).
Compound 3 (Chrysoeriol)
The flavonoidal band (Rf 0.06) separated by preparative PC (3MM, 15% acetic acid) and eluted by 90% methanol, gave after purification on Sephadex LH-20 column using 90% methanol, a single flavonoidal compound corresponding to that of Chrysoeriol (26 mg), which identified by TLC, PC, UV (Mabry et al., 1970), (Table 3), and EI-Ms [M+] 300 and fragment ions at m/z 285 (M+-CH3), 272(M+-CO), 269(M+-OCH3), 152 A1, 148 B1 which are characteristic for Chrysoeriol (Mabry & Markham, 1975).
Compound 4 (Isorhamnetin 3-O-galactoside)
The flavonoidal band (Rf 0.53), isolated from the ethyl acetate fraction was subjected for further purification using preparative thick layer chromatography using silica gel plates and developed with [ethyl acetate-formic acid-acetic acid-water (30: 1.2: 0.8:8)]. The main compound (Rf 0.47), was scrapped off and eluted by 85%, methanol. The eluent after purification by using centrifugally accelerated rotatory TLC using (silica gel 60 PF254), discs, and elution was carried out with chloroform / methanol (85:15), gave a single flavonoidal compound corresponding to that of isorhamnetin 3-O-galactoside (22 mg), which identified by TLC, PC, UV (Mabry et al., 1970), (Table, 2). Further confirmation was performed by carrying out 1H-NMR, which found to be identical as Isorhamnetin 3-O-galactoside. Acid hydrolysis (2N HCl) gave isorhamnetin which identified by TLC, PC, UV (Mabry et al., 1970); Mabry & Markham, 1975). and EI-Ms. The sugar moiety was identified as galactose by [PC. Whatman No. 1, ethyl acetate-pyridine-water (12:5:4) and n.butanol-benzene-pyridine-water (5:1:3:3)]. The spots were visualized by spraying with aniline phthalate spraying reagent.
Compound 5 (Kaempferol-3-O-rubinoside)
The flavonoid zone (Rf 0.7) isolated from the n-butanol fraction by preparative paper chromatography (3MM, 15% acetic acid), and eluted by 90%, methanol, gave upon purification on Sephadex LH-20 column using 90% methanol, a single flavonoidal compound Kaempferol-3-O-rubinoside (16.7 mg), which was identified by using PC and UV (Mabry et al., 1970), (Table, 2). Further confirmation was performed by carrying out 1H-NMR, which found to be identical with the reported data. Acid hydrolysis (2N HCl) gave Kaempferol which identified by TLC, PC, UV (Mabry & Markham, 1975) and EI-Ms [M+] 286 and fragments at m/e 258 (M+-CO), 152 A1, 153(A1 + 1),148 B1, 124 which are characteristic for that of Kaempferol. The sugar moiety was identified as rhamnose and galactose by [PC. Whatman No. 1, ethyl acetate-pyridine-water (12:5:4) and n.butanol-benzene-pyridine-water (5:1:3:3)]. The spots were visualized as mentioned before.
Compound 6 (Quercetin-3-O-rhamnoside)
The flavonoid compound (Rf 0.56), isolated from the n-butanol fraction by preparative paper chromatography (3MM, 15% acetic acid), and eluted by 85% methanol, gave after purification on centrifugally accelerated rotatory TLC using (silica gel 60 PF254) discs, and elution was carried out with chloroform / methanol (85:15), a single flavonoid compound (quercetin-3-O-rhamnoside, 17mg), which identified by PC, TLC and UV (Mabry et al., 1970), (Table, 2). Acid hydrolysis ( 2N HCl ) gave quercetin which identified by TLC, PC, UV and EI-MS [M+] 302 and fragment ions at m/z 274, 153, 152, 134, which are characteristic for quercetin. The sugar moiety was identified as rhamnose [PC, Whatman No. 1, ethyl acetate-pyridine-water (12:5:4), sprayed with aniline phthalate].
Compound 7 (Luteolin-7-O-glucoside)
The flavonoidal band (Rf = 0.17) isolated from the n-butanol fraction by preparative PC (3 MM, 15 % acetic acid) and eluted by 90 % methanol, gave after purification on Sephadex LH-20 column using 90% methanol, a single flavonoidal compound corresponding to that of luteolin-7-O-glucoside (19 mg) which identified by TLC, PC, UV (Mabry et al., 1970), (Table, 2). Acid hydrolysis (2N HCl) gave luteolin which was identified by TLC, PC, UV (Mabry et al., 1970), (Table, 2) and MS [M+] 286, in addition to fragment ions at m/z 258, 153, 152, 137, 134 which are characteristic to luteolin (Mabry & Markham, 1975). The sugar moiety was identified as glucose by PC [Whatman No. 1, ethyl acetate-pyridine-water (12:5:4) and other solvent systems].
