Abstract
Background
Sophora flavescens Ait. is a traditional Chinese medicine with a long history in China. It is mainly used in the treatment of heat dysentery and similar ailments in the clinical. The objective of this paper was to isolate, purify and identify alkaloids from Sophora flavescens Ait. and to explore their inhibitory effects on C6 glioma cells.
Materials and Methods
Column chromatography, extraction and NMR spectroscopy were used to structurally identify the isolated compounds. MTT assay and flow cytometry were used to detect the inhibitory effect of matrine on C6 cells.
Results
Three compounds were isolated from Sophora flavescens Ait., namely matrine, oxymatrine and lupeol. Different concentrations of matrine solution all had inhibitory effects on growth of C6 cell lines, which showed apparent dose-effect relationship. Compared with the control group, proportion of G0/G1 phase cells increased in each matrine concentration group to a maximum of 79.8%; proportion of S phase cells reduced, and proportion of G2/M phase cells declined slightly to a minimum of 6.3%, suggesting that after the action of matrine proliferation of C6 cells was significantly inhibited and the cells were arrested in the G1 phase.
Conclusion
We concluded that Sophora flavescens Ait. has an inhibitory effect on C6 cell proliferation.
Keywords: Sophora flavescens Ait., oxymatrine, lupeol, C6 glioma cells
Introduction
Chinese medicine Sophora flavescens Ait. is a plant in the genus Sophora of the family Leguminosae, which is a traditional Chinese medicine with a long history in China (Yuan et al., 2003), Sophora flavescens Ait. is recorded in the “Pharmacopoeia of the People's Republic of China” (2010 edition). It has heat-clearing, damp-drying (Zhao & Zhang, 1991), insecticidal and diuretic effects. Clinically, it is mainly used in the treatment of heat dysentery, hemafecia, jaundice, anuresis, leucorrhoea with reddish discharge, vulval swelling, pruritus vulvae, eczema, as well as external treatment of trichomonas vaginitis (Zhang et al., 2000; Zhu et al., 2000; Kang et al., 2003; Wang & Wang, 2000).
Matrine is one of main alkaloid constituents in Sophora flavescens Ait., which can also be isolated from the root of Sophora subprostrata, aerial parts of Sophora tonkinensis, whole plant of Sophora alopecuroides, and bark of Sophora chrysophylla and Sophora tetraptera. In this paper, three compounds from ethanol extract of Sophora flavescens Ait. were isolated by methods such as extraction, polyamide column chromatography, silica gel column chromatography and recrystallisation. Meanwhile, the effect of matrine on proliferation of human glioma cell line C6 was investigated.
Materials and Methods
Drugs and reagents
Sophora flavescens Ait. was purchased from medicine wholesale market, which was identified as Sophora flavescens Ait. in the genus Sophora of the family Leguminosae by Dr. Han Wang from Henan College of Traditional Chinese Meditation. Reagents included DMSO, MTT (Sigma, USA), fetal bovine serum (Gibco), and RPMI 1640 medium (Invitrogen).
Instruments
The main instruments used in the study include the following: CO-150 CO2 incubator (NBS, USA), EPICS-XL flow cytometer (Beckman Coulter, USA), SW-CJ-SF clean bench (Suzhou Purification Equipment Factory, Sujing Group); model 680 microplate reader (Bio-Rad, USA).
Results
Extraction and isolation of alkaloids from Sophora flavescens Ait.
3 kg of Sophora flavescens Ait. was weighed out, ground, and extracted three times with 95% ethanol. Each extraction lasted 1.5 h. The extracts were combined, and then recovered under reduced pressure until no ethanol was smelt. PH value of aqueous ammonia was adjusted to 10.0. Extraction was performed five times with chloroform. Then, the extracts were combined and solvent was recovered to give crude product of total alkaloids. The total alkaloids were column chromatographed on silica gel and gradient eluted with chloroform-methanol-aqueous to give compounds 1–3.
Structural identification of alkaloids from Sophora flavescens Ait.
