Abstract
Marking human saphenous vein graft (HSV) with a surgical skin marker to prevent twisting on implantation is a common practice in peripheral and coronary artery bypass procedures. This study is designed to examine the effects of surgical skin markers on the HSV smooth muscle and endothelial functional responses. De-identified HSV remnants were collected during peripheral and coronary artery bypass procedures. Physiologic responses of the HSV were measured using a muscle bath. Veins that were marked with surgical skin markers intraoperatively generated significantly less contractile force to depolarizing KCl (110 mM) and receptor-mediated contractile agonists than unmarked HSV, suggesting that surgical skin markers impaired HSV smooth muscle contractility. To directly access the effects of chemical components in the surgical skin markers, unmarked HSV was exposed to isopropyl alcohol (a solvent commonly used in surgical skin markers) or methylene blue (a dye). Smooth muscle contractility was significantly reduced by isopropyl alcohol and methylene blue. Endothelial-dependent relaxation to carbachol was significantly reduced after exposure to surgical skin markers. Our data demonstrated that marking HSV with surgical skin markers reduces smooth muscle and endothelial functional viability.
Over one million coronary artery bypass grafts (CABG) and peripheral revascularization procedures are performed each year. Despite the higher patency rate of the internal mammary artery when compared with the human saphenous vein (HSV) and increased interest in other arterial conduits, HSV remains the most widely used conduit for CABG procedures. Vein graft failure rate remains high, however, approaching 40 per cent at 18 months postoperatively.1
The predominant histological finding in failed vein grafts is intimal hyperplasia, which is thought to be the manifestation of cellular responses to injury.2 Vein graft injury during intraoperative graft preparation leads to intimal hyperplasia, accelerated atherosclerosis, and subsequent graft failure.2, 3 Injurious mechanisms during graft preparation include endovascular harvesting techniques, radial distension, and choice of storage solution.1, 4, 5 Optimal vein graft preparation before surgical anastomosis is hence important for long-term graft patency.6
Vein graft marking is a practice frequently used to properly orient the graft before implantation. Originally intended for use in marking the skin area around surgical sites, surgical skin markers are sterile, inexpensive, and readily available to surgeons for vein graft marking. The chemical constituents of these markers consist of alcohol-based solvents and the dyes methylene blue or gentian violet. Vein marking with methylene blue impairs both endothelium-dependent and endothelium-independent graft function.7 Similarly, gentian violet leads to a decrease in endothelium-independent relaxation to sodium nitroprusside.8 Although the deleterious effects of gentian violet and methylene blue are well established, the practice of using skin markers still remains widely accepted for CABG and peripheral vascular procedures. Although surgical skin markers are nontoxic in dermatology applications, little is known about the effects of applying the marker directly to the vein grafts. This study examined the effects of surgical skin markers on functional viability of HSV used for autologous bypass procedures.
Methods
Human Saphenous Vein Procurement
All human saphenous vein graft remnants were obtained after the approval of the Institutional Review Boards of the Vanderbilt University Medical Center and the Veterans Affairs Tennessee Valley Healthcare System, Nashville, Tennessee. De-identified remnant segments of HSV (n = 38) were collected from patients undergoing CABG and peripheral vascular bypass procedures. The veins were procured according to the surgeon’s discretion in terms of surgical and medical interventions, intraoperative graft handling such as distention, and the use of surgical skin markers. The veins were stored in heparinized saline solution until the end of the surgical procedure at which time they were placed in a cold University of Wisconsin transplant harvest buffer at 4°C (100 mM potassium lactobionate, 25 mM KH2PO4, 5 mM MgSO4, 30 mM raffinose, 5 mM adenosine, 3 mM glutathione, 1 mM allopurinol, 50g/L hydroxyethyl starch, pH 7.4). The vessels were tested within 24 hours of harvest. For endothelial-dependent relaxation experiments, HSV (n = 8) were collected immediately after surgical harvest without any further intraoperative manipulations such as the use of skin markers or distention and tested within 2 hours of surgical procurement.
