Abstract
OBJECTIVES
Bleeding is the most common complication of HeartMate II and is partially attributable to platelet dysfunction; however, antiplatelet therapy is arbitrary in most centres. We investigated how antiplatelet therapy adjustment with thrombelastography affects late-onset bleeding.
METHODS
Thrombelastography was used to adjust antiplatelet therapy in 57 HeartMate II recipients. Kaplan–Meier survival curves and Cox proportional hazard ratio model were used to identify predictors of late-onset bleeding in univariate and multivariate analysis. Finally, late-onset bleeding rate in our study was compared with the reported rates in other studies in the literature, all of which did not use any test to monitor or adjust antiplatelet therapy.
RESULTS
Mean follow-up was 347 days. Eighteen late-onset bleeding events occurred in 12 patients, a late-onset bleeding rate of 12/57 (21%) or 0.21 events/patient-year. The Kaplan–Meier survival curves demonstrated that late-onset bleeding was more common in the destination therapy cohort (P = 0.02), in patients older than 60 years (P = 0.04) and in females (P = 0.01), none of which was significant in multivariate analysis at a significance level of 0.05. To further investigate the higher bleeding rate in elderly patients, thrombelastography parameters were compared between younger and older patients at the age cut-off of 60 years which demonstrated a prothrombotic change the day after device implantation in younger patients that was absent in the elderly. There was also a trend towards higher requirement for antiplatelet therapy in younger patients while on device support, but the difference did not reach statistical significance. The average late-onset or gastrointestinal bleeding rate among seven comparable studies in the literature that did not use any monitoring test to adjust antiplatelet therapy was 0.49 events/patient-year.
CONCLUSIONS
Our study implicates that antiplatelet therapy adjustment with thrombelastography may reduce late-onset bleeding rate in HeartMate II recipients. Bleeding was more common in the elderly recipients and analysis of thrombelastography data suggests that a less aggressive antiplatelet therapy regimen could potentially lower bleeding rate in this vulnerable population.
Keywords: HeartMate II, Thrombelastography, Bleeding, Stroke, Thromboembolism
INTRODUCTION
HeartMate II (Thoratec Corporation, Pleasanton, CA, USA) is the most commonly implanted left ventricular assist device (LVAD) not only in the USA but worldwide [1]. Bleeding is the most common complication of HeartMate II [2–4]. In the early clinical phase of HeartMate II, an aggressive anticoagulation strategy with postoperative use of heparin, target international normalized ratio (INR) of 2.5 ± 0.5 and dual antiplatelet therapy (APT) with aspirin and dipyridamole was adapted in order to reduce thromboembolic (TE) complications [3]. Postapproval studies revealed a disproportionately higher rate of immediate postoperative and late-onset bleeding [5]. Following these observations, Thoratec investigators in their most recent statement opposed postoperative use of heparin and recommended a lower target INR of 2 ± 0.5 and single APT with 81–325 mg daily of aspirin [6]. While INR is used to monitor and adjust anticoagulation, normally no laboratory test is used to adjust APT. The effect of HeartMate II on platelet function is not well studied and the current evidence is controversial. Preapproval animal studies showed evidence of platelet activation from the shear stress generated by the pump; [7] conversely, the majority of real-life human studies of the effect of continuous-flow LVADs on platelet function have shown either no effect or evidence of platelet dysfunction. These studies are summarized by Eckman and John [8]. Additionally, chronic heart failure itself is an inflammatory state with endothelial activation, which indirectly affects platelet function [9]. Also, Majeed et al. [10] showed aspirin hyporesponsiveness and variable biological response to aspirin among LVAD population; nevertheless, this phenomenon has not been exclusively studied in HeartMate II recipients. Therefore, it is fairly obvious that the effect of LVAD and APT on platelet function is variable and unpredictable. We have used thrombelastography (TEG) to monitor and adjust APT among all HeartMate II recipients. The present study reports the rates of bleeding, TE and stroke complications using this approach and compares the rates of complications with the reported literature.
MATERIALS AND METHODS
Patient selection
The institutional review board approved this retrospective study and waived patient consent. Between April 2005 and May 2011, HeartMate II devices were implanted in 57 patients. All HeartMate II recipients had APT monitoring and adjustment with TEG.
