Abstract
Background
Venenum bufonis is the dried white secretion of the auricular and skin glands of Bufo gargarizans Cantor, or Bufo melanostictus Schneider, Bufonidae. It is used in the treatment of deep-rooted carbuncle, boils and swelling; pain in the throat, heart stroke, coma, abdominal pain, vomiting and diarrhea. The objective of this paper is to preliminarily observe the effects of ethanol extract of Venenum bufonis on growth, and proliferation of human osteosarcoma U2OS cell lines, and to provide a theoretical basis for an in-depth study of the clinical application of Venenum bufonis for osteosarcoma inhibition, with its mechanism of action.
Materials and Methods
SRB assay was used to determine the effect of Venenum bufonis ethanol extract on U2OS cell line activity, and to detect its inhibitory dose-effect on osteosarcoma cells. FCM was applied to determine the effect of Venenum bufonis ethanol extract on U2OS cell apoptosis and to perform cell cycle analysis.
Results
As results, different Venenum bufonis ethanol extracts showed apparent concentration-effect relationships on U2OS cell lines. FCM analysis showed that it had a U2OS apoptosis promoting effect, which increased with increasing concentration. Cell cycle analysis revealed that the Venenum bufonis ethanol extract mainly arrested U2OS in the G0/G1 phase, preventing the cells from progressing to the S phase.
Conclusion
The study concluded that Venenum bufonis ethanol extract has an inhibitory effect on proliferation of osteosarcoma U2OS cells.
Keywords: Venenum bufonis, U2OS, antitumor
Introduction
Osteosarcoma, also known as the osteogenic sarcoma, is the most common primary malignancy of bone tissue, which is derived from the mesenchymal tissue, and where osteoid tissue, and immature bone tissue are produced directly by malignant cells (Xu et al., 2001). It mostly occurs in adolescents, with its biological behavior as generally high-grade, and is prone to hematogenous metastasis in early stage. Before 1970, the standard treatment was amputation, but the five-year survival rate was around 10%. Over the past 25, years, with the wide application of neoadjuvant chemotherapy, and the improvement of operational methods, the five-year survival rate of osteosarcoma has reached 80%, but there is a considerable number of patients who died of pulmonary metastases in advanced stage (Bielack et al., 2002; Szendroi et al., 2000; Kandioler et al., 1998; Thompson et al., 2002). At present, it is believed internationally that the breakthrough on the improvement of the five-year, survival rate of patients with osteosarcoma is difficult with existing chemotherapy regimens (Marina et al., 2004). Moreover, due to the significant toxic, and side effects of chemotherapy, its wide application and efficacy is greatly limited. From the point of view of tumor treatment, Chinese medicines with significant curative effect and little side effects are attracting much attention. On the one hand, Chinese medicines can eliminate tumors; conversely, they can adjust systemic conditions of osteosarcoma patients, correct internal organ dysfunction, enhance the effect of chemotherapy and surgery, mitigate adverse reactions of chemo-radiotherapy, and enhance the patients' immune function, thereby improving the quality of life, with an improved survival rate.
Venenum bufonis is the dried white secretion of the auricular and skin glands of Bufo gargarizans Cantor or Bufo melanostictus Schneider, Bufonidae. It is mainly produced in China's Hebei, Shandong, Jiangsu and Zhejiang provinces. It is warm in nature, bitter and pungent in taste, and enters the heart meridian, with the effects of detoxification, pain relief and resuscitation induction. It is used in the treatment of deep-rooted carbuncle, boils, swelling and pain in the throat, heart stroke, coma, abdominal pain, vomiting and diarrhea. Recent studies have shown that Venenum bufonis not only ease pain, diminish inflammation, and is used in anesthesia, but also has multiple biological activities such as anti-cancer, anti-radiation and cardiotonic actions (Dong et al., 2003). Due to the definite pharmacological activity of Venenum bufonis in the clinical treatment of cancer, it has attracted widespread recognition. In this experiment, the effects of ethanol extract of Venenum bufonis on growth, and proliferation of human osteosarcoma U2OS cell lines were preliminarily observed, thus providing a theoretical basis for in-depth study of the clinical application of Venenum bufonis for osteosarcoma inhibition and its mechanism of action.