Compound 1: white columnar crystals (petroleum ether), easily soluble in methanol, chloroform, acetone, and slightly soluble in petroleum ether. m.p. 81–83°C. Data were attributed as follows: 1H-NMR (300 MHz, DMSO-d6) δ: 2.07 (1H, m, 2Ha), 2.83 (1H, m, 2He), 1.96 (1H, m, 3Ha), 1.46 (1H, m, 3He), 1.46–1.38 (m, 4Ha), 1.93 (m, 4He), 1.78–1.55 (m, 6H), 1.78–1.55 (m, 6H), 1.78–1.55 (m, 7H), 1.47–1.34 (m, 8H), 1.84 (m, 9Ha), 1.43–1.36 (m, 9He), 2.13 (m, 10Ha), 2.84 (m, 10He), 3.85 (dt, J=9.7, 6.0Hz), 1.54 (m, 12Ha), 1.95 (m, 12He), 1.76–1.55 (m, 13H), 2.23 (m, 14Ha), 2.44 (m, 14He), 3.08 (t, J=12.4Hz, 17Ha), 4.45 (dd, J=12.7, 4.2Hz, 17He). 13C-NMR (75 MHz, DMSO-d6) δ: 57.5 (C-2), 21.4 (C-3), 27.5 (C-4), 35.5 (C-5), 63.7 (C-6), 41.3 (C-7), 26.6 (C-8), 20.8 (C-9), 57.4 (C-10), 53.5 (C-11), 27.9 (C-12), 19.2 (C-13), 32.6 (C-14), 169.6 (C-15), 43.4 (C-17). Based on the above spectroscopic data and by contrasting the data with literature (Song et al., 2008), compound 1 was identified as matrine. The structure is shown in Figure 1.
Figure 1.
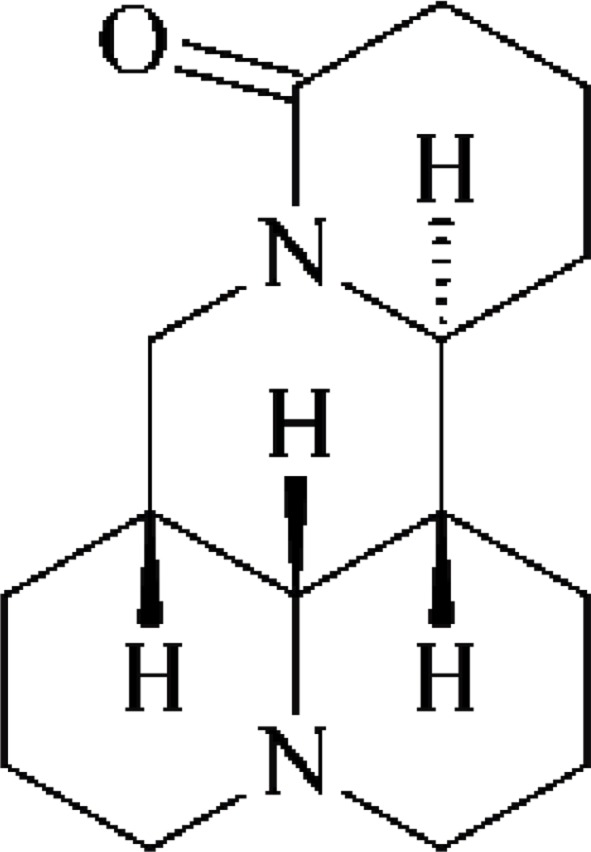
Matrine
Compound 2: white prismatic crystals (chloroform-ether), easily soluble in chloroform, methanol, slightly soluble in acetone, and insoluble in petroleum ether. m.p 207–208°C. Data were attributed as follows: 1H-NMR (300 MHz, DMSO-d6) δ: 3.12 (m, 2H), 2.73 (m, 3Ha), 1.87–1.54 (m), 1.86–1.54 (m, 4H), 1.86–1.54 (m, 5H), 3.12 (m, 6H), 1.86–1.53 (m, 7H), 1.86–1.53 (m, 8H), 2.08 (m, 8He), 2.73 (m, 9H), 3.13 (m, 10H) 5.14 (dt, J=10.2, 5.3Hz, 11H), 1.26 (m, 12Ha), 2.25 (m, 12He), 1.86–1.54 (m, 13H), 2.26 (m, 14Ha), 2.49 (m, 14He), 4.22 (t, J=12.6Hz, 17Ha), 4.43 (dd, J=12.6, 5.4Hz, 17He). 13C-NMR (75 MHz, DMSO-d6) δ: 69.5 (C-2), 17.4 (C-3), 26.5 (C-4), 34.5 (C-5), 67.4 (C-6), 42.3 (C-7), 24.5 (C-8), 17.7 (C-9), 69.5 (C-10), 53.3 (C-11), 28.4 (C-12), 18.4 (C-13), 32.6 (C-14), 170.5 (C-15), 41.3 (C-17). Based on the above spectroscopic data and by contrasting the data with literature (Li et al., 2004), compound 2 was identified as oxymatrine. The structure is shown in Figure 2.