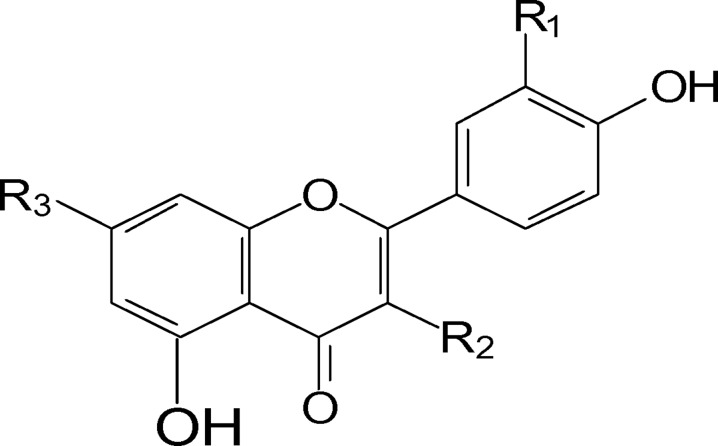
Comp. (2), R1=OMe; R2=OH; R3=OH (Isorhamnetin).
Comp. (3), R1=OMe; R2=H; R3=OH (chrysoeriol).
Comp. (4), R1=OMe; R2=O-Gal.; R3=OH. (isorhamnetin-3-O-galactoside).
Comp. (5), R1= H; R2=O-Rh-Gl.; R3=OH. (Kaempferol-3-O-rubinoside).
Comp. (6), R1=OH; R2= O-Rh; R3=OH. (Querecetin-3-O-Rhamnoside).
Comp. (7), R1=OH; R2=H; R3= O-GL. (Luteolin-7-O-glucoside).
Discussion
In the course of searching for natural antioxidants, the phyto-constituents of L. leucocephala (L.), the different extracts and the isolated compounds were evaluated for antioxidant activity against DPPH.
The study of the flavonoidal fraction revealed the isolation and identification of, Caffeic acid, Isorhamnetin, Chrysoeriol, Isorhamnetin 3-O-galactoside, Kaempferol-3-O-rubinoside, Quercetin-3-O-rhamnoside and Luteolin-7-glucoside. Their identification was proved by TLC, PC, UV and MS analysis, in addition to acid hydrolysis of the flavonoidal glycosides. The preparative centrifugally accelerated TLC using (silica gel 60 PF254) discs has been recommended as one of the best techniques for separation and purification for flavonoids. This is the first record of the flavonoids in the aerial parts of Leucaena leucocephala (L.) except Quercetin-3-O-rhamnoside.
The values of the radical scavenging activity (RSA%) of the tested extracts and isolated compounds on DPPH free radical were represented in (Table, 1). From the RSA% values represented in the table, we noticed that the ethyl acetate extract possess the highest (86.78%) antioxidant activity, due to the presence of the main flavonoids in this extract. Also, the chloroform extract showed moderate activity. As for the isolated compounds, we noticed that compound 7 showed the highest (90.31%) antioxidant activity followed by compounds 1 and 3, respectively.
In general, antioxidant activity of flavonoids depends on their structures and substitution pattern of hydroxyl groups. The essential requirement for effective radical scavenging is 3′, 4′ orthodihydroxy configuration in ring-B and 4-carbonyl group in ring-C. The presence of 3-OH group or 3-and-5-OH groups giving a catechol like structure in ring-C is also beneficial for the antioxidant activity of flavonoids. The presence of the C-2-C-3 double bond configured with a 4-keto arrangement is known to be responsible for electron delocalization from ring B and it increases the radical scavenging activity (Ye-ilada, et al., 2000).
The different extracts and isolated compounds of the plant exhibited no considerable cytotoxic activity against Ehrlich-ascitis carcinoma cell line at the tested concentrations (25µg/ml, 50µg/ml and 100µg/ml), as shown in Table (3).
