Abstract
OBJECTIVES
Ischaemia–reperfusion (I/R) injury is encountered in conditions that diminish intestinal blood flow. There is no clinically feasible technique available for mucosal preservation.
METHODS
One hundred Wistar rats were subjected to intestinal ischaemia for 15 and 60 min (I15′, I60′), followed by 1 and 7 days of reperfusion (R1d, R7d). Rats were subjected to ischaemia by clamping the superior mesenteric artery. Prostaglandin E1 (PGE1) (2.500 ng/kg intra-arterial bolus or 20 ng/kg intravenous infusion) was administered immediately prior to the commencement of the experimental period. Animals were divided into 20 groups: sham (laparotomy alone), sacrificed at 1 or 7 days; saline administration, 15 or 60 min of ischaemia, 1 or 7 days of reperfusion; prostaglandin E1 administration, 15 or 60 min of ischaemia, 1 or 7 days of reperfusion, each one for intra-arterial or intravenous administration. Ileal segments were excised and assessed for histopathological score, polymorphonuclear (PMN) leucocytes encountered and myeloperoxidase (MPO) activity measurement.
RESULTS
I/R caused deterioration of histological characteristics. Prophylactic administration of PGE1 resulted in a significant decrease in the histological score compared with the respective saline group (analysis of variance, P < 0.005). In groups treated with PGE1, PMN leucocyte infiltration was lower for the 60 min of ischaemia group (I60′/R1d *P = 0.026; I60′/R7d P = 0.015). I15′/R7d did not lead to a significant reduction in PMN infiltration (P = 0.061). Pretreatment with PGE1 attenuates MPO levels after intestinal I/R injury (P < 0.05). No differences were encountered between types of administration.
CONCLUSIONS
Results of this study showed that administration of prostaglandin E1 prevents I/R injury by diminishing histological damage parameters, inhibiting PMN leucocyte infiltration and attenuating MPO activity.
Keywords: Intestine, Ischaemia, Reperfusion injury, Prostaglandin E1
INTRODUCTION
Ischaemia–reperfusion (I/R) injury of the intestine is of importance in situations such as in cardiopulmonary bypass, strangulated hernias, intestinal transplantation, neonatal necrotizing enterocolitis, abdominal aortic aneurysm surgery and in hypovolemia and septic shock. I/R injury of the intestine is a systemic phenomenon resulting in bacterial translocation, endotoxemia, acute respiratory distress syndrome, acute hepatic injury, which may eventually lead to multiple organ dysfunction syndrome [1].
Although necessary to salvage the tissue, reperfusion of ischaemic intestine increases the hazardous effect of early ischaemic injury by releasing reactive oxygen species and accumulation of active neutrophils. Reperfusion leads to production of reactive oxygen species that enhance chemotactic activity of neutrophils. Once adhered to the endothelium, neutrophils increase endothelial damage by liberation of reactive oxygen species and proteolytic enzymes [2]. Reperfusion of an ischaemic organ is not only associated with local changes but also with systemic ones. These alterations include a progressive fall in the mean arterial blood pressure, the release of proinflammatory mediators from the reperfused tissue into the systemic circulation, the alteration of the function of remote organs, such as the heart and lungs and, ultimately, decreased survival [3].
Therapeutic strategies aimed at ameliorating I/R injury have focused both on preventing the effects of reactive oxygen species and on downregulating the signal transduction cascades related to the expression of proinflammatory genes. Prostaglandin E1 (PGE1), alprostadil, a biologically active lipid mediator produced by cells within the blood vessels (endothelium, smooth muscle, pericytes), interstitial cells (fibroblasts, mast cells) and blood cells (leucocytes, platelets), has been investigated in different models of I/R injury. In these investigations, PGE1-induced vasodilatation [4], inhibited platelet and leucocyte aggregation and activation, and decreased oedema formation during reperfusion [5].