Thrombelastography
TEG is a laboratory test that monitors the thrombodynamic properties of the whole blood as it forms clot in a simulated low shear stress environment. To perform the test, 1 ml of citrated blood is transferred to a vial containing Kaolin and mixed by inversion. Three hundred and forty microlitres of the activated blood is then transferred to a prewarmed cuvette and 20 μl of 0.2 mol/l calcium chloride is added to this cuvette and assayed in the TEG 5000 analyser manufactured by Haemoscope (Niles, IL, USA) or Haemonetics (Braintree, MA, USA). TEG analyser consists of a pin that is suspended from a torsion wire into the cuvette and the cuvette oscillates back and forth six times per minute. As the fibrin strands interact with the activated platelets on the surface of the pin, the rotational movement of the cuvette is transmitted to the pin. The stronger the clot the more the pin moves. These dynamic changes are transferred to a computer and are converted into a curve, which yields quantitative measures to assess different phases of coagulation. TEG maximum amplitude (TEG-MA) is a direct measure of the highest point on the TEG curve and represents clot strength. TEG-MA is dependent on platelet concentration, platelet function and platelet–fibrin interaction and can be used as a marker to monitor APT. This test is well described by Reikvam et al. [11].
Antiplatelet and anticoagulation strategy
APT was started within 12 h of operation, unless the patient had ongoing bleeding, and increased as needed to achieve a goal TEG-MA value of between 60 and 70 mm. This range was used arbitrarily and the normal value for TEG-MA is between 55 and 73 mm. In a stepwise manner, aspirin was started at 81 mg/day and increased to 325 mg/day, followed by 650 mg/day in divided doses, with addition of dipyridamole in escalating doses as required to a maximum of 1 g/day. Based on tolerability and adverse side effects in some cases, the provider might decide to selectively increase or decrease the dose of one medication over the other in order to achieve target TEG-MA. Anticoagulation with warfarin was started within 1–3 days of LVAD implantation and was adjusted to achieve a target INR of 2.0 ± 0.5. TEG-MA and INR values were checked prior to device implantation and then daily for several days in the intensive care unit. Thereafter, these values were recorded at each outpatient visit. For the current study, data were gathered at the following intervals: prior to LVAD implantation, Day 1, Weeks 2 and 4, then at Months 3, 6, 12, 18 and 24 after LVAD implantation.
Definitions
Bleeding, TE and stroke are defined according to the latest Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) registry's descriptions [12]. Bleeding is defined as the presence of an internal or external source of bleeding that results in death, reoperation, hospitalization or transfusion of greater than or equal to four units of blood within any 24-h period during the first 7 postoperative days or any transfusion after 7 days. Bleeding complications are categorized into two phases: early-onset (≤7 days) or late-onset (>7 days). An early-onset bleeding presents as increased chest tube drainage and is the consequence of haemostatic disturbances of cardiopulmonary bypass and surgical issues. It requires transfusion or in extreme cases re-exploration. It typically occurs before and delays the start on anticoagulation and APT. A late-onset bleeding, however, typically presents as gastrointestinal (GI) bleeding and is mainly affected by anticoagulation and APT regimen. Stroke is also defined according to the INTERMACS definition. In this article, the term stroke equals ischaemic stroke and if haemorrhagic stroke is meant it is specified. TE comprises stroke, transient ischaemic attack, pump thrombosis or peripheral TE. Follow-up data are only reported during the period of LVAD support and is censored at transplantation. Complications are reported as percentage of patients and events/patient-year (events/PY).
Literature search strategy
We performed an electronic literature search of MEDLINE (1950 to December 2012) using search terms HeartMate II, HeartMate 2, bleeding and GI bleeding alone or in combination. After filtering for human studies and eliminating duplicates, the initial search yielded 354 studies. Studies that included an adult population, full text was available in English, included at least 30 patients, differentiated between early- and late-onset bleeding and reported bleeding complications as events/PY were included. In the literature search, we did not use a specific cut-off to define early vs late-onset bleeding and as long as they were reported separately or GI bleeding rate was exclusively reported, the study was eligible for inclusion. Only studies that explicitly reported rate of late-onset bleeding as events/PY or reported the exact number of late-onset bleeding events and duration of LVAD support from which events/PY could be easily calculated by dividing number of events over PY of device support were included. Events/PY was used as the metric to compare complication rates in order to account for different durations of device support among studies.
