Abstract
OBJECTIVES
The treatment of persistent and long-standing persistent atrial fibrillation (AF) has unsatisfactory results using both medical therapy and/or catheter ablation, where incomplete ablation lines remain a significant problem. This study evaluates the feasibility, efficacy and safety of the sequential, two-staged hybrid treatment combining thoracoscopic surgical and transvenous catheter AF ablation.
METHODS
Thirty patients with persistent and long-standing persistent AF underwent surgical thoracoscopic radiofrequency (RF) ablation procedure using a predefined protocol (pulmonary veins isolation, box lesion, isthmus line lesion, dissection of the ligament of Marshall, left atrial appendage exclusion with an epicardial clip and ganglionated plexi ablation) followed by diagnostic catheterization and RF ablation 3 months later. In this session, electrical mapping of the left atrium was performed and any incomplete isolation lines were completed. Mitral and cavotricuspid isthmus ablation lines were performed during this session as well.
RESULTS
The preoperative mean duration time of AF was 33 ± 27 months with 17% patients with persistent and 83% patients with long-standing persistent AF. The mean size of the left atrium was 48 ± 5 mm. The complete surgical ablation protocol was achieved in 97% of patients, with no death, and no early stroke or pacemaker implantation in the early postoperative period. In 63% of patients, the left atrial appendage was excluded with an epicardial clip. An endocardial touch-up for achievement of bidirectional block of pulmonary veins was necessary in 10 patients (33%) and on the box, (roof and floor) lesions in 20 patients (67%). Freedom from atrial fibrillation was 77% after surgical ablation and 93% after the completed hybrid procedure.
CONCLUSIONS
The sequential, two-staged hybrid strategy (surgical thoracoscopic followed by catheter ablation) is feasible and safe with a high post-procedural success and seems to represent the optimal treatment with low risk load and potentially long-term benefit for patients with a persistent and long-standing persistent form atrial fibrillation.
Keywords: Atrial fibrillation, Persistent, Surgical treatment, Radiofrequency ablation, Hybrid procedure
INTRODUCTION
Atrial fibrillation (AF) represents the most common arrhythmia with higher incidence in aging population [1] and it is associated with increased mortality, risk of stroke and exacerbation of heart failure [2]. The pharmacological treatment of AF remains challenging with a long-term failure rate reaching 85% [3]. Catheter ablation as a first-line treatment for patients with drug-refractory AF has variable results with single-procedure success rates ranging from 16 to 84% [4]. Success rate decreases with the necessity for repeat procedures and higher economic costs in persistent forms of AF [5–8]. In these patients, the left atrium is often enlarged with fibrotic remodelling and the transvenous endocardial approach and creation of linear lesions is sometimes challenging. The other important point, mainly in the treatment of persistent AF, is exclusion of the left atrial appendage (LAA), which is not routinely closed during the catheter ablation procedure.
Surgical treatment of AF has shifted in the last decade towards minimally invasive procedures using endoscopic instruments to isolate pulmonary veins (PVs), create linear lesions and occlude the LAA on the beating heart. Although this approach achieves a higher arrhythmia-free event rate after a single procedure than catheter ablation [9], contiguous and transmural lesions cannot be always guaranteed and the mitral isthmus line as well as the tricuspid isthmus line also cannot be created from the epicardium [10].
The hybrid procedure combines the surgical and the catheter ablation to overcome the shortcomings of these two approaches and as an increased success rate could be expected, it can potentially lower the hospital costs.
This study evaluates the feasibility and safety of a two-staged hybrid treatment for persistent AF combining endoscopic surgical radiofrequency (RF) ablation of the left atrium with the occlusion of LAA, followed by an electrophysiologist (EP) diagnostic study and RF catheter ablation of the left and right atria after a 3-month period of healing. The short-term follow-up data are presented as well.