Other investigations have studied the effects of prostaglandin infusion on enteral ischaemia [6–8]. These articles, studied effects of PGI2, PGE2 and PGF2 alpha in porcine or dog models, with measurements of transmucosal resistance, epithelial barrier function or laser flowmeter. However, our investigation represents the first study on the effects of preischaemic intra-arterial and intravenous (IV) administration of PGE1.
In this study, we examined whether intra-arterial bolus administration or continuous IV administration of PGE1 could protect the intestinal mucosa from I/R injury. The intestinal I/R was assessed by changes in mucosal integrity, polymorphonuclear (PMN) leucocyte infiltration and myeloperoxidase (MPO) activity.
MATERIALS AND METHODS
Animal model and experimental design
One hundred Wistar rats, weighing 250–479 g, provided from our laboratory's rat colony, were used. They were housed in macrolon cages, five rats per cage, at 20–22°C room temperature, on a 12 h light:12 h darkness cycle and provided with commercial pelleted diet and tap water ad libitum. The facilities were in accordance with the Directive 86/609/EEC. The experiments were performed in adherence to the National Institutes of Health Guidelines on the Use of Laboratory Animals and the study was approved by our Institutional Animal Care and Use Committee.
The animals were randomly assigned to 20 groups of 5 animals each, as follows: Group 1, sham operation (rats were subjected to identical surgical procedures as the above groups except that the blood vessels were not occluded and the rats were maintained under anaesthesia for the duration of the experiment) and sacrificed after 24 h (Sham/1d); Group 2, 15 min of ischaemia and 24 h of reperfusion receiving saline (I15′/R1d saline); Group 3, 15 min of ischaemia and 24 h of reperfusion receiving PGE1 (I15′/R1d PGE1); Group 4, 60 min of ischaemia and 24 h of reperfusion receiving saline (I60′/R1d saline); Group 5, 60 min of ischaemia and 24 h of reperfusion receiving PGE1 (I60′/R1d PGE1); Group 6, sham operation and sacrificed after 7 days (Sham/7d); Group 7, 15 min of ischaemia and 7 days of reperfusion receiving saline (I15′/R7d saline); Group 8, 15 min of ischaemia and 7 days of reperfusion receiving PGE1 (I15′/R7d PGE1); Group 9, 60 min of ischaemia and 7 days of reperfusion receiving saline (I60′/R7d saline); Group 10, 60 min of ischaemia and 7 days of reperfusion receiving PGE1 (I60′/R7d PGE1). The doses of the treatment with PGE1 were a bolus of 2.5 μg/kg (=2500 ng/kg), or a 20 ng/kg/min given as IV infusion from the start of surgery (Groups 11–20 with the same I/R strategies). These doses used here to reduce I/R injury in the gut had previously been reported to reduce the tissue injury caused by I/R in the kidney [9, 10].
Surgical procedure
All animals were anaesthetized using intraperitoneal injection of ketamine 60 mg/kg. After skin shaving and preparation of the abdominal wall with 10% povidone–iodine solution, a midline laparotomy was performed. The small bowel was exteriorized. The ligament of Treitz was cut to dissect and expose the superior mesenteric artery (SMA). After saline or PGE1 administration immediately prior to the commencement of the experimental period, an atraumatic microvascular clamp (Aesculap, Tuttlingen, Germany) was then placed across the SMA just after its origin from the aorta for occlusion, avoiding occlusion of the superior mesenteric vein. The bowel was returned to the abdominal cavity, and the incision was closed with interrupted atraumatic 4/0 silk sutures. After 15 (Groups 2, 3, 7, 8) or 60 min (Groups 4, 5, 9, 10) of ischaemia, a relaparotomy was performed, the occluding clamp was removed and the abdominal incision was closed again in layers using 3-0 Prolene suture. Mesenteric reperfusion was confirmed with the restoration of pulsation and colour. Sham operation involved the same technique and exposure without clipping of the SMA. After a 24-h (Groups 1–5) or 7-day (Groups 6–10) reperfusion period, the abdominal wall was opened once more and samples of ileum were obtained for histological analyses. The animals were euthanized after the procedure was completed.