Physiological Measurements of Functional Viability
The HSV was dissected free of adipose and connective tissue and 1 mm rings were cut. The rings were weighed and their lengths were measured. To focus on smooth muscle responses, the endothelium was mechanically denuded by gently rolling the luminal surface of each ring at the tip of fine vascular forceps before suspension in a muscle bath containing a bicarbonate buffer (120 mM NaCl, 4.7 mM KCl, 1.0 mM MgSO4, 1.0 mM NaH2PO4, 10 mM glucose, 1.5 mM CaCl2, and 25 mM Na2HCO3, pH 7.4), equilibrated with 95 per cent O2 and 5 per cent CO2 at 37°C. The rings were progressively stretched to the optimal resting tension (approximately 1 gm) that would produce a maximal response to contractile agonists as described previously, and then maintained at the resting tension and equilibrated for a minimum of 2 hours.9, 10 Force measurements were obtained using a Radnoti Glass Technology (Monrovia, CA) force transducer (159901A) interfaced with a Powerlab data acquisition system and Chart software (AD Instruments, Colorado Springs, CO). Smooth muscle functional viability was determined by contracting the HSV rings repeatedly with 110 mM KCl (with equimolar replacement of NaCl in bicarbonate buffer) until the maximal response was generated. Potassium challenge (110 mM) causes depolarization of the membrane leading to contraction of functionally viable smooth muscle.11 Tissues that failed to respond to KCl (generated a contractile force equivalent to stress < 0.025 ×105 N/m2) were not tested further. Rings that generated stress of ≥ 0.025 × 105 N/m2 were washed to remove the KCl and equilibrated in bicarbonate buffer for 30 minutes.12 Concentration of physiologic agonists that would induce submaximal contractile responses was predetermined by treating the tissue with increasing doses (0.01, 0.1, and 1 μM) of norepinephrine (NE) or phenylephrine (PE). Tissues were then washed with bicarbonate buffer to remove the agonists and treated with 10−6 M NE or 10−6M PE (submaximal doses) to determine contractile responses.
Physiological Measurements of Endothelial-Derived Vasorelaxation
Rings from HSV that were not manipulated intraoperatively were prepared and tested essentially as described above except that the endothelium was preserved. Viable tissues were precontracted with 10−6 M PE and then treated with 5 × 10−7 M carbachol, and the maximal relaxation response was determined.13
Effect of Surgical Skin Markers on Vascular Reactivity
To study the effect of surgical skin markers and their chemical constituents, three commercially-available surgical skin markers were obtained (SSM1, Devon Skin Marker and Ruler, Covidien, Mansfield, MA; SSM2, Cardinal Health Corp., Dublin, OH; SSM3, MediChoice, Sunrise, FL). These marking pens were used at the institutions where HSV were collected for this study and all contained 50 per cent isopropyl alcohol and the dye gentian violet. Rings from unmarked HSV remnant segments were painted on the surface with either SSMs, or a cotton swab saturated with 1 per cent methylene blue (Akorn, Inc., Lake Forest, IL), or submerged in 50 per cent isopropyl alcohol at room temperature for 15 minutes. Contraction to 110 mM KCl and contractile agonists was measured in the muscle bath as described above.
To assess the effect of surgical skin markers on endothelial-dependent vasorelaxation responses, rings from minimally manipulated HSV were painted with surgical skin marking pens and incubated in 0.5 mL PlasmaLyte A (Baxter, Deerfield, IL), a pH balanced physiological solution used for storing HSV during graft preparation, at room temperature for 15 minutes. Vascular responses to 110 mM KCl and contractile agonists were measured in the muscle bath as described above.
Data Analysis
Contractile response was defined by stress ([105 Newtons (N)/m2] = force (g) × 0.0987/area, where area is equal to the wet weight [(mg)/length (mm at maximal length)] divided by 1.055), which was calculated using the force generated by the tissues.14 Any tissue that generated stress of 0.025 × 105 N/m2 or greater was considered functionally viable, which correlates to 0.5 g of force for a 10 mg, 1 mm thick, 4 mm diameter ring. Data was reported as mean responses ± Standard Error of the Mean. Unpaired t tests were conducted to determine the significance (P value) of each experiment using GraphPad Prism software (LaJolla, CA). A P value ≤ 0.05 was considered statistically significant.