MATERIALS AND METHODS
Thirty consecutive patients with symptomatic drug-refractory persistent and long-standing persistent AF underwent hybrid treatment of AF, while following a standardized protocol from July 2012 to January 2013. The study was approved by the institution's ethics committee. The definition of paroxysmal, persistent and long-standing persistent was based on recent guidelines [4]. The selection criteria were: AF refractory to at least one class I or class III antiarrhythmic therapy, absence of coronary artery disease, absence of moderate or severe heart valve disease, absence of stroke, absence of catheter ablation and thoracic surgery within the last 6 months, absence of thrombus in the LAA during transoesophageal examination and written informed consent to participation in the study. All patients were informed of alternative treatment options, including single thoracoscopic or catheter ablation. Patients were examined by transoesophageal echocardiography, cardiac computed tomography with contrast; pulmonary function tests and patients older than 50 years or patients with symptoms of angina pectoris underwent coronary angiography. Transoesophageal echocardiography as well as cardiac computed tomography with contrast was repeated before catheter ablation. Oral anticoagulation therapy was discontinued 7 days before surgery/catheter ablation and low-molecular-weight heparin was administered twice a day until the evening before the procedure.
Surgical technique
Surgery was performed under general anaesthesia with a double-lumen endotracheal tube for selective lung ventilation and external defibrillation pads were placed on intended positions. The procedure started on the right side with placement of endoscopic tools: a 10-mm camera port in the right fifth intercostal space mid-axillary line, a 5-mm working port placed in the third intercostal space anterior axillary line and a 10-mm working port placed in the seventh intercostal space mid-axillary line. Using single-lung ventilation, the pericardium was opened anterior to the phrenic nerve from the superior vena cava to the diaphragm and gently pulled with three stitches. The interatrial groove was dissected with endoscopic scissors as well as the access to the oblique sinus between the inferior vena cava and the right lower PV. An articulating and illuminating dissector (LumiTip Dissector, AtriCure, OH, USA) was used to surround the right PVs with a rubber tourniquet which is subsequently utilized as a guide for the insertion of the ablation clamp (Isolator Synergy, AtriCure, OH, USA). A series of ablations (minimally five times) of the right PVs was performed for electrical isolation, and entry block was verified. A roof line (connecting superior right-sided and left-sided PVs) and bottom (or floor) line (connecting inferior right-sided and left-sided PVs) were made using a linear pen device (Bipolar Linear Pen, AtriCure, OH, USA). In our series, an additional line on the roof of the left atrium ranging from the left superior PV to the non-coronary sinus of the aortic valve was made. This line was subsequently finished during catheter ablation, when the mitral isthmus line was created epicardially. After that, ganglionated plexi were located using high-frequency stimulation and observing a vagal response inducing bradycardia. ganglionated plexi were then ablated until the vagal response vanished. The procedure on the right side was finished by putting one approximating stich on the pericardium and placing a drain in the right pleural space.
The approach on the left side was similar to that on the right side, but ports were placed more posteriorly and also the pericardium was opened posterior to the phrenic nerve. The ligament of Marshall was dissected by scissors and both ends were burnt with the linear pen. The Lumitip with the rubber tourniquet was used to place the bipolar clamp and a series of ablations were made until entry block was documented. At this time, if the patient was in AF, electric cardioversion was performed and epicardial bidirectional block was confirmed for the PVs and for the box lesion. The LAA was excluded after measuring the size and using the AtriClip (Atricure, OH, USA). The drain was introduced in the right pleural space and the patient was transferred to the intensive care unit. Low-molecular-weight heparin was started 6 h after surgery and warfarin was administered after removal of pleural drains.
Catheter-based technique
All procedures were performed using the CARTO3 mapping system. If normal sinus rhythm was present at the beginning of the procedure, RF ablation of the cavotricuspid isthmus was performed before moving to the left atrium. Achievement of bidirectional block of conduction across the isthmus was ascertained by standard criteria. Then, after double trans-septal punctures, two steerable trans-septal sheaths (8F, Channel, BARD Electrophysiology, Lowell, MA, USA) were introduced into the left atrial (LA) and virtual reconstruction of the anatomy and a bipolar voltage map was conducted acquiring at least 300 points with the aim of detailed mapping of the whole LA. A circular mapping catheter (LASSO®, Biosense Webster, Inc., Diamond Bar, CA, USA) was used in all veins to confirm isolation or electrical reconnection.