Histopathological and morphometric examination
Biopsies of the distal ileum (10 cm from the ileocecal valve), ∼2 cm long, were excised and fixed immediately in 10% formalin, then embedded in paraffin wax, sectioned at 5 µm and stained with haematoxylin and eosin. For each animal, 10 sections of ileum were examined using standard light microscopy. Investigators reviewed each slide in a blinded fashion to prevent investigator bias. Histological mucosal damage in each preparation was classified according to microscopic criteria set for the grading of intestinal tissue injury (Grades 0–5) by Chiu et al. [11]. Villi height (difference between mucosal thickness and crypt depth), villi width, mucosal thickness (from the tip of the villi to the muscular layer) and crypt depth (from the villi base to the muscular layer) were measured. Morphometry was performed using the Nikon DS Camera Control Unit DS-L2® image analyzer software. PMN leucocytes were counted per Giemsa-stained high power field in 20 separate areas of each slide immediately superior to the muscularis mucosae, and the mean number of PMN leukocytes per high power field was determined for each animal.
Myeloperoxidase activity measurement
Tissue-associated MPO activity in the intestinal mucosa was determined following the method of Grisham et al. [12]. Samples of intestinal mucosa weighing 300 mg were homogenized in 5 ml of ice-cold 0.02 M ethylenediaminetetraacetic acid (pH: 4.7) for 60 s. Five millilitres of mucosal homogenate were centrifuged at 20 000 revolutions per minute for 15 min at 4°C to pellet the insoluble cellular debris. The supernatant, which contained <5% of total MPO activity, was discarded. The pellet was then rehomogenized in an equivalent volume of 0.05 M potassium phosphate buffer (pH: 6.0) containing 0.5% hexadecyltrimethylammonium bromide. MPO activity was assessed by measuring the H2O2-dependent oxidation of O-dianisidin. One unit of enzyme activity was defined as the amount of MPO present that caused a change in absorbance of 1.0/min at 410 nm and 37°C [13].
Immunohistochemistry
Rats were transcardially perfused with heparinized saline, followed by 4% paraformaldehyde, and then 20-µm-thick intestinal sections were cut on a cryostat. The adjacent sections were stained with haematoxylin and eosin to confirm the ischaemic boundary area. The effects were examined by MPO staining. The sections were then blocked with 3% normal goat serum in PBS, incubated once overnight with rabbit polyclonal antibodies against MPO diluted at 1 : 500 (DAKO, Baltimore, MD, USA) at 4°C, and incubated again with biotinylated goat rabbit IgG (Vector, CA, USA) at room temperature for 1 h. Endogenous tissue peroxidase activity was abolished by incubation with 0.3% H2O2 in methanol for 20 min. After washing in Tris-buffered saline, non-specific binding was blocked with 5% normal bovine serum in Tris-buffered saline. Positively stained cells for MPO were evaluated. The numbers of positively stained cells were counted in three randomly selected microscopic fields under ×100 magnification (1.33 mm2) in a blind fashion.
Statistical analyses
Data were analysed with SPSS 17.0 (SPSS, Inc., Software, Chicago, IL, USA) statistical software using one-way analysis of variance (ANOVA) and with the Tukey–Kramer test so as to determine comparison among groups, and differences among groups, respectively. All values were expressed as mean ± standard deviation (SD) and P < 0.05 was considered statistically significant.