Statistical analysis
Categorical variables are presented as frequency (percentage) and continuous variables as mean ± standard deviation (SD). Student's t-test or paired t-test was used to compare continuous variables and Pearson's χ2 was used to compare categorical variables as indicated. The log-rank test was used to compare bleeding-free survival curves. Multivariate Cox proportional hazard regression model was used to identify independent predictors of late-onset bleeding. Variables were selected in a stepwise forward selection manner; entry and retention sets with a P < 0.05 were considered significant. Due to the small sample size, only the three variables that were significant in univariate analysis meaning age (>60 vs ≤60), sex and device strategy (bridge to transplantation vs destination therapy) were entered into the multivariate Cox proportional hazard regression model. Level of APT therapy was not entered into the regression as a separate variable because all patients underwent continuous adjustment of APT to achieve target TEG-MA value. A P-value <0.05 was considered significant. Data analysis was performed using the SPSS version 17 (SPSS, Chicago, IL, USA) statistical software.
RESULTS
Study population
Table 1 shows the baseline characteristics of the patients. Thirty-four devices were implanted as bridge to transplantation and 23 as destination therapy (DT). The mean duration of LVAD support was 347 days (range 7–1516); median 188 days. The major outcomes at 6 months after device implantation were 8 deaths, 14 heart transplantations, 35 ongoing LVAD support (30 ≥6 months), no LVAD explanation due to recovery and no LVAD exchange for any reason.
Table 1:
Baseline characteristics of the 57 HeartMate II recipients
| Age (years) | 57.3 ± 15.2 |
| Male | 51 (90%) |
| Caucasian | 47 (83%) |
| Body mass index (kg/m2) | 27.9 ± 6.4 |
| Body surface area (m2) | 2.1 ± 0.2 |
| Ischaemic aetiology of heart failure | 30 (53%) |
| Left ventricular ejection fraction (%) | 16.4 ± 5.2 |
| Right ventricular stroke work index (mmHg ml/m2) | 577.3 ± 286.2 |
| New York Heart Association functional class | IV |
| Serum sodium (mmol/l) | 135.1 ± 4.1 |
| Serum albumin (g/dl) | 4.1 ± 1.8 |
| Prealbumin (mg/dl) | 18.2 ± 6.9 |
| Cholesterol (mg/dl) | 130.6 ± 43.1 |
| Serum creatinine (mg/dl) | 1.2 ± 0.4 |
| Estimated creatinine clearance (ml/min) | 71.8 ± 28.4 |
| Blood urea nitrogen (mg/dl) | 28.6 ± 15.4 |
| Alanine transaminase (IU/l) | 48.4 ± 84.9 |
| Aspartate transaminase (IU/l) | 33.7 ± 28.7 |
| Total bilirubin (mg/dl) | 3.4 ± 16.6 |
| Lactate dehydrogenase (mg/dl) | 312.3 ± 294.7 |
| Haematocrit (%) | 35 ± 4.5 |
| White blood count (1000/ml) | 8 ± 3.5 |
| Platelets (1000/ml) | 226.8 ± 84.5 |
| INR | 1.3 ± 0.2 |
| Two or more inotrope agents | 12 (21%) |
| Warfarin | 33 (58%) |
| Aspirin | 35 (61%) |
| Previous cardiac surgery | 14 (25%) |
| Cardiac resynchronization therapy | 39 (68%) |
| Implantable cardioverter-defibrillator | 50 (88%) |
| Intra-aortic balloon pump | 19 (33%) |
| Mechanical ventilation | 3 (5%) |
Bleeding complications
Seventeen patients had bleeding complication. Five patients had early-onset bleeding in the form of mediastinal haemorrhage requiring re-exploration. Twelve patients had 18 late-onset bleeding events >7 days after LVAD implantation including 16 GI bleeding and 2 epistaxis. The late-onset bleeding rate was 12/57 (21%) or 0.21 events/PY. The actuarial late-onset bleeding rate at 6, 12 and 24 months was 15, 23 and 31%, respectively. The Kaplan–Meier curves indicated that late-onset bleeding was more common in the DT cohort (Fig. 1A), in patients older than 60 years (Fig. 1B) and in females (Fig. 1C). In the multivariate analysis, Cox proportional hazard ratio model identified no independent predictor for late-onset bleeding at a significance level of 0.05, which is most likely due to the small sample size.