RF energy was applied using a 3.5 mm irrigated-tip ThermoCool® Smart Touch™ catheter (Biosense Webster, Inc.) and a software module that enables contact force sensing with a temperature limitation of 44°C and RF energy up to 35 W. If electrical reconnection of the PVs was suspected, detailed mapping of the PV antra was performed to find the earliest PV potential inside the presumed epicardial ablation line. Such regions were targeted for ablation first. The presence of a single spot of electrical reconnection (from now on referred to as a ‘gap’) corresponded to the complete abolition of the PV potentials after a single RF application at the spot with the earliest activation recorded on the ablation catheter. Similarly, multiple gaps were deemed to be present if either the conduction time to the PV prolonged (in such cases the abolition of the PV potentials was achieved usually in the adjoining segments) or the PV potentials suddenly showed a different activation pattern following RF application assuming that the electrical activity in the ablation catheter was recorded earlier than any of the PV potentials.
After achieving PV isolation of all veins (in case they were reconnected), posterior LA wall was mapped to confirm electrical isolation. For such purposes, the Lasso catheter was placed in such a way that it touched the posterior wall perpendicularly. If no potentials were recorded, the box lesion was deemed to be present (Fig. 1). If any potential was recorded, both superior and inferior connecting lines were mapped to look for a gap. All gaps were subsequently ablated.
Figure 1:
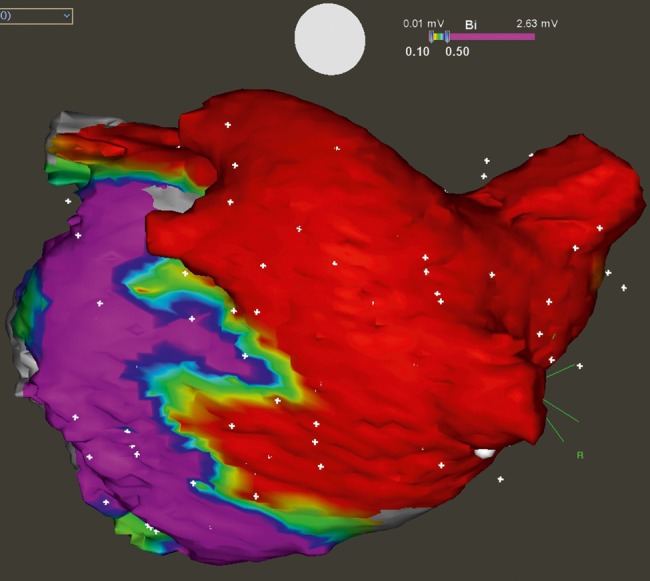
Box lesion with bidirectional block evaluated during catheter ablation. Colour-coded bipolar voltage map of the left atrium is showing complete abolition of the electrical signals from all four PVs and posterior wall (depicted by red colour with recorded voltage <0.1 mV), while inferior and lateral parts of the atrium remain electrically active (violet colour). The border zones of ablation lines are clearly depicted as yellow areas.
Following this step, the anterior mitral isthmus line was ablated starting close to the mitral annulus and extending the line towards the superior connecting line made by the surgeon. Conduction block of the mitral isthmus line was confirmed by standard criteria using pacing from the area of LAA lateral to the ablation line and proving clock-wise activation pattern around the mitral annulus towards the ablation line.
Furthermore, incremental atrial pacing up to 300 bpm was performed at the end of the procedure. In case any atrial tachycardia (AT) was induced, detailed activation remap of the left or right atrium was performed, mechanism of the arrhythmia was established and tachycardia was abolished by RF ablation. This step was done at first if sustained AT was recorded at the very beginning of the procedure.