RESULTS
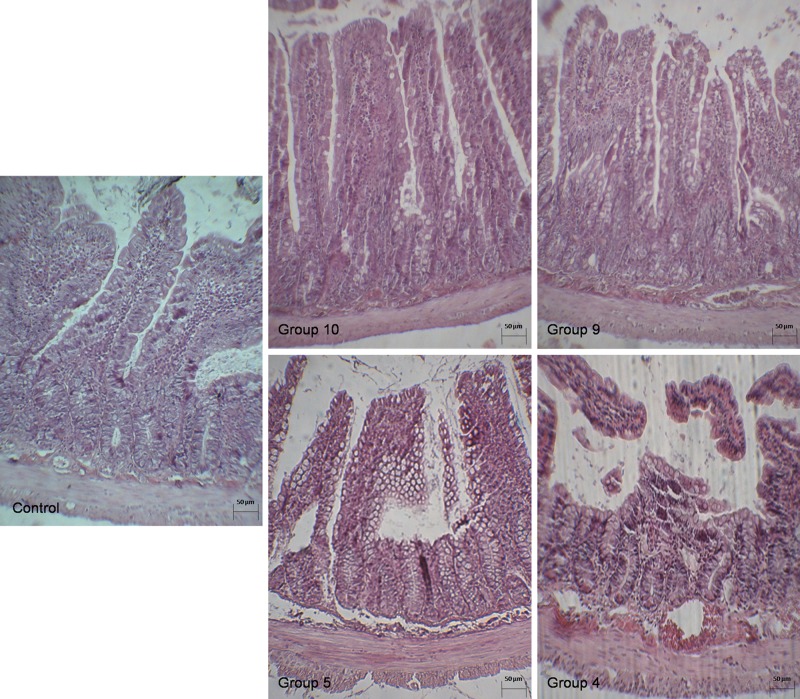
Microscopic slides of intestinal sections showed that I/R in our animal model was associated with significant structural damage (Fig. 1). There were no gross perforations in any of the rats. Although the villi of the sham animals were intact, the saline I/R group showed extensive mucosal sloughing, starting from the tips of the villi and proceeding towards the serosa. In sections from the group injured by I15′/R1d and with treatment with saline, mucosal damage and sloughing on part of the surface epithelium was obvious. At 7 days after reperfusion, a significant difference in mucosal appearance persisted between the groups. In some instances, as in the I60′/R1d group, sloughing was accompanied by mucosal ulceration, haemorrhage and necrosis. Mucosal injury in the I60′/R7d group was confirmed by histological grading, with the saline groups being associated with a higher score compared with the PGE1 groups indicative of increased tissue damage.
Figure 1:
Photomicrographs (×40, haematoxylin and eosin) of the small intestine at various stages of ischaemic damage. (Group 10) Normal non-ischaemic intestine Grade 0 (Group I60′/R7d, PGE1). (Group 9) Mild damage Grade 1 (Group I60′/R7d, saline). (Group 5) Sample of moderate damage Grade 2 (Group I60′/R1d, PGE1). (Group 4) Grade 3 severe damage (Group I60′/R1d, saline). See Materials and Methods for a complete description of histological grading.
