Abstract
Background
he present study aims to explore whether Schisandra chinensis Baill, a Chinese, medicinal herb can alleviates high-fat-diet-inducing non-alcoholic steatohepatitis in rats.
Materials and Methods
In the study, 24, male Wister rats with body weight between 180-220g, were included. The rats were randomly divided into four groups: model group, normal control group, rosiglitazone group, and Schisandra chinensis Baill group. The treatment lasted for 56, days. The high-fat diet used in the present study includes 25% lard, 2%, cholesterol 0.5%, sodium cholate, and 25%, Tween-80. The hepatic levels of superoxide dismutase (SOD), and malondialdehyde (MDA); the serum levels of total cholesterol (TC), and low-density lipoprotein cholesterol (LDLC), were detected.
Results
We found that the hepatic levels of SOD were significantly lower, and the serum levels of TC, LDLC as well as, the hepatic levels of MDA in model group were significantly higher than those of normal control group; rosiglitazone group and Schisandra chinensis Baill group (P<0.05), indicates that non-alcoholic steatohepatitis rats were successfully induced by high-fat diet. Schisandra chinensis Baill group presented a significant lower serum levels of LDLC, than rosiglitazone group (P<0.05); and the hepatic levels of SOD in Schisandra chinensis Baill group were significantly lower than rosiglitazone group (P<0.05). However, no significant difference existed between Schisandra chinensis Baill group, and rosiglitazone group on the hepatic levels of MDA and the serum levels of TC (P>0.05).
Conclusion
It is then concluded that Schisandra chinensis Baill can significantly alleviate the non-alcoholic steatohepatitis of the rats induced by high-fat diet, and it may be used as a complementary therapy for rosiglitazone.
Keywords: Non-alcoholic steatohepatitis, high-fat diet, Schisandra chinensis Baill
Introduction
With the epidemic of obesity, and the metabolic syndrome, non-alcoholic fatty liver disease has become the most common liver disease, and leading cause of altered liver enzymes in Western countries. (Bellentani et al., 2000; Browning et al., 2004). Due to the high prevalence of the non-alcoholic fatty liver disease, it is impossible to perform liver biopsy in a large population at risk to predict steatohepatitis (Musso et al., 2011). Unlike simple steatosis, which is characterized by a relatively favorable clinical caurse; non-alcoholic steatohepatitis is more likely to cirrhosis, and hepatocellular carcinoma. (Cohen et al., 2011; Starley et al., 2010) Non-alcoholic fatty liver disease has been found associated with oxidative hepatocellular damage, inflammation, and activation of fibro-genesis, such as non-alcoholic steato-hepatitis. (Day, 2006). It has been found that the “two-hit” mechanism is included in the development of non-alcoholic steato-hepatitis, in which, liver steatosis constitutes the “first hit”, and it is accompanied by obesity, and metabolic disruptions inducing excessive hepatic lipid accumulation. (Day and James, 1998) Liver steatosis was found to likely increase the vulnerability of the liver to a “second hit”, in the form of oxidative stress or, pro-inflammatory insults that result in non-alcoholic steato-hepatitis (Park et al., 2012). Non-alcoholic fatty liver disease includes a spectrum from simple steatosis to non-alcoholic steato-hepatitis. The simple steatosis, and non-alcoholic steato-hepatitis are different in histological, and patho-physiological viewpoint. (Yilmaz, 2012). The increased formation of reactive oxygen species from inflammatory cell activation, mitochondrial dysfunction, and the induction of microsomal, v-oxidation were found to change the intracellular redox state, and lead to hepatic lipid per-oxidation and liver injury. The liver injury has been found to be induced by the reactive oxygen species through interaction with bio-molecules, which will then change the function. (Mato et al., 2002). A “footprint”, for oxidative stress was found to increase in liver biopsy samples of the patients with non-alcoholic fatty liver disease. (Sanyal et al., 2001). The intervention, that function to suppress oxidative stress and pro-inflammatory responses may possess the potential to prevent the development of non-alcoholic steato-hepatitis (Park et al., 2012).