Results
Contractile Response of Human Saphenous Vein Grafts
Thirty-eight HSV surgical remnant segments were obtained and 22 (58%) had visible blue marking from surgical skin marker at the time of collection from the operating room. HSV segments that had no visible blue marking generated significantly (P < 0.0001) greater contractile responses to 110 mM KCl (0.174 ± 0.023 105 N/m2, n = 16) than those that were marked (0.047 ± 0.014 105 N/m2, n = 22) (Fig. 1A). Viable HSV rings (≥ 0.025 N/m2, n = 30) were then treated with a sub-maximal dose of the contractile agonist, norepinephrine (NE; 10−6 M). HSV without blue marking generated significantly (P = 0.0004) greater contractile responses to NE (0.1244 ± 0.02765 N/m2, n = 13) than those that had visible blue marking (0.02288 ± 0.006272 N/m2, n = 17) (Fig. 1B).
Fig. 1.
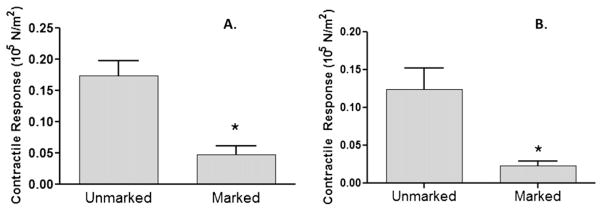
Human saphenous vein grafts with blue markings displayed impaired contractile responses. Remnant saphenous vein from patients undergoing coronary artery bypass or peripheral vascular revascularization surgery were collected (n = 38). Rings from each vein were suspended in a muscle bath, contracted with 110 mM KCl (A), 10−6 M norepinephrine (B), force was measured, and converted to stress (105 N/m2). Unmarked (n = 16) had no visible sign of markers when collected; marked (n =22) had visible sign of marking. The error bars show the standard error of the mean. * P < 0.0001.
Surgical Skin Marker Chemical Constituents Decreased HSV Smooth Muscle Contractile Response
We next determined whether isopropyl alcohol, a solvent used in surgical skin markers, impairs contractile responses of HSV. Rings were cut from HSV segments that were void of blue marking. These rings were either left untreated, marked with surgical skin marker SSM1, treated with 50 per cent isopropyl alcohol, or treated with 1 per cent methylene blue, and exposed to 110 mM KCl. HSV rings that were left untreated produced significantly greater contractile response (0.110 ± 0.014 105 N/m2, n = 12) than the rings that were marked with SSM1 (0.003 ± 0.001 105 N/m2, n = 5; P = 0.0002), 50 per cent isopropyl alcohol (0.005 ± 0.003 105 N/m2, n = 5; P =0.002), or methylene blue (0.014 ± 0.010 105 N/m2, n = 10; P < 0.0001) (Fig. 2).
Fig. 2.
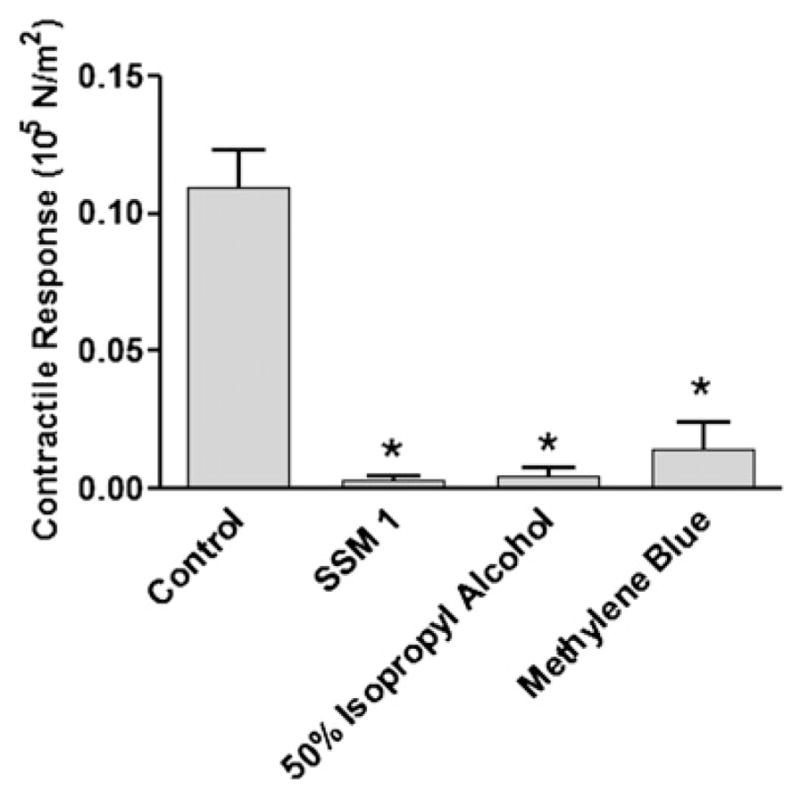
Surgical skin markers and its chemical constituents reduced contractility of remnant human saphenous vein grafts. Rings cut from remnant human saphenous vein grafts were left unmarked (control; n = 12), marked with a surgical skin marker (SSM1; n =5), treated with 50 per cent isopropyl alcohol (n =4), or with 1 per cent methylene blue (n = 10). The rings were then suspended in a muscle bath and exposed to 110 mM KCl. Force was measured and converted to stress (105 N/m2). The error bars show standard error of the mean. * P < 0.0002.