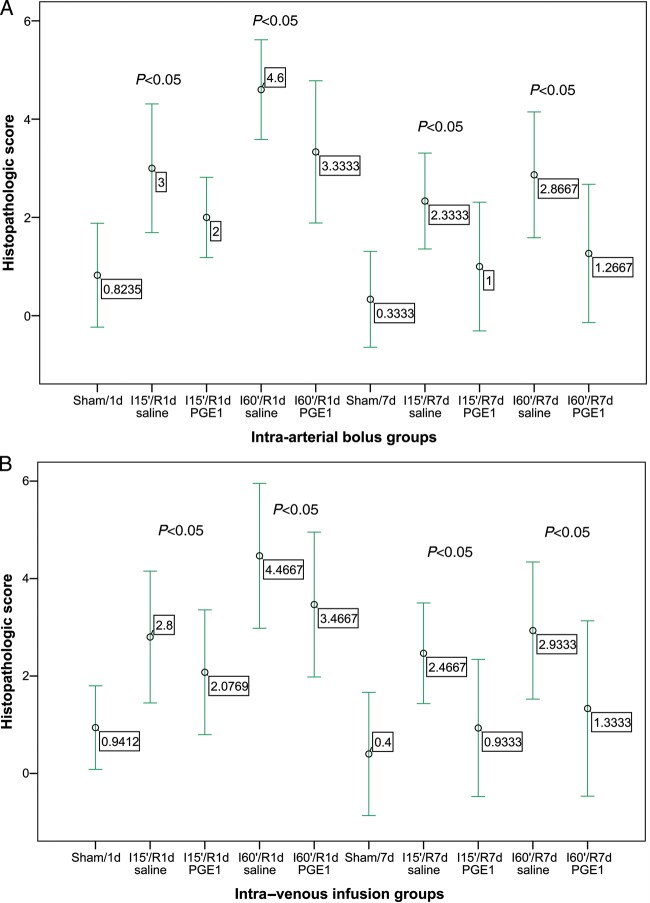
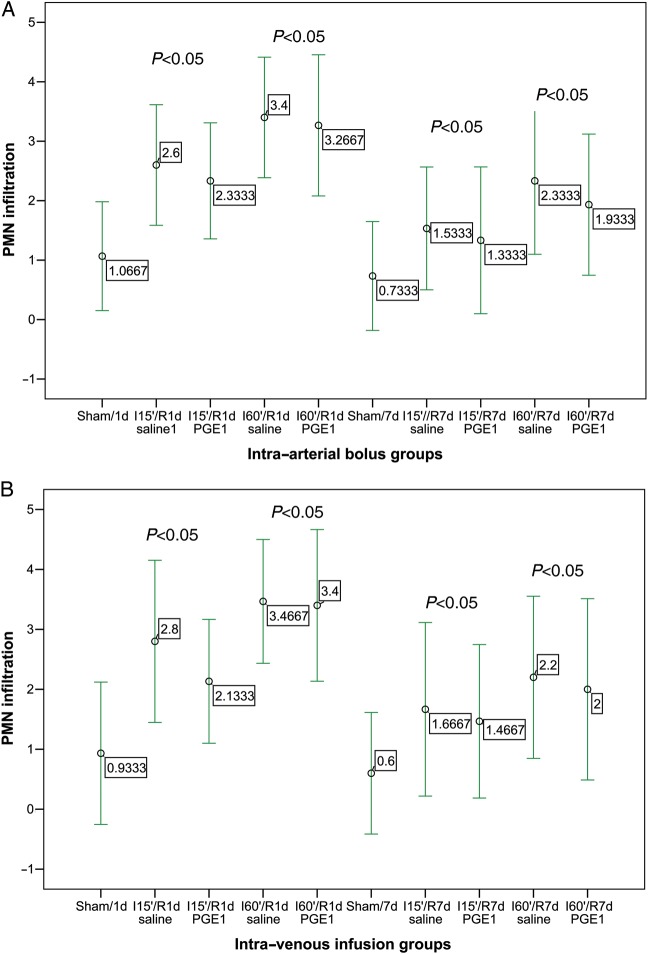
Mean villi height, villi width, intestinal mucosal thickness and crypt depth for all treatment groups are presented in Tables 1 and 2. There were no differences in mucosal thickness or in crypt depth. However, there were differences in villi height and villi width. In groups treated with PGE1, leucocyte PMN infiltration was lower for 60 min of ischaemia. I15′/R7d PGE1 did not lead to a significant reduction in PMN infiltration in comparison with the saline group (P = 0.061 and P = 0.073, in the intra-arterial and IV group, respectively) (Fig. 2). After reperfusion, the number of infiltrating PMNs increased rapidly in both groups. The highest PMN tissue infiltration was found at 60 min of ischaemia and 1 day of reperfusion, with clusters of PMNs found equally in the lamina propria and among crypts. At the later time points, the PMN leucocyte count decreased and infiltrating neutrophils were found mostly in the lower half of the villi. Neutrophil recruitment was less at all time points from pretreated subjects compared with controls (P < 0.05) (Fig. 3).