Postoperative care and follow-up
Patients underwent telemetric monitoring after the operation which lasted until discharge from the hospital. No antiarrhythmic drugs were administered in patients with sinus rhythm. If AF was documented, 900 mg of amiodarone was administered intravenously over 12 h followed by 200 mg dose once daily until the catheter procedure. Patients were followed up by 7-day Holter monitoring 3, 6, 9 and 12 months after the completion of the hybrid procedure. Success was defined as absence of AF and/or any other supraventricular arrhythmia lasting >30 s on the 7-day Holter monitor.
Clinical characteristics and perioperative and postoperative variables were collected prospectively. Patients were followed up according to the current guidelines [4]. Seven-day Holter was performed at 3-month intervals after completion of both procedures.
RESULTS
Preoperative characteristics of the 30 patients (20 males, 10 females) are summarized in Table 1. There were 17% of patients with persistent and 83% of patients with long-standing persistent AF, and 80% of patients had previous electric cardioversion. The mean size of LA was 48 ± 5 mm (long axis parasternal view).
Table 1:
Preoperative variables
| Variables | n = 30 |
|---|---|
| Male | 20 (67%) |
| Age (years) | 61 ± 8 |
| AF duration (months) | 33 ± 27 |
| Persistent AF | 4 (13%) |
| Long-standing persistent AF | 26 (87%) |
| Left atrium size (mm) (Plax) | 48 ± 5 |
| Left ventricular ejection fraction | 62 ± 8% |
| Body mass index (kg/m2) | 30.2 ± 4.8 |
| Hypertension | 20 (67%) |
| COPD | 6 (20%) |
| Previous failed electrical cardioversion | 24 (80%) |
Perioperative data are given in Table 2. The mean procedural time was 210 ± 30 min and the ablation protocol was achieved in 97%. In 1 patient with undiscovered diaphragmatic hernia, the right-sided lesions were not performed. Two patients underwent unplanned median sternotomy due to intraoperative bleeding from the left pulmonary artery. These two complications occurred early in our series just after the start-up of our thoracoscopic ablation program. Postoperative variables are presented in Table 3. There were no operative or postoperative deaths in the group as well as no in-hospital stroke or pacemaker dependency. Two phrenic nerve palsies with immobility of the right-sided diaphragm were diagnosed after completion of the surgical part of the procedure on skiascopy at the beginning of catheter RF ablation. Both of them continued to be present 12 months following the index surgical procedure. One patient was readmitted to the hospital with signs of cardiac tamponade with an international normalized ratio of 7.1 on Day 24 after surgery and he was successfully treated by pericardial drainage using the Seldinger method.
Table 2:
Surgical procedure variables
| Variables | n = 30 |
|---|---|
| Procedure time (min) | 201 ± 30 |
| Conversion to sternotomy | 2 (7%) |
| Operative mortality | 0 |
| Complete box lesion | 29 (97%) |
| Dissection of the ligament of Marshall | 29 (97%) |
| Mitral isthmus line | 26 (87%) |
| Occlusion of LAA with AtriClip | 19 (63%) |
Table 3:
Postoperative variables
| Variables | n = 30 |
|---|---|
| Haemothorax | 0 |
| Pneumothorax | 0 |
| Injury of phrenic nerve | 2 (7%) |
| Pacemaker dependency | 0 |
| Infection of wounds | 2 (7%) |
| In-hospital stroke | 0 |
| Hospital/30-day mortality | 0 |
There were 2 obese female patients (body mass index 43.2 and 34.4) with an infection of the unilateral wound who were treated with intravenous antibiotics. Seven patients underwent cardioversion during the hospital stay and 9 patients were dismissed with antiarrhythmic therapy. The average length of stay was 4.5 ± 3 days. One month after surgery, 77% of patients were in normal sinus rhythm.
Successful RF catheter ablation was achieved in 100% of patients with no complications and no in-hospital mortality or morbidity. During the electrophysiological evaluation, complete isolation of right PVs after the surgical ablation was found in 87% and complete isolation of left PVs in 77% of patients. Complete roof line after surgical procedure was found in 33% of patients and complete inferior line in 60% of patients. In patients with incomplete lines, the endocardial touch-up was performed to achieve bidirectional block with 100% success.