References
- 1.Ademola IO, Akanbi AI, Idowu SO. Comparative nematocidal activity of chromatographic fractions of Leucaena leucocephala seeds against gastrointestinal sheep nematodes. Pharmaceutical Biology. 2005;43(7):599–604. [Google Scholar]
- 2.Aderogba M A, Mc Gaw LJ, Bezabih BT, Abegaz BM. “Antioxidant activity and cytotoxicity study of Leucaena leucocephala (Lam.) de wit leaf extract constituents”. Nigerian Journal of Natural Products and Medicine. 2010;13.1:65–68. [Google Scholar]
- 3.Chang HC, Lee TH, Chuang LY, Yen MH, Hung WC. Inhibitory effect of mimosine on proliferation of human lung cancer cells is mediated by multiple mechanisms. Cancer Letters. 1999;145(1–2):1–8. doi: 10.1016/s0304-3835(99)00209-8. [DOI] [PubMed] [Google Scholar]
- 4.Deodhar UP, Paradkar AR, Purohit AP. Preliminary evaluation of Leucaena leucocephala seed gum as a tablet binder. Drug Dev Ind Pharm. 1998;24(6):577–582. doi: 10.3109/03639049809085662. [DOI] [PubMed] [Google Scholar]
- 5.Dugan L R. In: “Antioxidation in Food and Biological Systems”. Simic M G, Korel M, editors. New York: Plenum; 1979. Chapter 17. [Google Scholar]
- 6.El-Hossary M M, Fathy H A, Kassem Z A, Shehab G G. Bull Fac Pharm Cairo Univ. 2000;38:1. [Google Scholar]
- 7.Gamal-Eldeen AM, Amer H, Helmy WA, Ragab HM, Talaat RM. Antiproliferative and cancer-chemopreventive properties of sulfated glycosylated extract derived from Leucaena leucocephala. Indian J Pharm Sci. 2007;69:805–811. [Google Scholar]
- 8.Harborne J B. “Phytochemical Methods”. 1st Ed. London: Chapman and Hall; 1973. Toppan Company, limited, Tokyo, Japan. [Google Scholar]
- 9.Harborne J B. “Phytochemical Methods”. 2nd Ed. London, New York: Chapman and Hall; 1984. [Google Scholar]
- 10.Hassan RA, Radwan HM. “The Lipids and Volatile Oil Constituents of Leucaena glauca (L.) Benth. Growing inEgypt and Their Biological Activity”. Journal of Applied Sciences Research. 2010;6(5):478–482. [Google Scholar]
- 11.Hill GD. Leucaena leucocephala for Pastures in the Tropics. Herbae Abstracts. 1971;4:111–119. [Google Scholar]
- 12.Hylin JW, Sawait Ko. J of Biological Chem. 1984 Apr 4;239 [PubMed] [Google Scholar]
- 13.Mabberley DJ. The plant Book. 2nd Ed. UK: Cambridge University Press; 1997. ISBN 0-521-411421-0. [Google Scholar]
- 14.Mabry T J, Markham K R. “The Flavonoids”. London: Chapman and Hall; 1975. [Google Scholar]
- 15.Mabry T J, Markham K R, Thomas M B. “The Systematic Identification of Flavonoids”. Berlin: Springer Verlage; 1970. [Google Scholar]
- 16.MCLimans W F, Davis E V, Rake G W. Immunology. 1957;79:428. [PubMed] [Google Scholar]
- 17.Nicolaos N, Maria T. “Observation on the Estimation of scavenging activity of phenolic compounds using DPPH tests”. Jaocs. 2002;79(12) [Google Scholar]
- 18.Radwan H M, El-Missiry M M, Seif-El-Nasr M M. “Phytochemical Investigation of Ballota undulata”. Bull Fac Pharm Cairo Univ. 1997;35(1) [Google Scholar]
- 19.Syamsudin RS, Partomuan S. Antidiabetic activity of active fractions of Leucaena leucocephala (lmk) Dewit seeds in experiment model. European Journal of Scientific Research. 2010;43(3):384–391. [Google Scholar]
- 20.Verma PRP, Balkishen R. Studies on disintegrant action of Leucaena leucocephala seed gum in ibuprofen tablet and its mechanism. Journal of Scientific and Industrial Research. 2007;66:550–557. [Google Scholar]
- 21.Yamaguchi T, Takamura H, Terao J. “HPLC method for evaluation of the free radical-scavenging activity of food by using (DPPH)”. Bioscience, Biotechnology, Biochemistry. 1998;62:1201. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- 22.Ye-ilada E, Tsuchiya K, Takaishi Y, Kawazoe K. “Isolation and Characterization of free radical scavenging flavonoid glycosides from the flowers of Spartium junceum by activity guided fractionation”. J Ethnopharmcol. 2000;23(3):471–478. doi: 10.1016/s0378-8741(00)00327-5. [DOI] [PubMed] [Google Scholar]