Table 1:
Histological evaluation (villi height, villi width, mucosal thickness and crypt depth) of haematoxylin- and eosin-stained sections of intestinal mucosa at 15 and 60 min of ischaemia (I15′, I60′), and 1 and 7 days of reperfusion (R1d, R7d)
| Group | Villi height (µm) | Villi width (µm) | Mucosal thickness (µm) | Crypt depth (µm) |
|---|---|---|---|---|
| I15′/R1d, saline | 269.1 ± 52.0*,*** | 110.7 ± 25.3*** | 580.1 ± 45.4*,*** | 194.5 ± 39.4*** |
| I15′/R1d, PGE1 | 301.1 ± 40.0**,*** | 111.3 ± 35.5**,*** | 601.5 ± 72.0*,*** | 224.8 ± 43.1*** |
| I60′/R1d, saline | 248.0 ± 55.0* | 82.4 ± 15.3* | 511.2 ± 90.4* | 165.7 ± 30.5* |
| I60′/R1d, PGE1 | 277.7 ± 45.0** | 95.4 ± 13.4*,** | 499.7 ± 60.9* | 175.3 ± 11.7 |
| I15′/R7d, saline | 318.1 ± 47.6* | 115.9 ± 32.1*** | 674.4 ± 82.3*** | 227.5 ± 51.9*** |
| I15′/R7d, PGE1 | 337.4 ± 64.6 | 124.2 ± 25.8**,*** | 679.5 ± 61.3*** | 246.3 ± 39.2*** |
| I60′/R7d, saline | 301.5 ± 19.9* | 91.7 ± 24.9* | 520.5 ± 60.4* | 187.4 ± 15.6* |
| I60′/R7d, PGE1 | 331.8 ± 50.0** | 103.0 ± 12.8*,** | 519.5 ± 63.6* | 192.3 ± 25.0* |
| Sham/1d | 312.9 ± 45.8 | 129.5 ± 20.2 | 673.0 ± 34.9 | 213.3 ± 55.6 |
| Sham/7d | 345.6 ± 54.7 | 139.1 ± 24.7 | 683.3 ± 40.6 | 243.8 ± 36.8 |
Intra-arterial administration of saline and PGE1. All data are expressed as mean ± SD.
*P < 0.05 compared sham-operated group.
**P < 0.05 compared saline vs PGE1.
***P < 0.05 compared 15′ vs 60′.
Table 2:
Histological evaluation (villi height, villi width, mucosal thickness and crypt depth) of haematoxylin- and eosin-stained sections of intestinal mucosa at 15 and 60 min of ischaemia (I15′, I60′), and 1 and 7 days of reperfusion (R1d, R7d)
| Group | Villi height (µm) | Villi width (µm) | Mucosal thickness (µm) | Crypt depth (µm) |
|---|---|---|---|---|
| I15′/R1d, saline | 273.6 ± 73.0*,*** | 116.9 ± 14.2*,*** | 576.0 ± 39.4*** | 201.5 ± 39.1*,*** |
| I15′/R1d, PGE1 | 311.1 ± 23.4*,**,*** | 121.5 ± 18.2*,**,*** | 611.2 ± 65.3*** | 219.3 ± 48.3*,**,*** |
| I60′/R1d, saline | 233.0 ± 49.7* | 91.4 ± 22.3* | 517.5 ± 82.4* | 166.2 ± 39.9* |
| I60′/R1d, PGE1 | 256.4 ± 34.3** | 96.2 ± 11.7*,** | 511.3 ± 71.1* | 164.3 ± 17.9* |
| I15′/R7d, saline | 338.4 ± 41.0* | 133.2 ± 36.6*** | 685.7 ± 70.0*,*** | 223.4 ± 59.0*,*** |
| I15′/R7d, PGE1 | 343.1 ± 73.2*,**,*** | 131.2 ± 27.3*** | 683.1 ± 75.8*** | 253.4 ± 42.2*,*** |
| I60′/R7d, saline | 296.7 ± 33.5* | 101.2 ± 23.7* | 522.3 ± 69.0* | 176.6 ± 23.7 |
| I60′/R7d, PGE1 | 322.0 ± 57.3 | 111.0 ± 13.3** | 523.9 ± 53.9* | 199.6 ± 22.6 |
| Sham/1d | 306.8 ± 57.3 | 137.5 ± 11.2 | 696.1 ± 43.7 | 231.5 ± 57.1 |
| Sham/7d | 351.6 ± 59.4 | 128.2 ± 13.3 | 671.2 ± 32.6 | 248.4 ± 39.2 |
IV administration of saline and PGE1. All data are expressed as mean ± SD.