Figure 1:
Kaplan–Meier bleeding-free survival curve: (A) bridge to transplantation vs destination therapy; (B) age = <60 vs age >60 and (C) male vs female. The numbers at the bottom of the figures show numbers of patients at risk at the beginning of each interval.
The mean interval between LVAD implantation and first late-onset bleeding event was 284 days (range 15–1017), median 156 days. Three patients had recurrent GI bleeding; each patient had three bleeding episodes during follow-up. The source of GI bleeding was identified in 8/10 (80%) of cases. The diagnostic approach, aetiology, site and treatment for each case are summarized in Table 2.
Table 2:
Late-onset gastrointestinal (GI) bleeding (>7 days after device implantation)
| Diagnostic approach | Aetiology | Site | Treatment | |
|---|---|---|---|---|
| Patient 1 | Colonoscopy | Rectal haematoma (supra-INR) | Rectum | Transfusion |
| Patient 2 | EGD, colonoscopy, T-RBC and mesenteric angiography | AVM | Small bowel | Surgical repair |
| Patient 3 | EGD | AVM | Stomach | Cauterization |
| Patient 4 | EGD and colonoscopy | Not found | Not found | Transfusion |
| Patient 5a | EGD | Ulcerated polyp (PNET) | Duodenum | Resection |
| Patient 6a | EGD, colonoscopy and capsule endoscopy | Not found | Not found | Transfusion |
| Patient 7a | EGD, colonoscopy and capsule endoscopy | AVM | Stomach and small bowel | Cauterization |
| Patient 8 | EGD | AVM | Duodenum | Epinephrine injection |
| Patient 9 | EGD and colonoscopy | AVM and supra-INR | Duodenum | Cauterization |
| Patient 10 | EGD | AVM | Gastric | Cauterization |
Supra: supratherapeutic; EGD: oesophagogastroduodenoscopy; T-RBC: tagged RBC scan; AVM: arterio-venous malformation; PNET: pancreatic neuroendocrine tumour.
aPatients with recurrent GI bleeding.
Thromboembolic complications and stroke
Five patients suffered from six TE events, which consisted of suspected pump thrombosis (3), transient ischaemic attack (TIA) (2) and stroke (1). One patient suffered from both stroke and TIA. No peripheral embolic event occurred. TE event rate was 5/57 (9%) or 0.11 events/PY. The stroke rate was 1/57 (2%) or 0.018 events/PY. TE events are presented in Table 3. One patient experienced fatal haemorrhagic stroke after thrombolytic agent administration for suspected pump thrombosis as he was not considered appropriate for pump exchange.
Table 3:
Thromboembolic complications
| TE complication | Treatment | Outcome | |
|---|---|---|---|
| Patient 1 | Suspected pump thrombosis | Heparin; eptifibatide, t-PA | Death from ICH |
| Patient 2 | Suspected pump thrombosis | Heparin; eptifibatide, t-PA | Resolved |
| Patient 3 | Suspected pump thrombosis | Heparin; eptifibatide | Death from SDH |
| Patient 4 | Transient ischaemic attack | Conservative management | Resolved |
| Patient 5 | Transient ischaemic attack | Conservative management | Resolved |
| Patient 5 | Ischaemic stroke | Conservative management | Residual neurological deficit |
t-PA: tissue-plasminogen activator; ICH: intracerebral haemorrhage; SDH: subdural haematoma; TE: thromboembolic.
