Abstract
SIRT2 is a member of the mammalian sirtuin family (SIRT1–7). As compared with other sirtuins, SIRT2 is found primarily in the cytoplasm. It regulates multiple physiological processes. However, the precise role of SIRT2 in skin cancer remains unclear. Here, we show that SIRT2 is down-regulated in human skin cancer as compared with normal skin. SIRT2 deletion increases tumor growth in mice. SIRT2 knockdown up-regulates the stem cell marker Keratin 19 (K19) in keratinocytes. In mice, SIRT2 deletion up-regulates K19 and K15 while it down-regulates the differentiation marker Loricrin in both normal skin and tumors. In skin tumors but not normal skin SIRT2 deletion up-regulates the stem cell marker CD34 and increases the number of Ki67-positive cells. These findings indicate that SIRT2 is a tumor suppressor in the skin. Our findings add new insights into the role of SIRT2 in the molecular pathogenesis of skin cancer.
Keywords: SIRT2, Skin cancer, K19, Keratinocytes
Background
The members of the Sir2 family of NAD+-dependent deacetylases, also known as Sirtuins, are crucial factors in the response to metabolic, oxidative, or genotoxic stress. SIRT2 is a member of the mammalian sirtuin family (SIRT1–7). As compared with other sirtuins, SIRT2 is found primarily in the cytoplasm. It regulates multiple physiological processes by deacetylating several proteins, including alpha-tubulin (1), FOXO1 (2), deacetylates histone H4 at lysine 16 (3) and modulating the mitotic deposition of H4K20 methylation (4).
Questions addressed
SIRT2 plays an important role in cancer. In gliomas, melanomas, and gastric carcinomas, SIRT2 protein and RNA levels are decreased (5). However, the precise role of SIRT2 in skin cancer remains unclear.
Experimental design
Methods and materials can be found in the supporting information online.
Results
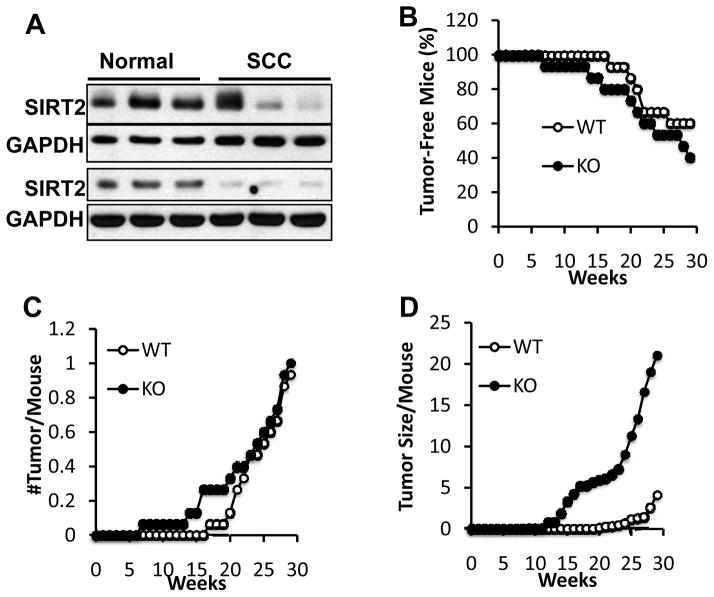
To determine whether SIRT2 has a role in skin cancer, we first analyzed SIRT2 protein levels in normal human skin and human squamous cell carcinoma (SCC). We found that SIRT2 protein levels were decreased in five out of six invasive SCCs from sun-exposed areas as compared with unpaired sun-protected normal human skin (Figure 1a), suggesting that SIRT2 is a tumor suppressor in human skin. To determine the role of SIRT2 in skin tumorigenesis, we treated wild-type mice (WT) and SIRT2 knockout mice (KO) once with 7,12-dimethylbenz(a)anthracene (DMBA) and then twice weekly for 30 weeks with 12-O-tetradecanoylphorbol-13-acetate (TPA). SIRT2 deletion slightly increased tumor onset and the number of tumor per mouse (Figure 1a–b; P > 0.05). Intriguingly, the tumor size for KO mice was significantly larger than for WT mice (Figure 1d; P < 0.05, two-way ANOVA), indicating that loss of SIRT2 increases tumor growth. The ratio of SCC to papilloma was higher in KO mice (25%) than in WT mice (8%), indicating that SIRT2 loss promotes tumor progression. These findings demonstrate that SIRT2 is a tumor suppressor in the skin.
Figure 1.