*P < 0.05 compared sham-operated group.
**P < 0.05 compared saline vs PGE1.
***P < 0.05 compared 15′ vs 60′.
Figure 2:
Effect of treatment with PGE1 on the histopathological score at 15 and 60 min of ischaemia (I15′, I60′), and 1 and 7 days of reperfusion (R1d, R7d). Mean ± 2 SDs. (A) Intra-arterial bolus groups. (B) Intra-venous infusion groups.
Figure 3:
Effect of treatment with PGE1 on PMN infiltration at 15 and 60 min of ischaemia (I15′, I60′), and 1 and 7 days of reperfusion (R1d, R7d). Mean ± 2 SDs. (A) Intra-arterial bolus groups. (B) Intra-venous infusion groups.
Histologically, the villi were denuded of mucosa, accompanied in some cases by haemorrhage. However, pretreatment with PGE1 ameliorated tissue damage associated to I/R, although the intestinal mucosa in these groups was distinguishable from normal bowel, with evidence of epithelial disruption (P < 0.05, compared with sham animals in all groups). Attenuation of tissue damage by PGE1 treatment was confirmed by histological grading. Thus, prophylactic administration of PGE1 resulted in a significant decrease in the histological score compared with the respective saline group (ANOVA, P < 0.005). The histological scores obtained for the PGE1-treated segments were different from non-I/R sham surgery control values (P = 0.0001).
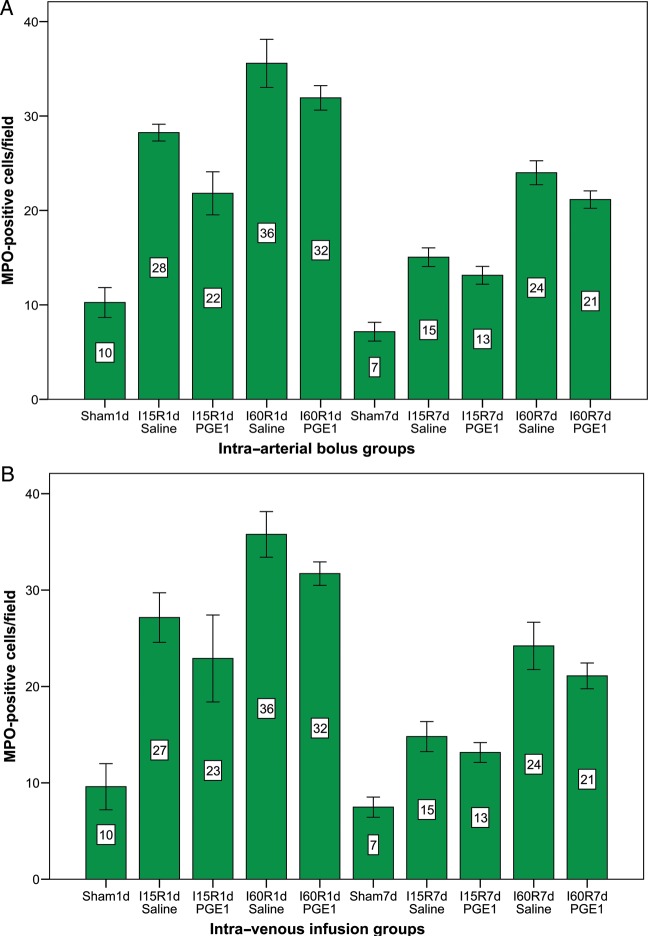
Table 3 compares the tissue-associated MPO responses to I/R in untreated animals and in animals pretreated with saline and PGE1. I/R alone (saline groups) produced a significant increase in ileal MPO activity compared with the sham-operated group (P < 0.05). The response to I/R was significantly reduced with PGE1 pretreated groups compared with the saline groups, indicating that PMNs might be an important regulator of I/R-induced mucosal injury (Fig. 4). Pretreatment of PGE1 attenuated MPO levels after intestinal I/R injury. The histological injury grading and neutrophil recruitment after 7 days of reperfusion were less compared with groups with 1 day of reperfusion, demonstrating the mucosal recovery during the reperfusion period. MPO-positivity was frequently observed in the ischaemic areas. PGE1 treatment significantly reduced the number of MPO-positive cells (Fig. 5).