Anticoagulation and antiplatelet therapy
Two hundred and seventy-one TEG-MA values (mean = 65.6; SD = 7.4) and 279 INR values (mean = 1.9; SD = 1.7) were recorded following device implantation at the previously described sample collection intervals. Fifty-two percent of TEG-MA and 50% of INR values were within the target range and 83% of TEG-MA measurements were between 55 and 75 mm. There was no significant difference between INR or TEG-MA values at the time of bleeding or TE complication vs routine follow-up as shown in Fig. 2. There was wide interindividual variability in APT throughout follow-up. Figure 3 shows the interindividual variability by showing the maximum dose of APT a patient received ≥4 weeks after device implantation (4 weeks was arbitrarily chosen as the cut-off to mainly reflect outpatient regimen). Also in order to study the effect of APT on bleeding, we divided patients into three categories based on the maximum dose of outpatient APT. Those who only received aspirin (n = 16), those who received aspirin plus ≤225 mg/day of dipyridamole (n = 19) and those who received aspirin plus >225 mg/day of dipyridamole (n = 16). Kaplan–Meier bleeding-free survival curves demonstrated that there was no difference in the rate of late-onset bleeding between these three groups (log rank P = 0.65).
Figure 2:

Comparison between TEG-MA (A) and INR (B) values at routine follow-up vs at the time of bleeding or TE complication.
Figure 3:

Maximum outpatient APT during follow-up. ASA, aspirin; doses are mg/day; n represents number of patients.
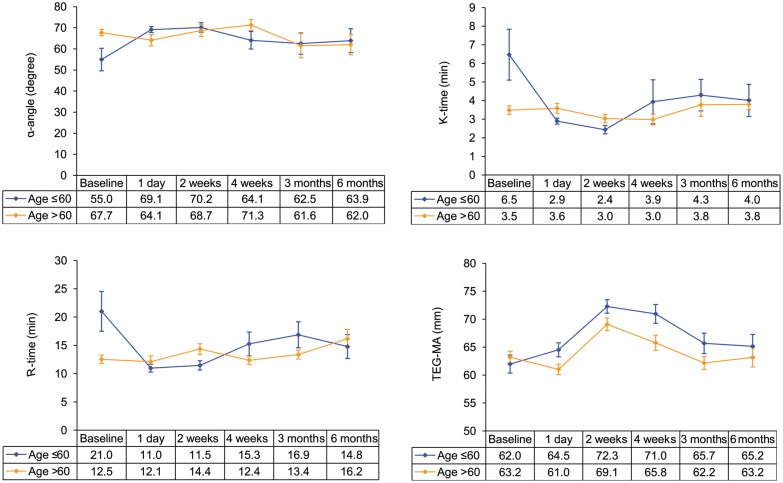
Age-specific analysis of thrombelastography data and antiplatelet therapy
Since late-onset bleeding was more common in the elderly, we decided to conduct an age-specific analysis of the TEG data to investigate whether there was a difference in the effect of LVAD on platelet function between younger and older patients to account for more bleeding in the elderly. Patients were divided into a younger cohort (age ≤60 years old) and an older cohort (age >60 years old). TEG profile of the two cohorts is shown at baseline and various follow-up intervals in Fig. 4. In the younger cohort, we observed a significant prothrombotic change in the haemostatic parameters of TEG the day after VAD implantation, which was absent in the older cohort. In the younger cohort, TEG-MA was 62 ± 7.8 at baseline vs 69.3 ± 5.8 mm at Day 1 (paired t-test P = 0.01); K-time was 6.4 ± 6.7 at baseline vs 2.9 ± 0.82 min at Day 1 (paired t-test P = 0.02); R-time was 21 ± 17.1 at baseline vs 11 ± 3.6 min at Day 1 (paired t-test P = 0.02) and α-angle was 56 ± 19.7 vs 68 ± 5 degree at Day 1 (paired t-test P = 0.06). Also analysis of TEG-MA data in the younger cohort showed that TEG-MA was significantly higher compared with baseline at Day 1 and 2 weeks after device implantation (paired t-test P = 0.01 and < 0.0001, respectively), but was not significantly different from baseline at 4 weeks, 3 or 6 months (paired t-test P = 0.90; 0.24 and 0.78, respectively) after device implantation. In the older cohort, TEG-MA was significantly higher at 2 and 4 weeks postimplantation compared with baseline (paired t-test P < 0.0001 and 0.02, respectively); however, TEG-MA was not significantly different from baseline at 1 day, 3 or 6 months after implantation (paired t-test P = 0.80; 0.20 and 0.54, respectively). TEG data on the first day after device implantation compared with baseline more accurately reflect the effect of the LVAD on platelet function since at subsequent follow-up intervals, APT is being actively adjusted to achieve target TEG-MA and patients might be on different regimen and dose of APT. Following discharge 75% of the younger cohort received 325 mg or more of aspirin daily vs 56% of the older cohort (χ2 P = 0.15). Seventy-five percent of the younger cohort received dipyridamole during follow-up vs 63% of the older cohort (χ2 P = 0.35) and 61% of the younger cohort received 225 mg or more of dipyridamole daily vs 29% of the older cohort (χ2 P = 0.06).