The mean follow-up was 208 ± 29 days. No patient was lost during follow-up. None of the patients died or experienced a late stroke. After completion of the hybrid procedure, freedom from AF was 90% without antiarrhythmic medication and 93% on drugs. Two patients had AT despite medication and completed hybrid procedure. One of the patients was successfully reablated and the other remains in right AT despite a reablation catheter procedure (rate control with permanent anticoagulation was finally chosen as a treatment strategy).
DISCUSSION
Patients with persistent or even long-standing persistent AF represent the most difficult patient subgroup to treat due to high recurrent rates of arrhythmia. According to the latest guidelines, catheter ablation has become the first-line treatment method in patients with paroxysmal AF and no structural heart disease taking into account also the patient's wish and level of experience of the operator/centre [4]. The success rate of catheter ablation in patients with persistent or long-lasting persistent AF, where fibrotic alteration of the atrial wall and enlargement of the LA is usually present, is reported to be as low as 45% after a single procedure. Even after redo ablation the success rate does not exceed 60% after 1 year and decreases quickly with time [5–8].
Surgical alternatives to catheter-based treatment have arisen due to a strenuous effort to lower the invasiveness of surgery and several types of modified Cox maze procedures have been introduced [11]. This evolution during the last decade has brought the shift of ablation therapy from unipolar to bipolar RF energy [12, 13] and from isolated PV ablation [14–16] to an expanded set of lesions involving both the left and the right atrium [17]. The other ambition of the surgical procedure was the ablation of ganglionated plexi which, as a part of autonomic system, seem to play an important role in AF initiation and maintenance [4]. The systematic ablation of ganglionated plexi is easily performed during the endoscopic procedure [18], but still there is no evidence whether it improves the long-term outcomes of the procedure [19] and surgeons tend to skip this part of the protocol [20].
The hybrid procedure is usually described as a simultaneous procedure in the hybrid operating theatre [21]. We have decided to adopt the method as a two-stage procedure for several reasons. Currently, our centre is not equipped with a hybrid operating theatre, which would allow us to work simultaneously with the electrophysiologists. Secondly, we expect an advantage of a 3-month healing period between the two procedures—the scars created by the RF ablation tools in the LA become a mature with no residual oedema, which is always presented after the surgical ablation and which can affect the sensitivity of endocardial mapping. There is also a clear evidence of an increased complication rate of simultaneous procedures due to extremely long general anaesthesia time, risk of bleeding due to prolonged heparinization and infection risk.
LAA exclusion was planned as an integral part of our operation protocol wherever deemed feasible and safe by the surgeon. LAA occlusion may play an important role in stroke reduction [4]. It is mostly performed either by stapler excision or by the LAA clip as described by our group. We find the clip a safe and feasible method for exclusion and electrical isolation of the LAA. In our study, only 63% of patients had LAA exclusion with the AtriClip. This issue is applied to the learning curve and to the type of instruments available to us. In the beginning of the study, we used the first generation AtriClip device, which did not include distal device articulation as part of deployment. With this first generation, our implantation rate was only ∼50%. In the last 10 patients, we started utilizing the second generation of AtriClip (AtriClip Pro), which we found preferable for thoracoscopic surgery and which has raised our exclusion rate to >90%.
In our group, there were 2 cases of phrenic nerve injury with absent movement on the skiascopy recorded at the beginning of the RF catheter ablation. This is a very unpleasant complication, which can be caused by two surgical mistakes. The first is mechanical injury made during opening of the pericardium very close to the phrenic nerve. After placing pericardial stitches and pulling the pericardium towards the surgeon to improve the view, handling with endoscopic tools, mainly clamps or linear pen when performing the inferior line, the pericardium can be easily stretched or torn, and with it the nerve. The second potential cause is a heat injury during the ablation. There is evidence, that if the phrenic nerve is not completely damaged, the possibility of its healing is ∼70% after 1 year and ∼90% after 2 years [22]. The first patient from our group was highly symptomatic and underwent a plication of diaphragm 6 months after surgical ablation. The second patient was completely asymptomatic with preserved lung function on the spirometry. There is a lack of available information about this complication from the literature. One-side injury of the phrenic nerve with limited/no movement of the diaphragm does not necessarily lead to sensation of dyspnoea. Thus, such a complication is most likely underestimated in the surgical literature as consistent follow-up examination aimed at detection of diaphragm movement on skiascopy is usually missing.