Surgical Skin Markers Impaired HSV Endothelial-Dependent Relaxation
The effect of marking with surgical skin marker on the endothelium-dependent vasorelaxation ability of the vein grafts was determined. Pressure distension during surgical harvest can lead to loss of endothelial-dependent functions; therefore, HSV segments were collected immediately after harvest and before being subjected to any intraoperative manipulation.15 Rings from the HSV were either left unmarked or marked with surgical skin markers SSM2 or SSM3. Contractile response to high potassium chloride was significantly reduced by 20 to 30 per cent (Fig. 3A; n = 4; P < 0.03) in segments marked with SSM2 (0.06681 ± 0.02713) and SSM3 (0.07455 ± 0.02901) when compared with the untreated segments (0.09638 ± 0.02261). A similar effect was seen in the agonist-induced contractile response to phenylephrine. Marking with SSM2 (0.04277 ± 0.01956 N/m2) or SSM3 (0.04773 ± 0.01935 N/m2) significantly reduced contractile force generation by 40 to 50 per cent (P < 0.03) when compared with the unmarked segments (0.07760 ± 0.01586 N/m2) after exposure to PE (Fig. 3B). When the segments were precontracted with PE, endothelium-dependent vasorelaxation to carbachol was significantly reduced in HSV (Fig. 4; n = 3; P < 0.02) marked with either SSM2 (3.560 ± 1.672%) or SSM3 (7.334 ± 2.192%) when compared with the unmarked segments (19.94 ± 1.529%).
Fig. 3.
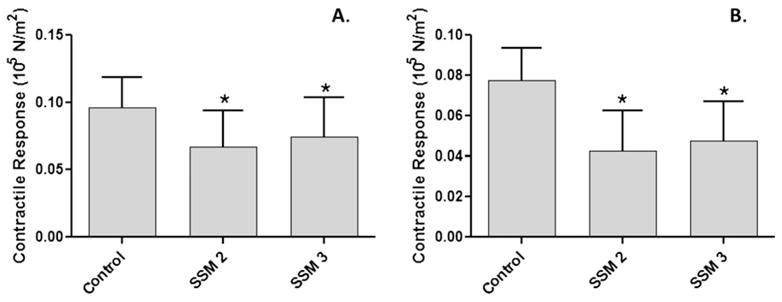
Surgical skin markers impaired contractile response in minimally manipulated human saphenous vein grafts. Saphenous veins from patients undergoing coronary artery bypass or peripheral vascular revascularization surgery were collected before any intraoperative preparation (n =4). Rings from each vein were either left untreated (control), or treated with one of two different surgical skin markers (SSM2 or SSM3), and suspended in a muscle bath. Rings were contracted with 110 mM KCl (A), or 10 −6 M phenylephrine (B), force was measured and converted to stress (105 N/m2). The error bars show standard error of the mean. *P < 0.003.
Fig. 4.
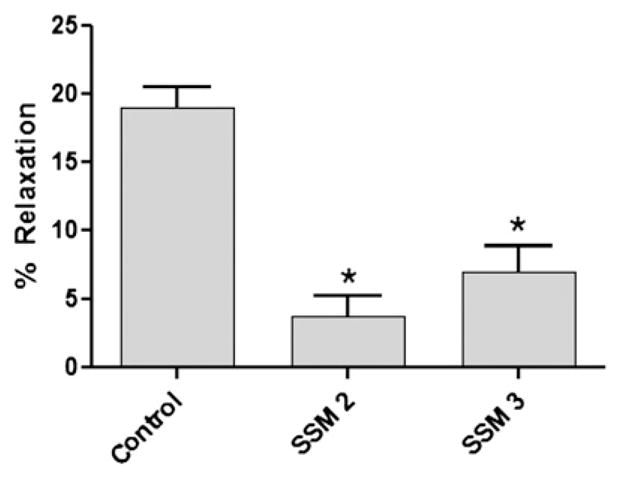
Surgical skin markers impaired endothelial-dependent relaxation in minimally manipulated human saphenous vein grafts. Saphenous veins from patients undergoing coronary artery bypass or peripheral vascular revascularization surgery were collected before any intraoperative preparation (n = 3). Rings from each vein were either left untreated (control), or treated with one of two different surgical skin markers (SSM2 or SSM3), and suspended in a muscle bath. Rings were precontracted with 10−6 M phenylephrine and then exposed to 5 × 10−7 M carbachol. Force was measured and converted to stress (105 N/m2). The error bars show standard error of the mean. * P < 0.002.5.