Schisandra chinensis Baill, as a key Chinese medicinal herb, is native to northern and northeastern China. Its Chinese name emanates from the fact that, its berries possess all the basic five flavors: salty, sweet, sour, and pungent (spicy), and bitter. The active components in Schisandra chinensis Baill includes: deoxyschisandrin, gamma-schisandrin, schizandrin, and gomisin A. Both deoxyschisandrin, and gamma-schisandrin were proved to have protective effect against CCl4-induced hepatic damage, and ultra-high pressure extraction (UHPE), is an efficient extraction method for the extraction of effective ingredients from Schisandra chinensis Baill (Liu et al., 2009), and the aqueous extract of Schisandra chinensis Baill has been found to possess dual effects on the enzyme activity of CYP3A in rats in vivo (Chen et al., 2010).
The present study was designated to explore whether Schisandra chinensis Baill, a Chinese medicinal herb, can alleviates high-fat-diet-inducing non-alcoholic steatohepatitis in rats.
Materials and methods
Animals and group
Twenty-four male Wister rats (weighing 180–220g), were provided by the Laboratory, Animal Center of Zhejiang University of Chinese Medicine (Hangzhou, China). The animals were kept in a room under a 12hrs light-12hrs, dark cycle and environmentally controlled conditions of 22±2°C. The research was carried out according to the National Research Council's protocol for the care and use of laboratory animals. After all the animals were acclimatized a week before the experiment, the rats were randomly divided into four groups: model group (high-fat diet + intra-gastric distilled water for 56, days), normal control group (standard diet + intra-gastric distilled water for 56 days), rosiglitazone group (high-fat diet + intragastric rosiglitazone, 0.5mg/kg/day for 56, days), and Schisandra chinensis Baill group (high-fat diet + intra-gastric Schisandra chinensis Baill, 100mg/kg/day for 56, days). Food intake was recorded throughout the 8-week treatment. A high-fat diet includes 25%, lard, 2%, cholesterol 0.5%, sodium cholate and 25%, Tween-80, which were provided by the Laboratory, Animal Center of Zhejiang University of Chinese Medicine (Hangzhou, China). Schisandra chinensis Baill was provided, and identified by Zhejiang University of Chinese Medicine (Hangzhou, China). 20g, of Schisandra chinensis Baill was extracted by refluxing with 250ml of water for 30min., followed by filtration. The same extraction procedures were repeated once. The obtained solution was combined, and condensed to a concentration of 1g/ml, which was then freeze dried, and extract of Schisandra chinensis Baill gotten. The rosiglitazone used in the present study was purchased from Jinan Zhongke Yitong Chemical Co., Ltd., China.
Sample collection and detection of parameters
On the day following the final administration, all the rats were sacrificed after 12hrs of fasting. The serum and liver samples were taken after the rats were anesthetized with diethyl ether. The serum obtained was stored at −80 °C, until assay. The liver tissues were rapidly dissected, cut and fixed in 10%, formaldehyde saline solution for the determination of the hepatic levels of malondialdehyde (MDA), and superoxide dismutase (SOD), using commercial assay kits (Sigma-Aldrich, USA), according to manufacturer instructions. The serum levels of total cholesterol (TC), and low-density lipoprotein cholesterol (LDLC), were determined by enzymatic colorimetric method using commercial standard enzymatic assay kits (Biosino, Bio-technology & Sience Inc.), according to the manufacturer's instruction. All the measurements were carried out in duplicate and conducted according to the manufacturer's instruction. Both intra-and inter-assay coefficients of variation were less than 10%.
Statistical analysis
All the data were analyzed with Statistical Package for Social Sciences (SPSS 13.0 for Windows). Analysis of variance (ANOVA), was employed for analyzing all the data. A 5%, significance level (P<0.05), and two-tailed tests were used for all hypothesis tests.