Figure 2.
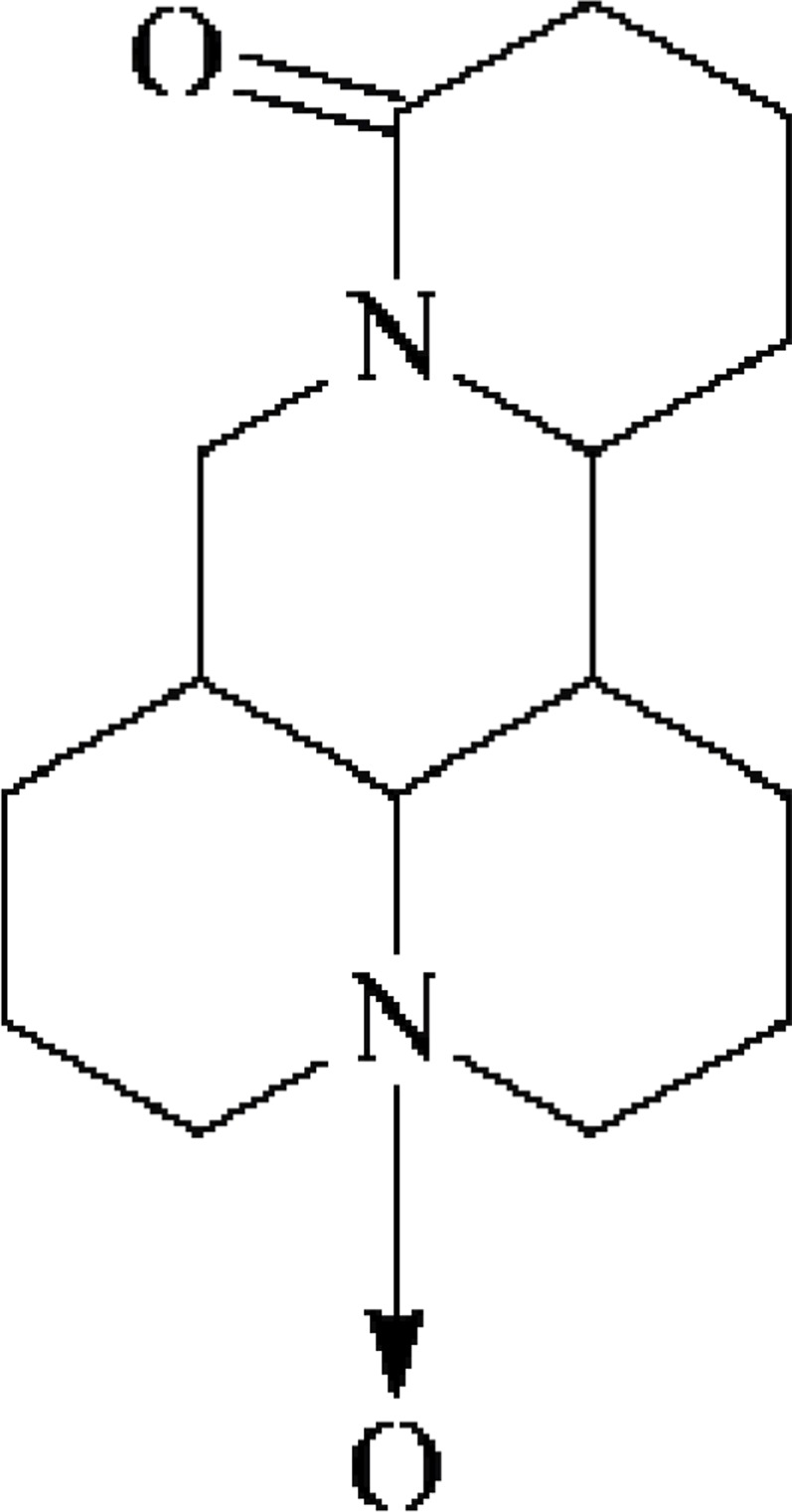
Oxymatrine
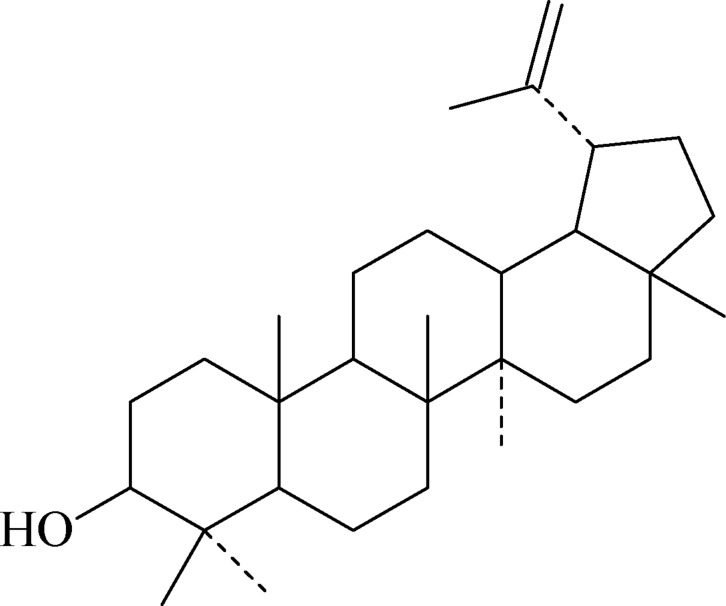
Compound 3: white needle crystals (CHCL3), m.p.: 173.4–174.5°C, easily cinsoluble in chloroform, diethyl ether, petroleum ether; hardly soluble in methanol, acetone. Data were attributed as follows: 1H-NMR (300 MHz, DMSO-d6) δ: 3.21 (dd, 1.3, 7.3Hz, 3H), 2.35 (m, 19H), 4.68 (s, 20Ha), 4.56 (s, 20Hb); 13C-NMR (75 MHz, DMSO-d6) δ: 38.8 (C-1), 27.6 (C-2), 79.2 (C-3), 38.7 (C-4), 55.5 (C-5), 18.4 (C-6), 34.3 (C-7), 40.7 (C-8), 50.6 (C-9), 37.2 (C-10), 21.2 (C-11), 25.3 (C-12), 38.1 (C-13), 42.7 (C-14), 28.1 (C-15), 35.7 (C-16), 43.1 (C-17), 48.4 (C-18), 48.2 (C-19), 151.2 (C-20), 29.9 (C-21), 40.1 (C-22), 29.9 (C-23), 15.6 (C-24), 16.1 (C-25), 16.0 (C-26), 14.6 (C-27), 18.1 (C-28), 109.4 (C-29), 19.5 (C-30). Based on the above data, and by contrasting with the literature (Cheng, 1999), compound 3 was identified as lupeol.
Cell cultivation
C6 glioma cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum, and placed in the incubator set at 37°, 5% CO2 and subcultured routinely. The logarithmic growth phase cells were used in the experiment.
Determination of inhibitory effect of matrine on C6 cell growth by MTT assay
Logarithmic growth phase cells were digested, collected, made into a cell suspension with a concentration of 5×104 cells/ml, and seeded in 96-well plates at 200 uL per well. 24 h later, matrine solutions that were prepared in RPMI 1640 medium were added. Their final concentrations were 0.2 mg/L, 0.4 mg/L, 0.6 mg/L, 0.8 mg/L and 1.0 mg/L, respectively. 6 replicate wells were set up for each concentration; cultivation was continued for additional 24, 48 and 72 h, respectively. After supernatant was discarded, each well was added with 20 µl of MTT, mixed uniformly, and the plates were incubated at 37°C in 5% CO2 incubator for another 4 h. Then, the culture medium was carefully aspirated, and 150 µl of DMSO was added to each well. The plates were shaken for 10 min, and absorbance value (A value) of each well was measured at 570 nm using microplate reader. The rate for cell proliferation inhibition rate was calculated with the following formula:
Cell proliferation inhibition rate (%) = (1 − D value of the experimental group / D value of the control group) × 100%.