Discussion
Vein graft failure after coronary artery bypass procedures has been attributed to injury to the endothelial and/or medial layers of the saphenous vein and remains an enigmatic, morbid, and expensive problem. Vein graft failure leads to myocardial infarction, heart failure, repeat hospitalizations, and repeat surgical or percutaneous interventions. Injury to veins by mechanical damage, including distension and endovascular harvesting techniques, lead to thrombosis, intimal hyperplasia, and ultimately vein graft failure.16 We have previously reported that vein grafts used in revascularization procedures display variable contractile function and viability.12 A (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, or MTT, live/dead assay illustrated that the loss of the contractility of smooth muscle directly correlates to the loss of cellular viability in HSV suggesting surgical preparation and manipulation lead to cell death and hence significant loss of conduit function. Before implantation, HSV is prepared on a “back table” where marking vein grafts is routinely used for graft orientation and prevention of graft kinking. Although mechanical damage to HSV grafts has been described in detail, there remains a paucity of literature outlining the effects of surgical marking pens on vein graft function. Consequently, the widely accepted technique of vascular marking with a sterile surgical skin marker persists, despite unknown effects on vein grafts. The current study offers evidence that exposure of HSV to surgical skin markers contribute to impaired graft function and viability.
Vein grafts fail in 5 to 10 per cent of patients as early as the first postoperative week and are associated with adverse outcomes.17–19 A common theme for early vein graft failure (occurs within 1 month) is vascular endothelial injury.20–22 Manchio et al.22 showed that, at 5 days post coronary artery bypass surgery, thrombosed veins had only 10 per cent of the endothelial layer intact, compared with 50 per cent intact endothelium in patent vein grafts, implicating endothelial damage as a cause of early vein graft failure. Additionally, reendothelialization attenuates intimal hyperplasia after distension injury.23 Intact endothelium expresses nitric oxide synthase, eNOS, which is responsible for the conversion of L-arginine to nitric oxide. Nitric oxide inhibits platelet aggregation and vascular smooth muscle proliferation in addition to modulating vascular tone. During graft procurement and preparation, disruption of vascular endothelium exposes collagen, a substrate for thrombosis independent of platelet activation or presence of a hypercoagulable state.22, 24
By 12 to 18 months post CABG, vein graft failure is observed in approximately 40 per cent of patients.1 Although loss of endothelium integrity correlates with early vein graft failure, the primary histologic finding in late vein graft failure (>1 month postbypass) is intimal hyperplasia (IH).3 Evidence of IH may be seen as early as 3 weeks to 3 months after coronary artery bypass surgery.25, 26 Characterized by vascular smooth muscle cell proliferation, IH is a response to injury and the inciting substrate for accelerated atherogenesis associated with late graft failure.3 Phenotypic changes in vascular smooth muscle cells include conversion of a contractile, filament-rich structure to a metabolically active, organelle rich phenotype. Synthesis of extracellular matrix proteins, an important component of vein graft failure, is also increased.27
Numerous attempts to ameliorate IH, including E2F decoys, chemotherapeutic, and antiplatelet agents, have failed.2, 28, 29 However, preservation of vein graft integrity does prevent morphologic changes associated with IH. A “no-touch” method of vein graft harvesting seems to preserve vein structure and function and slows the rate of atherosclerosis formation after coronary artery bypass grafting.30 Long-term evaluation of vein grafts (>8 years) after noninjurious harvesting methods have significantly fewer and smaller atherosclerotic plaques and significantly less IH compared with conventional harvesting.30 In addition to mechanical injury, chemical damage from storage solutions, such as normal saline, also leads to IH.31 Grohs et al.5 demonstrated a marked decrease in receptor-independent depolarization and agonist-mediated contractility with prolonged storage in various storage media. Taken together, a protective role of intact endothelium and a functional, viable medial layer in vein graft patency is critical.