Results
The hepatic levels of SOD
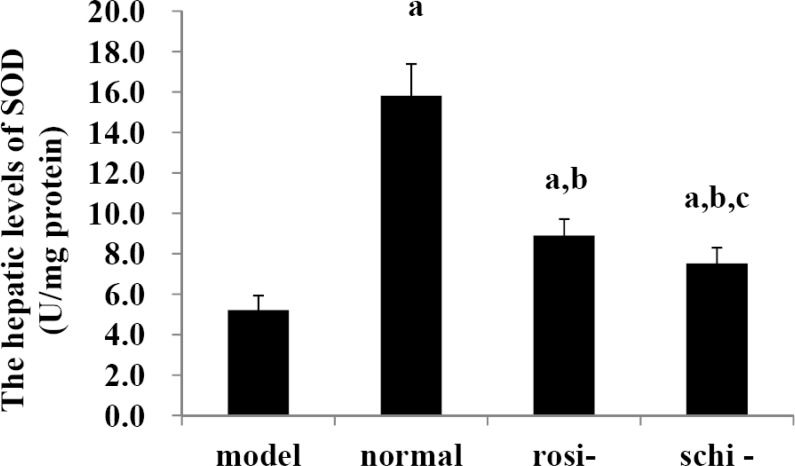
As shown in Figure 1, in model group, the hepatic levels of SOD were notably lower than those of normal control group, rosiglitazone group, and Schisandra chinensis Baill group (P<0.05). In the normal control group, the hepatic levels of SOD were significantly higher than those of rosiglitazone group, and Schisandra chinensis Baill group (P<0.05). The hepatic levels of SOD in Schisandra chinensis Baill group were significantly lower than rosiglitazone group (P<0.05).
Figure 1.
The hepatic levels of superoxide dismutase (SOD): model group (high-fat diet + intragastric distilled water for 56, days), normal control group (standard diet + intragastric distilled water for 56 days), rosiglitazone group (rosi-group: high-fat diet + intra-gastric rosiglitazone, 0.5mg/kg/day for 56, days) and Schisandra chinensis Baill group (schi-group: high-fat diet + intragastric Schisandra chinensis Baill, 100mg/kg/day for 56, days). Data were shown as mean ± SD. (n=6 in each group). The significant difference was set at a P<0.05, compared with the model group; b p <0.05, compared with normal control group; c P<0.05, compared with rosiglitazone group.
The hepatic levels of MDA
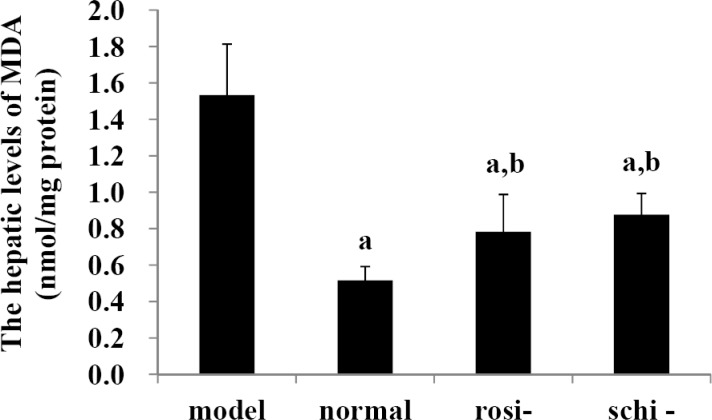
As shown in Figure 2, in the model group, the hepatic levels of MDA were remarkably higher than those of normal control group; rosiglitazone group, and Schisandra chinensis Baill group (P<0.05). In the normal control group, the hepatic levels of MDA were significantly lower than those of rosiglitazone group and Schisandra chinensis Baill group (P<0.05). There existed no significant difference on the hepatic levels of MDA between Schisandra chinensis Baill group and rosiglitazone group (P>0.05).