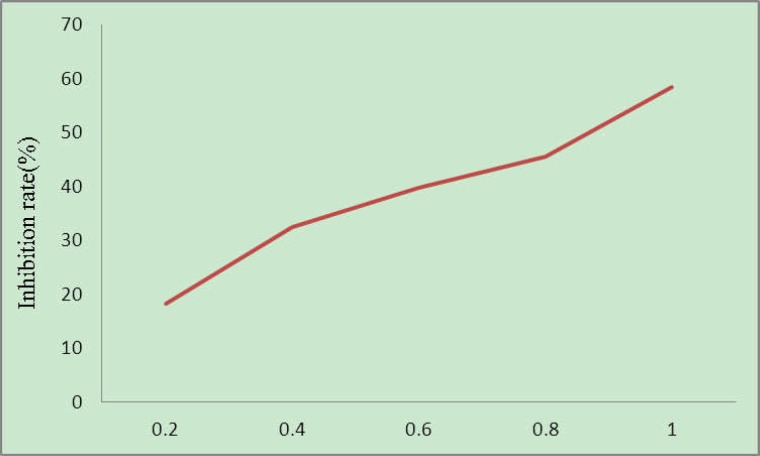
The experimental results are shown in Fig. 4. As can be seen, the culture media containing different concentrations of matrine could all inhibit the growth of C6 cell lines, where apparent dose-effect relationship was seen. Such results indicate that matrine can effectively inhibit the proliferation of C6 cell lines in vitro.
Figure 4.
Inhibitory effect of matrine on C6 cell growth (mg/L)
Determination of apoptosis rate by flow cytometry
C6 cells in the matrine treatment groups and control group which were cultured for 72 h were collected. Cell concentration was adjusted to 5×106, and the cells were washed 3 times with PBS, and fixed with 70% ice-cold ethanol at −20°C overnight. They were then washed 3 times again with PBS, and stained at 4°C under dark conditions by addition of PI dye for 30 min. Cell concentration was adjusted appropriately, and the cells were passed through 300-mesh nylon sieve for six times. After filtration, cells were loaded on flow cytometry, and changes in the cell cycle after drug treatment were analysed.
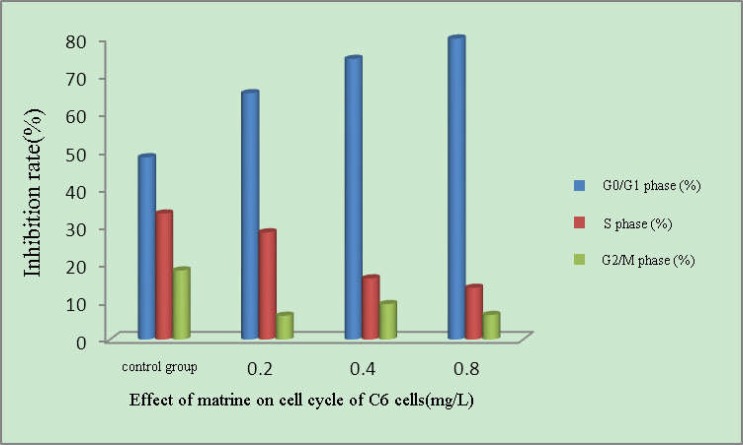
As can be seen from the results in Fig. 5, compared with the control group, proportion of G0/G1 phase cells increased in each matrine concentration group to a maximum of 79.8%. S phase reduced and G2/M phase declined slightly to a minimum of 6.3%, suggesting that after the action of matrine, C6 cell proliferation was significantly inhibited and the cells were arrested in the G1 phase.
Figure 5.
Effect of different concentrations of matrine on cell cycle of C6 cells
Discussion
In this experiment, alkaloids were extracted and isolated from commonly used Chinese medicine Sophora flavescens Ait. Three compounds were isolated from it. The isolated compounds were structurally identified by column chromatography, extraction and NMR spectroscopy. The three compounds were matrine, oxymatrine and lupeol.