Given the crucial association between IH and vein graft failure, we examined the effects of surgical marking on function and viability of vein graft. In the remnant human saphenous vein grafts examined in this study, almost 60 per cent had been marked intraoperatively. The marked veins demonstrated a 73 per cent reduction in vascular smooth muscle contractility compared with HSV with no visible blue marking. HSV that were not manipulated after harvesting had a ~50 per cent decrease in smooth muscle contractile function after exposure to surgical skin marker (SSM). The difference in functional loss seen in those that were marked by the surgeons intraoperatively compared with those marked experimentally implicates that marking with surgical skin marker alone did not explain the decrease in smooth muscle function. Other graft preparation techniques may also account for more severe functional impairment observed in veins marked intraoperatively.
Because cellular viability correlates with functional viability of HSV,12 our results infer that vein marking with surgical skin markers is deleterious to the conduit and that the loss of HSV contractile response to both receptor-dependent contraction and depolarization can be attributed in part by the use of SSM and its chemical composition, particularly ~50 per cent isopropyl alcohol. Although short-term dermal application is non-toxic, isopropyl alcohol intoxication via chronic dermal exposure has been reported.32 In addition, isopropyl alcohol is converted by alcohol dehydrogenase to acetone, a solvent used to fix tissues.33 Alcohol dehydrogenase is present in human vessels with majority of the activity found in the medial layer.34 Treating the vein with isopropyl alcohol or direct contact of the vein with a surgical skin marker essentially converts the tissue to a decellularized vein, analogous to cryopreserved veins. When used for peripheral vascular reconstructions, cryopreserved veins have dismal 30 per cent and 18 per cent patency rates at 1 and 2 years, respectively.35
Because endothelial function is often abolished due to surgical harvest and intraoperative handling,21 we obtained HSV specimens that were collected immediately after surgical harvest and before “back table” preparation and subsequently marked with SSM in the laboratory. Our results indicated that marking with surgical skin markers profoundly impaired endothelial-dependent vasorelaxation to carbachol in minimally manipulated HSV. Shoemaker et al.8 previously showed that gentian violet did not affect endothelium-dependent response to < 10−6 M acetycholine. The reason for the different findings may lie in the presence of isopropyl alcohol in the SSM or the surgical procurement and manipulation of the HSV specimens used in the separate studies. Notwithstanding, our data demonstrated that the endothelium is rendered nonfunctional when exposed to these two surgical skin markers.
It is logical that cellular death and graft organ injury may result from exposure to SSM in a time and dose-dependent manner. Saphenous veins are typically marked on the conduit surface after initial harvesting and are often stored at room temperature for up to 3 hours before implantation. How much isopropyl alcohol is absorbed into the vein and the time frame during which conversion to acetone peaks within the tissue is not known. However, the fact that endothelial and medial damage was observed after a 15 minute exposure to the SSM in this study suggests that contents of the SSM is readily absorbed through the full thickness of the vein. Furthermore, the extent of vein marking varies among surgeons. We observed that noncontiguous marking along the length of HSV resulted in greater smooth muscle functional viability of vein graft compared with heavily marked veins (data not shown). At the conclusion of the procedure, the chest is often thoroughly irrigated with normal saline; leeching of alcohol in and around the graft may result in untoward effects on HSV viability.
The current study is limited by the use of de-identified tissues and the lack of intraoperative data regarding procurement techniques and graft preparation method. Additionally, patient demographics were not available which may contribute to the variability of vascular responses to injury and experimental conditions. It is also limited by the lack of long-term follow-up to infer how marking with surgical skin markers affects HSV graft patency.
In conclusion, our findings suggest that marking human saphenous veins with a surgical skin marker before implantation causes a profound decrease in contraction and relaxation function of these grafts and interferes with the activation of cellular signaling pathways involved in normal physiological responses in endothelial and vascular smooth muscle. Nevertheless, implications of these findings on development of IH graft patency remain to be determined. Until alternatives become available, restricted use of surgical skin markers for vein graft marking is advised.