Figure 2.
The hepatic levels of malondialdehyde (MDA): model group (high-fat diet + intra-gastric distilled water for 56, days); normal control group (standard diet + intra-gastric distilled water for 56, days); rosiglitazone group (rosi-group: high-fat diet + intra-gastric rosiglitazone, 0.5mg/kg/day for 56, days); and Schisandra chinensis Baill group (schi-group: high-fat diet + intra-gastric Schisandra chinensis Baill, 100mg/kg/day for 56, days). Data were shown as mean ± SD. (n=6 in each group). The significant difference was set at a P<0.05, compared with model group; b p <0.05, compared with normal control group; c P<0.05, compared with rosiglitazone group.
The serum levels of TC
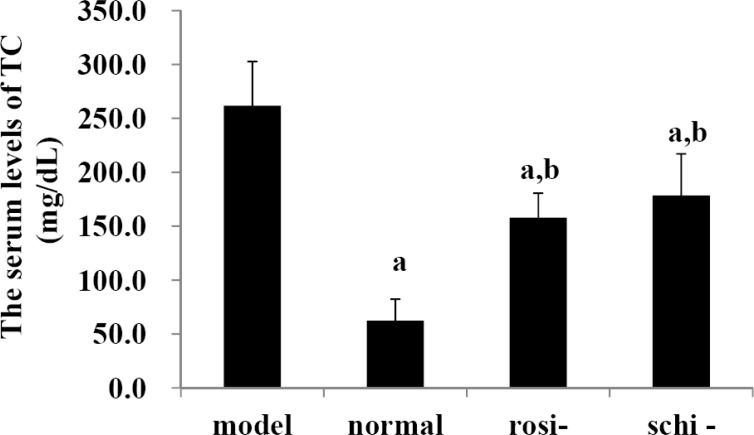
As shown in Figure 3, the serum levels of TC in model group were significantly higher than those of normal control group, rosiglitazone group, and Schisandra chinensis Baill group (P<0.05). In the normal control group, the serum levels of TC were significantly lower than those of rosiglitazone group and Schisandra chinensis Baill group (P<0.05). No significant difference existed between Schisandra chinensis Baill group, and rosiglitazone group on the serum levels of TC (P>0.05).
Figure 3.
The serum levels of total cholesterol (TC): model group (high-fat diet + intragastric distilled water for 56, days); normal control group (standard diet + intra-gastric distilled water for 56, days), rosiglitazone group (rosi-group: high-fat diet + intra-gastric rosiglitazone, 0.5mg/kg/day for 56, days); and Schisandra chinensis Baill group, (schi-group: high-fat diet + intragastric Schisandra chinensis Baill, 100mg/kg/day for 56, days). Data were shown as mean ± SD. (n=6 in each group). The significant difference was set at a P<0.05, compared with model group; b p <0.05, compared with normal control group; c P<0.05, compared with rosiglitazone group.
The serum levels of LDLC
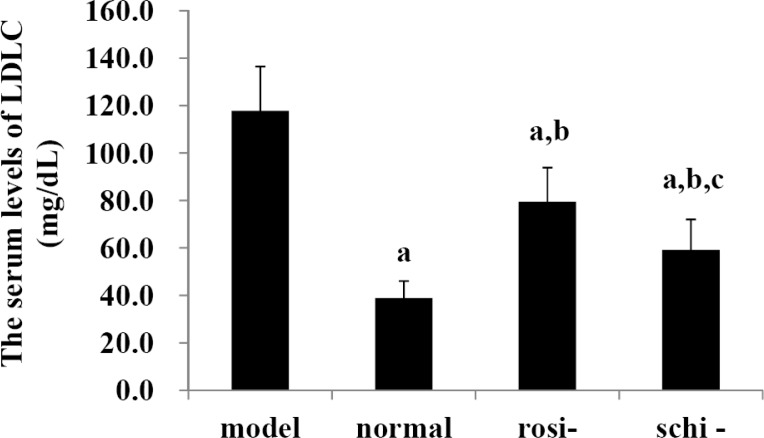
As shown in Figure 4, in the model group, the serum levels of LDLC were markedly higher than those of normal control group, rosiglitazone group, and Schisandra chinensis Baill group (P<0.05). The serum levels of LDLC in the normal control group were significantly lower than those of rosiglitazone group and Schisandra chinensis Baill group (P<0.05). Schisandra chinensis Baill group presented significantly lower serum levels of LDLC than rosiglitazone group (P<0.05).