Approximately one-third of our patients had a partial recovery of at least one PV identified during the electrophysiological evaluation. There was a higher number of linear lesion failures after the surgical procedure: 40% of patients had gap in the inferior line and 67% in the roof line of the box lesion leading to incomplete box lesion in two-thirds of patients. The possible explanation of failure could be a lack of transmurality mainly in patients with enlarged and fibrotic LA or in patients with fatty heart, where a higher number of ablation is needed to reach the entry/exit block. Gaps that were found during the electrophysiology part of the procedure were small gaps that needed only a single or double endocardial ablation to complete for exit/entry block. The completeness of all lesions (pulmonary vein isolation, box lesion) is always checked during surgery by entry/exit block testing. The completeness of the lesions during surgery does not always mean completeness of the lesion after the follow-up period, where a recovery of atrial tissue can be found. The critical importance of this information is that a small gap in a lesion does not always mean the recurrence of AF postoperatively and that ablation by surgeons from the epicardial site can facilitate the achievement of permanent transmurality that is easily completed from the endocardial site.
CONCLUSIONS
Hybrid treatment of AF has encouraging short-term results and could be an alternative to a single surgical procedure or RF catheter therapy in patients with persistent or long-standing persistent AF. A staged hybrid approach may have several advantages over a single-step hybrid procedure. After overcoming of the learning curve of endoscopic surgery for AF, the hybrid approach offers low morbidity and mortality rate comparable with repeated RF catheter ablations. However, a randomized trial with an emphasis on safety, success rates and economic implications in a long-term follow-up period of 2–5 years, comparing the hybrid approach with the catheter ablation as a first-line treatment of AF is necessary to support this statement.
Funding
This study was partially funded by the Faculty of Health and Social Studies, University of South Bohemia in České Budějovice (BOV 2012_001).
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr S. Salzberg (Zurich, Switzerland): The heart team approach also needs to be applied to the problem of atrial fibrillation, because we can learn from the EPs, and the EPs can learn from us. And therefore, I think that your two-stage hybrid treatment approach is really the way to go. Whether this is done in one procedure or in two procedures, I think further data will show. I have two comments in regard to your data, because I am pretty astonished by your paper. Intraoperatively, you confirmed entrance and exit block of the PVs; however, three months after, when patients got touched up, only 66% had isolated pulmonary veins. What is your explanation for that?
Dr Kurfirst: Yes. I think that during our surgical procedure, when you are testing the transmurality of the lesion, you are testing it by the decline of impedance of the clamps. And also, it has been shown that clamping of pulmonary veins alone, without any energy, has the potential for provoking some kind of exit or entry block for a while, maybe for some hours. So I think that especially in dilated left atria with fibrous tissue, we need more lesions. So these are our results from the beginning of this programme, and I hope that with adding more lesions, we can also increase the success rate of PV isolation.
Dr Salzberg: We do a single hybrid approach in Amsterdam, and we clamp the PVs until the impedance drops within 5 seconds, and then we do epicardial entrance and exit block testing. And I was just wondering why these lesions weren't complete over time.
My second question, more importantly, concerns the left atrial appendage. You were able to exclude 66% of left atrial appendages, which in my opinion is not enough. Because when the patient comes to surgery, we are not sure if we are going to be able to treat the rhythm properly, but we can at least get rid of that appendage. Why only 66% success rate for this?
Dr Kurfirst: Yes you are completely right; this has something to do with the learning curve of our procedure. So in the beginning, in the first 10 patients, it was maybe about 40 or 50% of patients. And at this time, it is more than 90%. So I think that with the learning curve, we will also be more successful in this.