Materials and Methods
Drugs and reagents
Venenum bufonis identified by professor Liu Yishan of the Shandong University of Traditional Chinese Medicine (purchased from the Nepstar Chain Drugstore Ltd.); DMEM medium (GIBCOBRL); fluorouracil (Hengrui Medicine Co., Ltd., Jiangsu); Sulforhodamine B (SRB) (Sigma); other reagents were all of analytical grade.
Main instruments and Materials
AE31, inverted phase contrast microscope (Motic); SW-CJ-IF clean bench (Suzhou Purification Equipment Factory); fully automatic micro-plate reader (Mod550, BioRad, USA); low-temperature refrigerated centrifuge (Eppendorf, Germany); electronic balance (Sartorius AG, Beijing); blood counting chamber (Qiujing Biochemical Instrument Factory, Shanghai). Human osteosarcoma cell line U2OS. Was provided by the Department of Pharmacology of our College.
Preparation of Venenum bufonis extract
Dried Venenum bufonis material was crushed, and an appropriate amount weighed; a 25-fold amount of 70% ethanol was added, and soaked for 30min., then ultrasonically extracted three times, [each time lasted 30min]. The extracts were combined, ethanol removed, and the remaining was concentrated at low-temperature, and freeze-dried to get the Venenum bufonis extract powder. When it was ready for use, the right amount of powder was accurately weighed, and diluted with culture medium to the desired concentration.
Cell cultivation
The proliferating cells in the logarithmic growth phase were dispersed with 0.25%, trypsin-EDTA, and then counted. It was prepared into cell suspension, and seeded in a 96-well plate at 180µL/well, after pre-incubation for 24hrs, each well was added with 20µL of drug (final concentration of 1/10, of the prepared concentration), total liquid volume of each well was 200µL, six replicate wells were set up for each drug concentration, and blank control group was set up as well, the plates were placed in an 37 °C, 5% CO2 incubator, and cultured under full wet conditions for 48hrs.
Detection of Anticancer Sensitivity by SRB, colorimetric assay
With reference to the Skehan's method in (Skehan et al., 1990), after the completion of cell culture, each well was added with 50µL, of 50%, trichloroacetic acid (TCA), to fix the cells. The final concentration of TCA was 10%, which was gently added to the liquid surface of each well, and then the plate was placed in a 4°C, refrigerator for 1hr. Each well of the culture plate was washed with deionized water, five times to remove TCA. After drying in air, each well was added with 100µL, of 0.4%, SRB, and allowed to stand at room temperature for 10∼30min. Liquid in each well was discarded, and unbound dye was removed by five washes with 1% acetate, after drying in air, the remaining was dissolved with 10mmol/L unbuffered Tris base (Tris (hydroxymethyl), amino methane), with a pH of 10.5, then oscillated in an oscillator for 5min, and OD value was measured at a wavelength of 490nm, in a microplate reader using the value of blank control for zero adjustment. The obtained tumor cell growth inhibition rate was defined as the in vitro inhibition rate of the drug on tumor cells.
Tumor inhibition rate (%) = (OD value of drug-free cell control well - OD value of drug well), / OD value of drug-free cell control well × 100%.
Colony forming assays (Yin et al., 2008)
U2OS cells were seeded in 10mL culture dishes at 100, cells/dish, and cultured for 24hrs. Venenum bufonis ethanol extract was added to make the concentration 25.0, 50.0, 100, and 200mg/L respectively. Blank control group, and 5-Fu group (50 mg/L), were also set up, and cultivation was further continued for 10 days. Culture solution was discarded, cells were rinsed trice with PBS, and fixed with 10% methanol, 15min., later, fixative was discarded, and cells were stained with 0.25%, gentian violet. Number of colonies greater than 50, cells was counted under a microscope, and the rate of colony formation was calculated.
Colony formation rate (%) = number of colonies / number of seeded cells × 100%.