Figure 4.
The serum levels of low-density lipoprotein cholesterol (LDLC): model group (high-fat diet + intragastric distilled water for 56, days); normal control group (standard diet + intra-gastric distilled water for 56, days); rosiglitazone group (rosi-group: high-fat diet + intra-gastric rosiglitazone, 0.5mg/kg/day for 56, days); and Schisandra chinensis Baill group (schi-group: high-fat diet + intra-gastric Schisandra chinensis Baill, 100mg/kg/day for 56, days). Data were shown as mean ± SD. (n=6 in each group). The significant difference was set at a P<0.05, compared with model group; b p <0.05, compared with normal control group; c P<0.05, compared with rosiglitazone group.
Discussion
Non-alcoholic fatty liver disease, defined by the presence of liver fat deposit associated with the metabolic syndrome as well as, the systemic insulin resistance (Alisi et al., 2012; Alisi and Nobili, 2012), is a major public health issue for the high prevalence. The oxidative, and nitrosative stress were also found in the animal experiment. (Armutcu et al., 2005) Consequently, it is important to create some noninvasive methods for the diagnosis, and follow-up of non-alcoholic steato-hepatitis; and effective treatment to alleviate the progression of non-alcoholic steato-hepatitis are urgently needed. Some promising results have been shown for anti-oxidants (Armstrong et al., 2010; Sanyal et al., 2010; Violi and Cangemi, 2010). However, no therapeutic trial has yielded to demonstrate the results in the progression of liver damage. (Musso et al., 2010)
In 2005, a simple, economic, and effective non-aqueous capillary electrophoresis separation and detection method, has been established for the quantification of deoxyschizandrin, and gamma-schizandrin in Schisandra chinensis Baill (Anjia et al., 2005). In a previous study, four bioactive lignans (schisandrin, schisantherin, deoxyschizandrin, and gamma-schizandrin) in Schisandra chinensis Baill were determined using Slice-scan method together with the new quantitative strategy, and satisfactory results have been obtained (Qi et al., 2009) Schisandrin, the main active ingredient isolated from Schisandra chinensis Baill, has been found to have anti-inflammatory properties through inhibition of nitric oxide (NO), production, prostaglandin E(2) (PGE(2)), release, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression, which in turn results from the inhibition of nuclear factor-kappaB (NF-kappaB), c-Jun N-terminal kinase (JNK), and p 38, mitogen-activated protein kinase (MAPK), activities in a RAW 264.7, macrophage cell line. (Guo et al., 2008). In the present study, we found that the hepatic levels of SOD were significantly lower, and the serum levels of TC, LDLC as well as, the hepatic levels of MDA in model group were significantly higher than those of normal control group, rosiglitazone group, and Schisandra chinensis Baill group, indicating the non-alcoholic steatohepatitis rats were successfully induced by high-fat diet. Schisandra chinensis Baill group presented significant lower serum levels of LDLC, than rosiglitazone group; and the hepatic levels of SOD in Schisandra chinensis Baill group were significantly lower than rosiglitazone group. However, no significant difference existed between Schisandra chinensis Baill group and rosiglitazone group on the hepatic levels of MDA and the serum levels of TC. Although Schisandra chinensis Baill failed to alter some parameters as marked as rosiglitazone, we found it can significantly alleviate the non-alcoholic steatohepatitis of the rats induced by high-fat diet, hence merits further study. Further study will be conducted on the mechanism involved in the process of Schisandra chinensis Baill alleviating the non-alcoholic steatohepatitis of the rats induced by high-fat diet.