Figure 4:
TEG profile of younger and older patients at various follow-up intervals. Error bars represent one standard error around mean.
Literature search
Based on our search criteria, seven studies were eligible for comparison with our study. These studies are summarized in Table 4. Definition of late-onset bleeding or GI bleeding was variable between the studies. Miller et al. [3], Pagani et al. [4] and Slaughter et al. [13] defined late-onset bleeding as an event requiring operation or two or more packed red blood cells (p-RBC) transfusion in a 24-h period 30 days after surgery. Uriel et al. [14] defined late-onset bleeding as an event requiring at least one unit of p-RBC transfusion >7 days after implantation. Boyle et al. [5], Kushnir et al. [15] and Menon et al. [16] exclusively reported GI bleeding rate. Boyle et al. [5] defined GI bleeding as an event requiring at least two units of p-RBC transfusion in 24 h. Kushnir et al. [15] defined GI bleeding as development of overt bleeding from the upper or lower GI tract, or occult bleeding with ≥2 g/dl drop in haemoglobin from baseline and haemoccult positive stool with no alternative explanation for anaemia, and Menon et al. [16] defined GI bleeding as an event occurring anytime after surgery requiring greater than two units of p-RBC transfusion in a 24-h period. Target and achieved INR were comparable between studies but APT was arbitrary and different (Table 4). The average late-onset or GI bleeding rate among these studies was 0.49 events/PY vs 0.21 in our study. The average TE rate was 0.16 events/PY vs 0.11 in our study and the average stroke rate was 0.065 events/PY vs 0.018 in our study. Notwithstanding the limitations in comparing our results with the literature, the late-onset bleeding rate in our study is lower than those of six of the seven comparable studies we found in the literature and the TE rate is lower than or similar to those of three and higher than those of three of the studies and one study [15] did not report the TE rate.
Table 4:
Literature search for late-onset bleeding in HeartMate II recipients
| First author | Journal, publication, year | Patientsa | Device strategy | F/U mean ± SD (day) | Target INR | Achieved INR | APT | Bleeding (events/PY) | TE (events/PY) | Stroke (events/PY) |
|---|---|---|---|---|---|---|---|---|---|---|
| Present study | — | 57 | BTT or DT | 347 ± 371 | 2 ± 0.5 | 2.1 ± 1.8 | Adjusted with TEG monitoring | 0.21 | 0.11 | 0.018 |
| Miller et al. [3] | N Engl J Med, 2007 | 133 | BTT | 168 ± 148 | 2.5 ± 0.5 | 2.2 ± 0.7 | ASA 81–325 mg/day and dipyridamole 75 mg three times a day | 0.91 | 0.41 | 0.13 |
| Pagani et al. [4] | J Am Coll Cardiol, 2009 | 281 | BTT | 155 (median) | 2.5 ± 0.5 | 2.1 ± 0.9 | ASA 81–325 mg/day and dipyridamole 75 mg three times a day | 0.75 | 0.29 | 0.09 |
| Boyle et al. [5] | J Heart Lung Transplant, 2009 | 331 | BTT | 272 ± 201 | 2.5 ± 0.5 | 1.9 (median) | ASA in all and dipyridamole in half (dose not specified) | 0.23 | 0.055b | 0.041 |
| Slaughter et al. [13] | J Heart Lung Transplant, 2010 | 418 | BTT | 293 ± 263 | 2.5 ± 0.5 | ASA 81–325 mg/day and dipyridamole 75 mg three times a day; a small number received clopidogrel or pentoxifylline | 0.67c | 0.031c | 0.05 | |
| Uriel et al. [14] | J Am Coll Cardiol, 2010 | 79 | BTT or DT | 370 ± 486 | 2.5 ± 0.5 | 1.7 ± 0.5d | 0.44a | 0.06 | 0.02 | |
| Kushnir et al. [15] | Gastrointest Endosc, 2012 | 112 | BTT or DT | 2.5 ± 0.5 | ASA 81–325 mg/day; clopidogrel, dipyridamole or both were arbitrarily added | 0.34 | ||||