Flow cytometry (FCM) analysis (Yin et al., 2004)
U2OS cells treated with different drug concentrations were collected, with each group containing approximately 1×105 cells, and fixed with 70% cold ethanol at 4°C, overnight, washed twice with PBS, digested at 37°C, with lmg/ml RNase A (Sigma) for 30min., stained with 100µg/ml propidium iodide for 20min., then cell ratio, and apoptosis rate of each phase under the action of different concentrations of drug were determined by FCM.
Statistical methods
The data were analyzed using SPSS 13.0 software, and comparisons between two groups were performed by t-test, while pairwise comparisons among groups were performed using one-way ANOVA.
Results
SRB colorimetric assay
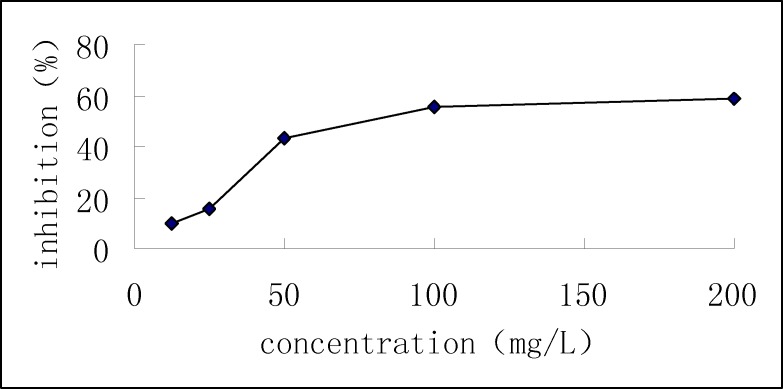
The activity of human osteosarcoma U2OS cells after treatment with different concentrations of Venenum bufonis ethanol extracts that were short-term cultured in a 96-well culture plate was studied, and the results are shown in Fig. 1. When human osteosarcoma U2OS cell lines were between 6×104cells/mL, to 1×106cells/mL, with the increase in the number of cells, OD values obtained by SRB assay also increased. The inhibitory effect of Venenum bufonis ethanol extract on U2OS cells presented a certain dose-effect relationship. The greater the concentration of the extracts, the higher the inhibition rate.
Figure 1.
Dose-effect curve of Venenum bufonis extract on U2OS
Colony forming assay results
The effects of different doses of Venenum bufonis ethanol extracts on U2OS colony formation are shown in Tab. 1. As shown [Table. 1], five Venenum bufonis ethanol extract dose groups all had different degrees of inhibitory effects on colony formation of U2OS cell lines, and the differences were all significant when each dose group was compared with the blank control group.
Table 1.
Inhibitory effect of Venenum bufonis ethanol extract on U2OS colony formation
| Group | Concentration (mg/L) | Colony formation rate (%) |
| Blank control group | - | 96.8±1.3 |
| 5-Fu group | 50 | 79.1±3.6 |
| Venenum bufonis ethanol extract 1 | 12.5 | 90.3±4.1 |
| Venenum bufonis ethanol extract 2 | 25 | 89.4±2.7 |
| Venenum bufonis ethanol extract 3 | 50 | 85.5±4.8 |
| Venenum bufonis ethanol extract 4 | 100 | 82.8±3.1 |
| Venenum bufonis ethanol extract 5 | 200 | 80.2±3.2 |
Note: comparison of each dose group with the blank control group, P<0.05.
Flow cytometry (FCM) analysis results
FCM analysis results are as shown in Table 2, compared with the blank control group, after induction of cells by different concentrations of Venenum bufonis ethanol extracts, in the G0/G1 phase, cells increased with the increase of drug concentration, changes were not evident in the S phase, which were around the levels of 100mg/L to 200mg/L, in the G2/M phase, cells were significantly reduced, indicating that the cells may mainly be arrested in the G0/G1 phase. Apoptosis rate increased significantly with increasing concentration.
Table 2.