Dr Salzberg: I think the previous speaker, with the talk on the stapler, showed the importance that we surgeons learn that we have different tools to be able to deal with different types of anatomy. I don't think that one size fits all, and that's why having more options available (as do the cardiologists in Europe with the Watchman and the Amplatzer and the LARIAT) I think will help us.
Dr S. Benussi (Milan, Italy): I have only one more question. Sasha talked about the 60%, nearly 70% success rate on isolation of the pulmonary veins. I really don't understand why you would pursue a trigone line which in your experience has achieved a successful ablation in only one case. So why do that at all? The EP cardiologists like to do that line in totally another place, not where you suggest they do so. You're probably just creating some scars, which are quite likely the cause of post-ablation tachycardia, like the one you experienced in your series.
Dr Kurfirst: You are totally right. The issue is that in our protocol, we only had the partial isthmus line, because it was as a favour by our EPs that they wanted to help with this mitral isthmus line. So it was in a protocol that we go to only part of it, and they will do the rest. So they don't have to do a 7 cm lesion, but only a 3 or a 2.
Dr T. Hanke (Lübeck, Germany): As I understand it from an anatomical standpoint, the trigone line is different than the EP's mitral isthmus line, right?
Dr Kurfirst: Yes.
Dr Hanke: It goes from the left pulmonary vein down through the mitral, and it is P1, and the trigone line goes to A1. So do you call this finalization?
Dr Kurfirst: We call it finalization.
Dr Benussi: You are actually right about the second mitral line.
Dr Kurfirst: So that's my mistake. Sorry.
Dr N. Ad (Falls Church, VA, USA): I have two comments. One is that we have to remember that ablation lines that are being applied across the Bachmann bundle as a part of the Dallas lesion set are very hard to establish as the atria are very thick there. It is basically a very similar line to the maze I which was abandoned because it created a block and a significant delay between the activation of the right and left atrium. So you may end up with an 180 to 280 millisecond delay between the right and left atrium. I don't understand this line at all.
Secondly, we all have to go back to the original mapping of human atria and remember that the isthmus line across the coronary sinus is necessary not only to abolish atypical atrial fibrillation, but also because AF can originate around the coronary sinus. But I believe that the main issue here is not the heart team concept and the collaboration; it is about the fact that we surgeons don't have reliable tools to ablate epicardially on a beating heart and create reliable transmural lesions.
So in our institute, the biggest opponents of the hybrid procedures are the EPs. And we have a hybrid EP suite, an operating room that is equipped like an EP Lab where we can do everything. Why is that? Because we see time after time that all those lines that look okay during the operation are not okay and create a lot of arrhythmias, both new and recurrent.
So as a stage to a good hybrid procedure that makes sense; it is not about the lesion, it is about the devices. And currently, except for the bipolar clamp to the pulmonary veins, nothing really works, and Stefano can say more about it than I can say; we have no device that can transmit reliable transmural lesions epicardially.
So we can do a fancy minimally invasive procedure and basically throw away all our essential work that is actually good for the patients. The procedure shouldn't be done so the patient can drink coffee in the afternoon; the procedure should be done so the patient can drink coffee ten years from now, A Fib free. This is our challenge as surgeons.