Role of SIRT2 in human skin cancer and DMBA/TPA-induced mouse skin tumorigenesis. (a) Immunoblot analysis of SIRT2 and GAPDH protein levels in human normal skin and squamous cell carcinoma. (b, c, d) Percentage (%) of tumor-free mice (b), tumor number per mouse (c) and tumor size per mouse (d, mm3) in SIRT2 WT and KO mice (n=15) following DMBA/TPA treatment.
To determine the molecular mechanism in the action of SIRT2, we measured the role of SIRT2 in critical processes involved in skin tumorigenesis, including DNA damage repair, apoptosis and proliferation in normal human epidermal keratinocytes (NHEK), transfected with negative control (siNC), or siRNA targeting SIRT2 (siSIRT2) (Figure S1a–d). We found that SIRT2 had no effect on the repair of UVB-induced cyclobutane pyrimidine dimers (CPD), the major DNA lesions (Figure S1a), or the levels of proteins involved in UVB-induced DNA damage repair, including XPC, DDB1 or DDB2 (Figure S1b). SIRT2 did not significantly affect p53 acetylation or p53 levels (Figure S1b), UVB-induced apoptosis or cell proliferation (Figure S1c–d).
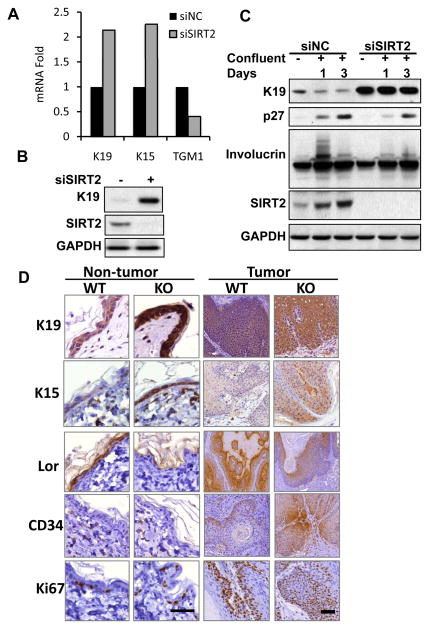
To determine the molecular function of SIRT2, we carried out a microarray analysis of gene expression in siNC and siSIRT2-transfected NHEK cells. Among the genes that were up- or down-regulated by SIRT2 knockdown (Supplemental Table 1), several were genes involved in keratinocyte differentiation. SIRT2 inhibition increased the expression of keratin (K19) and keratin 15 (K15), while it decreased the expression of transglutaminase 1 (TGM1) (Figure 2a). Immunoblot analysis indicated that SIRT2 knockdown increases K19 protein levels (Figure 2b), suggesting that SIRT2 plays a role in keratinocyte differentiation. To determine the role of SIRT2 in keratinocyte differentiation induced by confluence, we measured the levels of SIRT2, K19, and the differentiation markers involucrin and p27 in NHEK cells at subconfluence, confluence and postconfluence. As compared with their subconfluent cells, while SIRT2 was up-regulated, K19 was down-regulated in NHEK cells transfected with siNC in confluence for 1 and 3 days, in parallel with the up-regulation of p27 and involucrin (Figure 2c). Knockdown of SIRT2 delayed up-regulation of p27 and involucrin after confluence, and increased K19 levels before and after confluence (Figure 2c). To determine the role of SIRT2 in keratinocyte differentiation and stemness, we assessed the difference in the levels of K19, the differentiation marker Loricrin, the stem cell marker CD34 and K15, and the number of Ki67-positive cells in normal skin and tumors between SIRT2 WT and KO mice. As compared with SIRT2 WT mice, in SIRT2 KO mice K19 and K15 were increased in both normal skin and tumors (Figure 2d), while Loricrin was decreased. In tumors but not in normal skin, SIRT2 deletion increased CD34 expression and number of K67-positive cells (Figure. 2d). These results indicate that SIRT2 promotes keratinocyte differentiation and that SIRT2 loss promotes tumor cell stemness and proliferation.
Figure 2.
Role of SIRT2 in cell differentiation, proliferation, and stemness in keratinocytes, normal skin, and tumors. (a) Gene array analysis of mRNA levels of K19, K15, and TGM1 in siNC and siSIRT2 NHEK cells. (b) Immunoblot analysis of K19, SIRT2 and GAPDH in NHEK cells transfected with NC/siSIRT2. (c) Immunoblot analysis of K19, p27, Involucrin, SIRT2, and GAPDH in NHEK cells at subconfluence or confluence for 1 day and 3 days following transfection with siNC or siSIRT2. (d) Immunohistochemical analysis of K19, K15, Loricrin (Lor), CD34, and Ki67 in normal skin and tumors from SIRT2 WT and KO mice. Representative images from three independent specimens were shown. Scale bar: 50 μm.