Conclusion
Schisandra chinensis Baill can significantly alleviate the non-alcoholic steatohepatitis of the rats induced by high-fat diet, and may be used as complementary therapy for rosiglitazone.
Acknowledgements
The present research was partly funded by the grants for TCM, Zhejiang Province (No.2010ZB083, and 2008YA023).
References
- 1.Alisi A, Cianfarani S, Manco M, Agostoni C, Nobili V. Non-alcoholic fatty liver disease and metabolic syndrome in adolescents: pathogenetic role of genetic background and intrauterine environment. Ann Med. 2012;44:29–40. doi: 10.3109/07853890.2010.547869. [DOI] [PubMed] [Google Scholar]
- 2.Alisi A, Nobili V. Non-alcoholic fatty liver disease in children now: lifestyle changes and pharmacologic treatments. Nutrition. 2012;28:722–726. doi: 10.1016/j.nut.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Anjia C, Cunhong L, Wenhua G, Zhide H, Xingguo C. Separation and determination of active components in Schisandra chinensis Baill. and its medicinal preparations by non-aqueous capillary electrophoresis. Biomed Chromatogr. 2005;19:481–487. doi: 10.1002/bmc.464. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong MJ, Houlihan DD, Rowe IA. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;363:1185. doi: 10.1056/NEJMc1006581. author reply 1186. [DOI] [PubMed] [Google Scholar]
- 5.Armutcu F, Coskun O, Gurel A, Kanter M, Can M, Ucar F, Unalacak M. Thymosin alpha 1 attenuates lipid peroxidation and improves fructose-induced steatohepatitis in rats. Clin Biochem. 2005;38:540–547. doi: 10.1016/j.clinbiochem.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 7.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Wu YJ, Cheng NN, Li YL, Wang YM. [Dual effects of extract of Schisandra chinensis Baill on rat hepatic CYP3A] Yao Xue Xue Bao. 2010;45:1194–1198. [PubMed] [Google Scholar]
- 9.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–210. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 12.Guo LY, Hung TM, Bae KH, Shin EM, Zhou HY, Hong YN, Kang SS, Kim HP, Kim YS. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur J Pharmacol. 2008;591:293–299. doi: 10.1016/j.ejphar.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Zhang S, Wu H. Non-thermal extraction of effective ingredients from Schisandra chinensis Baill and the antioxidant activity of its extract. Nat Prod Res. 2009;23:1390–1401. doi: 10.1080/14786410902726100. [DOI] [PubMed] [Google Scholar]
- 14.Mato JM, Corrales FJ, Lu SC, Avila MA. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- 15.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 16.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 17.Park HJ, Lee JY, Chung MY, Park YK, Bower AM, Koo SI, Giardina C, Bruno RS. Green tea extract suppresses NFkappaB activation and inflammatory responses in diet-induced obese rats with nonalcoholic steatohepatitis. J Nutr. 2012;142:57–63. doi: 10.3945/jn.111.148544. [DOI] [PubMed] [Google Scholar]
- 18.Qi Y, Song Y, Fan G, Wu Y, Chai Y, Lu F. Resolution of two-way chromatographic data of Schisandra chinensis Baill. using a modified slice-scan method for direct determination of the components in overlapping peaks. Anal Sci. 2009;25:541–545. doi: 10.2116/analsci.25.541. [DOI] [PubMed] [Google Scholar]
- 19.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 22.Violi F, Cangemi R. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;363:1185–1186. doi: 10.1056/NEJMc1006581. author reply 1186. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz Y. Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36:815–823. doi: 10.1111/apt.12046. [DOI] [PubMed] [Google Scholar]