| Menon et al. [16] | Eur J Cardiothorac Surg, 2012 | 40 | BTT or DT | 245 | 2.5 (2008–2009) and 2.0–2.5 since 2010 | 2 | ASA 50–100 mg/day only in patients <55 years old or in case of severe atherosclerotic disease of the right coronary artery | 0.1e | 0.1 | 0.06 |
aData are extracted from Table 2 of the article.
bTE rate is derived from Table 3 by adding pump thrombosis and stroke.
cData are extracted from Table 2 of the study by averaging groups A, B and C. TE rate includes pump thrombosis and stroke.
dINR at the time of bleeding event is reported.
eData are extracted from Table 4 of the article. Device support time was 10 566 days in the text.
DISCUSSION
The interaction between HeartMate II and platelets is complex and poorly understood but is not uniform among all recipients. Therefore, we used TEG to adjust APT in all patients throughout LVAD support and achieved late-onset bleeding rate of half of the average rate of seven comparable studies in the literature with similar rate of stroke and TE complications. Our study also demonstrates inter- and intra-individual variability in APT during device support, showing the variable and dynamic effect of HeartMate II on platelet activation and the need to actively adjust APT. Our study does not show any difference in TEG-MA and INR values at the time of bleeding complication compared with routine follow-up which indicates that other factors also play a role such as the effect of loss of pulsatile flow on the development of AV malformations [17] or other haemostatic derangements not monitored by TEG or INR such as development of acquired von Willebrand disease [14].
Previous investigators have shown that older LVAD recipients have higher incidence of bleeding but have not studied why [14, 15]. Similarly our study patients older than 60 years had a higher incidence of late-onset bleeding. Analysis of TEG data in our study shows a prothrombotic shift in TEG parameters of younger patients the day after LVAD implantation that was absent in elderly recipients. This prothrombotic change is observed in R-time, which reflects enzymatic reaction of the coagulation cascade; TEG-MA, which reflects platelet function, fibrinogen and platelet–fibrin interaction and K-time, which reflects both enzymatic reaction and platelet function. Although TEG parameters the day after LVAD implantation are most likely affected by coagulopathic effects of cardiopulmonary bypass and intraoperative transfusion of blood products, the selective prothrombotic shift in TEG profile observed in younger patients is of clinical importance. We also observed higher APT requirement in the younger population throughout follow-up, for which the dose of dipyridamole almost reached statistical significance (P = 0.06). Our results suggest that younger recipients with healthier and more functional platelet and coagulation system show a robust and prothrombotic response to HeartMate II implantation that is absent in the elderly and perhaps the APT and/or anticoagulation regimen should be less aggressive in the elderly population. Interestingly, in our review of the literature the only study that reported a lower bleeding rate is the study by Menon et al. [16], in which HeartMate II recipients ≥55 years old only received APT in case of severe atherosclerotic disease of the right coronary artery and platelet count >100 000. In this study, TE and stroke rates are comparable with those of other studies, but this study is also the smallest study with a relatively short follow-up. We also observed a higher bleeding rate in females, which was statistically significant but only 6 out of 57 patients in our study were female. This finding needs validation in a larger sample of female VAD patients.