Acknowledgments
This study was supported by National Institute of Health RO1HL70715 and a Veteran Affairs Merit Review to Colleen Brophy and American Heart Association 10crp2550035 to Susan Eagle.
Footnotes
Presented at the Annual Scientific Meeting and Postgraduate Course Program, Southeastern Surgical Congress, Chattanooga, TN, February 12–15, 2011.
References
- 1.Lopes RD, Hafley GE, Allen KB, et al. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N Engl J Med. 2009;361:235–44. doi: 10.1056/NEJMoa0900708. [DOI] [PubMed] [Google Scholar]
- 2.Wallitt EJ, Jevon M, Hornick PI. Therapeutics of vein graft intimal hyperplasia: 100 years on. Ann Thorac Surg. 2007;84:317–23. doi: 10.1016/j.athoracsur.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Zhang C. Neointimal hyperplasia, vein graft remodeling, and long-term patency. Am J Physiol Heart Circ Physiol. 2009;297:H1194–5. doi: 10.1152/ajpheart.00703.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rousou LJ, Taylor KB, Lu XG, et al. Saphenous vein conduits harvested by endoscopic technique exhibit structural and functional damage. Ann Thorac Surg. 2009;87:62–70. doi: 10.1016/j.athoracsur.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 5.Grohs JG, Kadletz M, Wodratzka M, et al. Contractile function of human veins after long-term storage in different media. J Cardiovasc Pharmacol. 1996;28:89–93. doi: 10.1097/00005344-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Raja SG, Haider Z, Ahmad M, Zaman H. Saphenous vein grafts: to use or not to use? Heart Lung Circ. 2004;13:403–9. doi: 10.1016/j.hlc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Barber DA, Rubin JW, Zumbro GL, Tackett RL. The use of methylene blue as an extravascular surgical marker impairs vascular responses of human saphenous veins. J Thorac Cardiovasc Surg. 1995;109:21–9. doi: 10.1016/S0022-5223(95)70417-5. [DOI] [PubMed] [Google Scholar]
- 8.Shoemaker K, Rubin J, Zumbro GL, Tackett R. Evans blue and gentian violet: alternatives to methylene blue as a surgical marker dye. J Thorac Cardiovasc Surg. 1996;112:542–4. doi: 10.1016/s0022-5223(96)70286-6. [DOI] [PubMed] [Google Scholar]
- 9.Wingard CJ, Browne AK, Murphy RA. Dependence of force on length at constant cross-bridge phosphorylation in the swine carotid media. J Physiol. 1995;488:729–39. doi: 10.1113/jphysiol.1995.sp021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai TR, Bates JH, Brusasco V, et al. On the terminology for describing the length-force relationship and its changes in airway smooth muscle. J Appl Physiol. 2004;97:2029–34. doi: 10.1152/japplphysiol.00884.2004. [DOI] [PubMed] [Google Scholar]
- 11.Herlihy JT, Murphy RA. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res. 1973;33:275–83. doi: 10.1161/01.res.33.3.275. [DOI] [PubMed] [Google Scholar]
- 12.Hocking KM, Brophy CM, Rizvi SZ, et al. Detrimental effects of mechanical stretch on smooth muscle function in saphenous veins. J Vasc Surg. 2011;53:454–60. doi: 10.1016/j.jvs.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 14.Khalil RA, Crews JK, Novak J, et al. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension. 1998;31:1065–9. doi: 10.1161/01.hyp.31.5.1065. [DOI] [PubMed] [Google Scholar]
- 15.Viaro F, Capellini VK, Celotto AC, et al. Immunohistochemical evaluation of three nitric oxide synthase isoforms in human saphenous vein exposed to different degrees of distension pressures. Cardiovasc Pathol. 2010;19:e211–20. doi: 10.1016/j.carpath.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Conte MS. Technical factors in lower-extremity vein bypass surgery: how can we improve outcomes? Semin Vasc Surg. 2009;22:227–33. doi: 10.1053/j.semvascsurg.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Halabi AR, Alexander JH, Shaw LK, et al. Relation of early saphenous vein graft failure to outcomes following coronary artery bypass surgery. Am J Cardiol. 2005;96:1254–9. doi: 10.1016/j.amjcard.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 18.Fabricius AM, Gerber W, Hanke M, et al. Early angiographic control of perioperative ischemia after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2001;19:853–8. doi: 10.1016/s1010-7940(01)00692-3. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–26. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 20.Tsui JC, Souza DS, Filbey D, et al. Localization of nitric oxide synthase in saphenous vein grafts harvested with a novel “no-touch” technique: potential role of nitric oxide contribution to improved early graft patency rates. J Vasc Surg. 2002;35:356–62. doi: 10.1067/mva.2002.121072. [DOI] [PubMed] [Google Scholar]