Discussion
Our findings demonstrate that SIRT2 functions as a tumor suppressor in skin, consistent with a key role of SIRT2 in recent studies (4, 6) reported that the SIRT2 loss is associated with a greater risk of tumorigenesis. The major difference between our study and the recent work by Serrano et al (4) is that we did not detect tumor metastasis or the formation of fibrosarcoma. This may be due to the higher concentration of TPA used in their studies. Knockdown of SIRT2 increased keratin 19, but did not affect UVB-induced DNA damage repair and apoptosis. K19 has been considered to be a putative marker for epidermal stem cells in the hair follicle bulge (7). Its expression is associated with the retention of stem cell characteristics or a state of poor differentiation and a more aggressive behavior in human skin and hepatocellular tumors (8, 9). As loss of differentiation is known to promote tumorigenesis (10, 11), our data indicate that inhibition of SIRT2 may promote tumor growth in skin through inhibition of differentiation and increasing stemness by regulating K19. This is in contrast with the role of SIRT2 in differentiation of adipocytes (12), where SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1, and in differentiation of oligodendroglia (13), where SIRT2 decelerates cell differentiation through deacetylating a-Tubulin. However, SIRT2 was found to enhance CG4 oligodendroglial differentiation by enhancing the expression of myelin basic protein (14). These discrepancies may be due to a cell type-dependent role of SIRT2.
In summary, our results indicate that SIRT2 suppresses skin tumorigenesis, and also suggest that SIRT2 promotes keratinocyte differentiation and suppresses tumor cell stemness in association with down-regulating K19. Our findings add new insight into the role of SIRT2 in the molecular pathogenesis of non-melanoma skin cancer.
Supplementary Material
Acknowledgments
This work was supported by the NIH/NIEHS grant ES016936 (YYH), the American Cancer Society (ACS) grant RSG-13-078-01 (YYH), the NIH grants P30 CA014599 and UL1RR024999, and the University of Chicago Friends of Dermatology Endowment Fund. We thank Terri Li for K19, K15, CD34, Loricrin, and Ki67 immunohistochemical analysis.
Footnotes
Author contribution
MM and YYH designed the study, analyzes the data, and wrote the paper. MM performed the experimental work. QL and BZ provided essential tools.
CONFLICT OF INTERESTS
The authors state no conflict of interest.
References
- 1.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 2.Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007;6:105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaquero A, Scher MB, Lee DH, et al. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006;20:1256–1261. doi: 10.1101/gad.1412706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrano L, Martinez-Redondo P, Marazuela-Duque A, et al. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev. 2013;27:639–653. doi: 10.1101/gad.211342.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Oliveira RM, Sarkander J, Kazantsev AG, Outeiro TF. SIRT2 as a Therapeutic Target for Age-Related Disorders. Front Pharmacol. 3:82. doi: 10.3389/fphar.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HS, Vassilopoulos A, Wang RH, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20:487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel M, Torok N, Godbout MJ, et al. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109 ( Pt 5):1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Takahara M, Kido M, et al. Increased expression of an epidermal stem cell marker, cytokeratin 19, in cutaneous squamous cell carcinoma. Br J Dermatol. 2008;159:952–955. doi: 10.1111/j.1365-2133.2008.08731.x. [DOI] [PubMed] [Google Scholar]
- 9.van Sprundel RG, van den Ingh TS, Desmet VJ, et al. Keratin 19 marks poor differentiation and a more aggressive behaviour in canine and human hepatocellular tumours. Comp Hepatol. 2010;9:4. doi: 10.1186/1476-5926-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aymard E, Barruche V, Naves T, et al. Autophagy in human keratinocytes: an early step of the differentiation? Exp Dermatol. 2011;20:263–268. doi: 10.1111/j.1600-0625.2010.01157.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, Cho KH, Lee JH, Chang MS, Cho S. Intratumoral mast cell number is negatively correlated with tumor size and mitosis in dermatofibrosarcoma protuberans. Exp Dermatol. 2012;21:559–561. doi: 10.1111/j.1600-0625.2012.01530.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Tong Q. SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1’s repressive interaction with PPARgamma. Mol Biol Cell. 2009;20:801–808. doi: 10.1091/mbc.E08-06-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Zhang B, Tang J, et al. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci. 2007;27:2606–2616. doi: 10.1523/JNEUROSCI.4181-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji S, Doucette JR, Nazarali AJ. Sirt2 is a novel in vivo downstream target of Nkx2. 2 and enhances oligodendroglial cell differentiation. J Mol Cell Biol. 2011;3:351–359. doi: 10.1093/jmcb/mjr009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.