Percentage of each cycle after induction of U2OS cells by different concentrations of Venenum bufonis extracts for 48 h (%)
| Concentration (mg/L) | G0/G1 phase | S phase | G2/M phase | Apoptosis rate |
| 0 | 43.78±3.6 | 38.87±2.1 | 17.27±0.9 | 2.2±1.1 |
| 50 | 51.28±4.7 | 29.67±3.2 | 20.11±2.7 | 8.9±2.5 |
| 100 | 57.43±5.5 | 36.28±4.1 | 6.3±1.8* | 28.2±4.8* |
| 200 | 62.27±5.2 | 31.92± | 5.97±1.3* | 33.7±5.6* |
Comparison with the blank control group: "*" indicates P<0.05
Discussion
The in vitro assay of tumor inhibitory effect of anticancer drugs have significant values in these aspects such as screening of effective anti-cancer drugs, assisting in estimation of anticancer spectrum of anti-cancer drugs, and individualization of clinical treatment with anti-cancer drugs. Among various methods, comparatively speaking, MTT is simple, sensitive, rapid, and semi-automated, which is considered to be of good practical value, it is also a method widely used at present. As for SRB assay, a new method for screening of anticancer drugs by NCI, the time required for experimental operation is even shorter compared with MTT. TCA cell fixation needs 1hr, and SRB staining only needs 10∼30min., whereas, in the MTT assay, after cells were added to MTT, an additional 4hrs is needed. SRB assay is more sensitive than the MTT assay, if there are only 150, cells in a well, inhibition rate can be detected by SRB assay, while MTT assay can't (Haselsberger et al., 1996).
We found through the study of Venenum bufonis ethanol extract that, it has an apparent inhibitory effect on growth of osteosarcoma U2OS cell lines. The effect of the drug on cell viability is investigated by SRB assay, and colony formation assay, and it was found that different Venenum bufonis ethanol extracts all showed apparent concentration-effect relationships on U2OS cell lines. FCM analysis showed that the extract had a U2OS apoptosis promoting effect, which increased with increasing concentration. Cell cycle analysis found that the Venenum bufonis ethanol extract mainly arrested U2OS, in the G0/G1 phase, preventing them from progressing to the S phase.
Acknowledgements
The work was supported by The National Natural Science Foundation of China (No. 30901576 and 30672200).
References
- 1.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. Journal of Clinical Oncology. 2002;20(3):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 2.Dong WB, Wu BL, Zhu MH. Clinical efficacy and functional characteristics of toad venom preparation. Chinese Medical & Pharmaceutical Journal. 2003;2(10):26–27. [Google Scholar]
- 3.Haselsberger K, Peterson DC, Thomas DGT, Darling JL. Assay of anticancer drugs in tissue culture: comparison of a tetrazolium-based assay and a protein binding dye assay in short-term cultures derived from human malignant glioma. Anticancer Drug. 1996;7(3):331–338. [PubMed] [Google Scholar]
- 4.Kandioler D, Kromer E, Tuchler H, End A, Müller MR, Wolner E, Eckersberger F. Long-term results after repeated surgical removal of pulmonary metastases. Annual Thoracic Surgery. 1998;65(4):909–912. doi: 10.1016/s0003-4975(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 5.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422–441. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 6.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J of Nat Cancer Ins. 1990;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 7.Szendroi M, Papai Z, Koos R, Illes T. (2000) Limb-saving surgery, survival, and prognostic factors for osteosarcoma: The Hungarian experience. Journal of Surgery Oncology. 73(2):87-–94. doi: 10.1002/(sici)1096-9098(200002)73:2<87::aid-jso6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RC, Cheng EY, Clohisy DR, Perentesis J, Manivel C, Le CT. Results of treatment for metastatic osteosarcoma with neoadjuvant chemotherapy and surgery. Clinical Orthopedic and related research. 2002;397:240–247. doi: 10.1097/00003086-200204000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Xu WP, Feng CH. Orthopedic Oncology. People's Military Medical Press. 2001;4:39–40. [Google Scholar]
- 10.Yin CP, Fan LC, Zhang LD, Lin ZC, He JW, Liu MN, Wang YQ. The inhibiting effect of extracts in Radix Ranunculi Ternati on the growth of human breast cancer cells in vitro. Chinese Journal of Hospital Pharmacy. 2008;28(2):93–96. [Google Scholar]
- 11.Yin JQ, Shen JN. In vitro studies of the water extract of Venenum bufonis mediated apoptosis in human osteosarcoma cell line U2OS. Chinese Journal of Bone Tumor & Bone Disease. 2004;3(5):303–305s. [Google Scholar]