REFERENCES
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Wolf PA, D´Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 3.Marcus GM, Sung RJ. Antiarrhythmic agents in facilitating electrical cardioversion of atrial fibrillation and promoting maintenance of sinus rhythm. Cardiology. 2001;95:1–8. doi: 10.1159/000047335. [DOI] [PubMed] [Google Scholar]
- 4.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial designs (A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Hearth Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, The American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society) Heart Rhythm. 2012;9:632–96. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Fiala M, Skňouřil L, Toman O, Pindor J, Bulková V, Chovančík J, et al. Long-term results of catheter ablation for atrial fibrillation in 866 patients. Cor et Vasa. 2012;54:e361–8. [Google Scholar]
- 6.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–6. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 7.Cheema A, Chandrasekhar R, Vasamreddy CR, Dalal D, Marine JE, Dong J, et al. Long-term single procedure efficacy of catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2006;15:145–55. doi: 10.1007/s10840-006-9005-9. [DOI] [PubMed] [Google Scholar]
- 8.Lo LW, Tai CT, Lin YJ, Chang SL, Udyavar AR, Hu YF, et al. Predicting factors for atrial fibrillation acute termination during catheter ablation procedures: implications for catheter ablation strategy and long-term outcome. Heart Rhythm. 2009;6:311–8. doi: 10.1016/j.hrthm.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Boersma LV, Castella M, van Boven W, Berruezo A, Yilmaz A, Nadal M, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012;125:23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 10.Castellá M, García-Valentin A, Pereda D, Colli A, Martinez A, Martinez D, et al. Anatomic aspects of the atrioventricular junction influencing radiophrequency Cox maze IV procedures. J Thorac Cardiovasc Surg. 2008;136:419–23. doi: 10.1016/j.jtcvs.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 11.Gaynor SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, Bailey MS, et al. A prospective, single-center clinical trial of a modified Cox Maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–42. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Melby SJ, Gaynor SL, Lubahn JG, Lee AM, Rahgozar P, Caruthers SD, et al. Efficacy and safety of right and left atrial ablations on the beating heart with irrigated bipolar radiofrequency energy: a long-term animal study. J Thorac Cardiovasc Surg. 2006;132:853–60. doi: 10.1016/j.jtcvs.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Cappato R, Negroni S, Pecora D, Bentivegna S, Lupo PP, Carolei A, et al. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003;108:1599–604. doi: 10.1161/01.CIR.0000091081.19465.F1. [DOI] [PubMed] [Google Scholar]
- 14.Wolf RK, Schneeberger EW, Osterday R, Miller D, Merrill W, Flege JB, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg. 2005;130:797–802. doi: 10.1016/j.jtcvs.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 15.Bage L, Blomstrom P, Nilsson L, Einarsson GM, Jedéus L, Blomstrom-Lundqvist C. Epicardial off-pump pulmonary vein isolation and vagal denervation improve long-term outcome and quality of life in patients with atrial fibrillation. J Thorac Cardiovasc Surg. 2008;137:1265–71. doi: 10.1016/j.jtcvs.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Puskas J, Lin E, Bailey D, Gyuton R. Thoracoscopic radiofrequency pulmonary vein isolation and atrial appendage occlusion. Ann Thorac Surg. 2007;83:1870–2. doi: 10.1016/j.athoracsur.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Shirak J, Schwartzman D. Interim results of the 5-box thoracoscopic maze procedure. Ann Thorac Surg. 2012;94:1880–5. doi: 10.1016/j.athoracsur.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz A, Geuzebroek GS, Van Putte BP, Boersma LVA, Sonker U, De Bakker JMT, et al. Completely thoracoscopic pulmonary vein isolation with ganglionic plexus ablation and left atrial appendage amputation for treatment of atrial fibrillation. Eur J Cardiothorac Surg. 2010;38:356–60. doi: 10.1016/j.ejcts.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 19.Schauerte P, Scherlag BJ, Patterson E, Scherlag MD, Matsudaria K, Nakagawa H, et al. Focal atrial fibrillation: experimental evidence for pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol. 2001;12:592–9. doi: 10.1046/j.1540-8167.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- 20.Weimar T, Vosseler M, Czelsa M, Boscheinen M, Hemmer WB, Doll KN. Approaching a paradigm shift: endoscopic ablation of lone atrial fibrillation on the beating heart. Ann Thorac Surg. 2012;94:1886–93. doi: 10.1016/j.athoracsur.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Pison L, La Meir M, van Opstal J, Blaauw Y, Maessen J, Crijins HJ. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. JACC. 2012;60:54–61. doi: 10.1016/j.jacc.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 22.Kolář P. Rehabilitation in Clinical Praxis. Prague: Galén; 2011. pp. 572–6. [Google Scholar]