- 21.Dashwood MR, Savage K, Dooley A, et al. Effect of vein graft harvesting on endothelial nitric oxide synthase and nitric oxide production. Ann Thorac Surg. 2005;80:939–44. doi: 10.1016/j.athoracsur.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 22.Manchio JV, Gu J, Romar L, et al. Disruption of graft endothelium correlates with early failure after off-pump coronary artery bypass surgery. Ann Thorac Surg. 2005;79:1991–8. doi: 10.1016/j.athoracsur.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 23.Asahara T, Bauters C, Pastore C, et al. Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation. 1995;91:2793–801. doi: 10.1161/01.cir.91.11.2793. [DOI] [PubMed] [Google Scholar]
- 24.Poston R, Gu J, Brown J, et al. Hypercoagulability affecting early vein graft patency does not exist after off-pump coronary artery bypass. J Cardiothorac Vasc Anesth. 2005;19:11–8. doi: 10.1053/j.jvca.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Makuuchi H, Naruse Y, et al. Assessment of saphenous vein graft wall characteristics with intravascular ultrasound imaging. Jpn J Thorac Cardiovasc Surg. 1998;46:701–6. doi: 10.1007/BF03217805. [DOI] [PubMed] [Google Scholar]
- 26.Yamada T, Itoh T, Nakano S, Tokunaga O. Time-dependent thickening of the intima in aortocoronary saphenous vein grafts: clinicopathological analysis of 24 patients. Heart Vessels. 1995;10:41–5. doi: 10.1007/BF01745076. [DOI] [PubMed] [Google Scholar]
- 27.Sharony R, Pintucci G, Saunders PC, et al. Matrix metalloproteinase expression in vein grafts: role of inflammatory mediators and extracellular signal-regulated kinases-1 and -2. Am J Physiol Heart Circ Physiol. 2006;290:H1651–9. doi: 10.1152/ajpheart.00530.2005. [DOI] [PubMed] [Google Scholar]
- 28.Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 29.Chu WW, Rha SW, Kuchulakanti PK, et al. Efficacy of sirolimus-eluting stents compared with bare metal stents for saphenous vein graft intervention. Am J Cardiol. 2006;97:34–7. doi: 10.1016/j.amjcard.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Johansson BL, Souza DS, Bodin L, et al. Slower progression of atherosclerosis in vein grafts harvested with ‘no touch’ technique compared with conventional harvesting technique in coronary artery bypass grafting: an angiographic and intravascular ultrasound study. Eur J Cardiothorac Surg. 2010;38:414–9. doi: 10.1016/j.ejcts.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Davies MG, Hagen PO. Influence of perioperative storage solutions on long-term vein graft function and morphology. Ann Vasc Surg. 1994;8:150–7. doi: 10.1007/BF02018863. [DOI] [PubMed] [Google Scholar]
- 32.Martinez TT, Jaeger RW, deCastro FJ, et al. A comparison of the absorption and metabolism of isopropyl alcohol by oral, dermal and inhalation routes. Vet Hum Toxicol. 1986;28:233–6. [PubMed] [Google Scholar]
- 33.Kapp RW, Jr, Bevan C, Gardiner TH, et al. Isopropanol: summary of TSCA test rule studies and relevance to hazard identification. Regul Toxicol Pharmacol. 1996;23:183–92. doi: 10.1006/rtph.1996.0042. [DOI] [PubMed] [Google Scholar]
- 34.Allali-Hassani A, Martinez SE, Peralba JM, et al. Alcohol dehydrogenase of human and rat blood vessels. Role in ethanol metabolism. FEBS Lett. 1997;405:26–30. doi: 10.1016/s0014-5793(97)00151-8. [DOI] [PubMed] [Google Scholar]
- 35.Farber A, Major K, Wagner WH, et al. Cryopreserved saphenous vein allografts in infrainguinal revascularization: analysis of 240 grafts. J Vasc Surg. 2003;38:15–21. doi: 10.1016/s0741-5214(03)00330-6. [DOI] [PubMed] [Google Scholar]