Our study has several major limitations. Our comparison with the literature is crude and not statistically tested; however, this is the best comparison that can be conducted with current evidence in the literature, since no previous investigator has conducted a direct comparison between TEG-adjusted and fixed APT. Also the definition of late-onset or GI bleeding is variable among studies although we used a more inclusive definition than most studies. Additionally, target INR is slightly different between studies although achieved INR is very similar. Furthermore, it is known that the TEG-MA is an indirect measurement of APT as it mainly measures platelet function and platelet–fibrin interaction. We are hopeful that the newer modified TEG that allows direct measurement of APT inhibition will prove fertile ground for further investigation. Despite these limitations, our study suggests that APT monitoring with TEG lowers the rate of late-onset bleeding and calls for a direct comparative randomized controlled trial to truly elucidate its benefit. Additionally, a less aggressive APT and/or anticoagulation regimen may reduce bleeding in elderly HeartMate II recipients.
Acknowledgements
We thank Jessica Bell and Jana Reid for assiduous review and adjustment of APT.
Conflict of interest: none declared.
REFERENCES
- 1.Garbade J, Bittner HB, Barten MJ, Mohr FW. Current trends in implantable left ventricular assist devices. Cardiol Res Pract. 2011;2011:290561. doi: 10.4061/2011/290561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuousflow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 4.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–21. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 5.Boyle AJ, Russell SD, Teuteberg JJ, Slaughter MS, Moazami N, Pagani FD, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant. 2009;28:881–7. doi: 10.1016/j.healun.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Snyder TA, Watach MJ, Litwak KN, Wagner WR. Platelet activation, aggregation, and life span in calves implanted with axial flow ventricular assist devices. Ann Thorac Surg. 2002;73:1933–8. doi: 10.1016/s0003-4975(02)03549-x. [DOI] [PubMed] [Google Scholar]
- 8.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125:3038–47. doi: 10.1161/CIRCULATIONAHA.111.040246. [DOI] [PubMed] [Google Scholar]
- 9.John R, Panch S, Hrabe J, Wei P, Solovey A, Joyce L, et al. Activation of endothelial and coagulation systems in left ventricular assist device recipients. Ann Thorac Surg. 2009;88:1171–9. doi: 10.1016/j.athoracsur.2009.06.095. [DOI] [PubMed] [Google Scholar]
- 10.Majeed F, Kop WJ, Poston RS, Kallam S, Mehra MR. Prospective, observational study of antiplatelet and coagulation biomarkers as predictors of thromboembolic events after implantation of ventricular assist devices. Nat Clin Pract Cardiovasc Med. 2009;6:147–57. doi: 10.1038/ncpcardio1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reikvam H, Steien E, Hauge B, Liseth K, Hagen KG, Størkson R, et al. Thrombelastography. Transfus Apher Sci. 2009;40:119–23. doi: 10.1016/j.transci.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Interagency registry for mechanically assisted circulatory support manual of operations version 2.3. published 30 October 2008 http://www.uab.edu/ctsresearch/intermacs/manuals.htm. 30 August 2012, date last accessed.
- 13.Slaughter MS, Naka Y, John R, Boyle A, Conte JV, Russell SD, et al. Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy. J Heart Lung Transplant. 2010;29:616–24. doi: 10.1016/j.healun.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Uriel N, Pak SW, Jorde UP, Jude B, Susen S, Vincentelli A, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–13. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Kushnir VM, Sharma S, Ewald GA, Seccombe J, Novak E, Wang IW, et al. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest Endosc. 2012;75:973–9. doi: 10.1016/j.gie.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menon AK, Götzenich A, Sassmannshausen H, Haushofer M, Autschbach R, Spillner JW. Low stroke rate and few thromboembolic events after HeartMate II implantation under mild anticoagulation. Eur J Cardiothorac Surg. 2012;42:319–23. doi: 10.1093/ejcts/ezr312. discussion 323. [DOI] [PubMed] [Google Scholar]
- 17.Demirozu ZT, Radovancevic R, Hochman LF, Gregoric ID, Letsou GV, Kar B, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2011;30:849–53. doi: 10.1016/j.healun.2011.03.008. [DOI] [PubMed] [Google Scholar]