To investigate the anti-glioma effect of Sophora flavescens Ait., this paper used different concentrations of self-prepared matrine to act on C6 cells. MTT assay results showed inhibition of C6 cell growth by different concentrations of matrine. Compared with the control group, each matrine experimental group all had a significant inhibitory effect on proliferation of C6 cells, which showed apparent dose-effect relationship as well.
Apoptosis is the programmed cell death regulated by related genes due to internal and external environmental changes. It can eliminate aging cells and potential abnormal growth cells in the body to achieve body's normal metabolism and avoid inflammation, which is an important guarantee for the maintenance of homeostasis in the body. Apoptosis regulation disorder can lead to a variety of human diseases, and thus is also an important cause of tumorigenesis (Thorbum., 2004; Green & Kroemer, 2004; Liu & Jiang, 2006).
It can be seen from the flow cytometric analysis of cell cycle that the proportion of S phase and G2/M phase C6 cells decreased after 72 h action of matrine to the minimum of 13.7% and 6.3%, respectively. The proportion of G0/G1 phase cells increased apparently, reaching 79.8%, suggesting that after the action of matrine, proliferation of C6 cells was significantly inhibited and that the cells were arrested in the G1 phase.
Figure 3.
Lupeol
Acknowledgements
The work was supported by the Natural Science Foundation of Hebei Province (No. 2012201136), and Medical Science Special Foundation of Hebei University (No. 2012B2004) and Hebei postdoctoral fund (No. 111867).
References
- 1.Cheng Jie-kai. Study on chemical constituents of Petasites tricholobus Franch. Chinese Pharmaceutical Journal. 1999;34(11):734–736. [Google Scholar]
- 2.Green DR, Kroemer G. The pathopliysiology of mitocliondrial cell death. Science. 2004;305(5684):626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 3.Kang Wen-zhen, Xie Yu-mei, Nie Qing-he, Zhang Yan, Hao Chun-qiu, Wang Jiu-ping. Effect of oxymatrine on experimental liver fibrosis. World Chinese Journal of Digestology. 2003;11(2):195–198. [Google Scholar]
- 4.Li Kai, Wang Yin-lan, Zhu Hui-qin. Determination of three kinds of alkaloids in Kudouzi by HPLC. Northwest Pharmaceutical Journal. 2004;l9(2):53–54. [Google Scholar]
- 5.Liu XS, Jiang J. Molecular mechanism of matrine-induced apoptosis in leukemia K562 cells. Am J Chin Med. 2006;34:1095–1103. doi: 10.1142/S0192415X06004557. [DOI] [PubMed] [Google Scholar]
- 6.Song Yu-qin, Wei Yu-hui, Liu Wen-jing, Wu Xin-an. Simultaneous Determination of Sophoridine, Matrine and Sophocarpine in Sophora Alopecuroides Cream by HPLC. China Pharmacy. 2008;19(24):1878–1879. [Google Scholar]
- 7.Thorbum A. Death receptor-induced cell killing. CeII Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Wang Jun-xue, Wang Guo-jun. Pharmacological effects and clinical application of matrine and oxymatrine. Chinese Hepatology. 2000;5(2):116–117. [Google Scholar]
- 9.Yuan Jing, Zhang Zong-jian, Cong Bin. Biological activity of matrine and its research progress. Pesticides. 2003;42(7):1–4. [Google Scholar]
- 10.Zhang Jun-ling, Chen Xue-rong, Yin Jin-zhu. Study on Apoptosis Induced by Oxymatrin in Cultured Keratinocytes. The Chinese Journal of Dermatovenereology. 2000;14(6):367–368. [Google Scholar]
- 11.Zhao Yu-ying, Zhang Ru-yi. Overview of studies on chemical constituents of Sophora flavescens Ait. Natural Product Research and Development. 1991;3(3):93–102. [Google Scholar]
- 12.Zhu Liang, Song Jian, Zhang Xing-rong, Shen Jian-wei, Zhang Xian-kang. Effect of kwoninone on fibroblastic proliferation, morphology and expression of TGF-β1. Chinese Journal of New Drugs and Clinical Remedies. 2000;19(6):461–463. [Google Scholar]