Table 3:
Myeloperoxidase (MPO) activity
| Intra-arterial | IV | |
|---|---|---|
| Group | MPO activity (U/g) | MPO activity (U/g) |
| I15′/R1d, saline | 3.79 ± 0.88*,*** | 3.67 ± 0.21*,*** |
| I15′/R1d, PGE1 | 2.11 ± 0.45*,**,*** | 2.04 ± 0.25*,**,*** |
| I60′/R1d, saline | 4.97 ± 0.35* | 4.83 ± 0.43* |
| I60′/R1d, PGE1 | 3.39 ± 0.77*,** | 3.45 ± 0.88*,** |
| I15′/R7d, saline | 2.04 ± 0.20*,*** | 2.11 ± 0.31*** |
| I15′/R7d, PGE1 | 1.28 ± 0.53*,*** | 1.22 ± 0.67*,**,*** |
| I60′/R7d, saline | 2.36 ± 0.82* | 2.40 ± 0.71* |
| I60′/R7d, PGE1 | 1.95 ± 0.79*,** | 2.03 ± 0.64*,** |
| Sham/1d | 0.63 ± 0.22 | 0.69 ± 0.53 |
| Sham/7d | 0.27 ± 0.76 | 0.24 ± 0.71 |
Intra-arterial and IV administration of saline and PGE1. All data are expressed as mean ± SD.
*P < 0.05 compared sham-operated group.
**P < 0.05 compared saline vs PGE1.
***P < 0.05 compared 15′ vs 60′.
Figure 4:
Quantitative analysis of the number of MPO-positive cells by immunohistochemistry. Data are the mean ± 2 SDs. (A) Intra-arterial bolus groups. (B) Intra-venous infusion groups.
Figure 5:
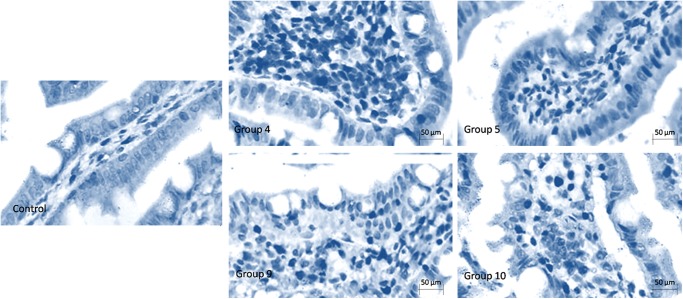
Pictures of inmunohistochemical staining of MPO. MPO+ intestinal cells were stained dark. A few MPO+ cells were found in the control group, while there were many apoptotic cells in the groups treated with saline. However, fewer MPO+ cells were observed in the PGE1 groups.
There were no significant side effects except for 14 cases of body oedema.
DISCUSSION
We have examined the effects of pretreatment with PGE1 on the intestinal responses to mesenteric I/R. Histological damage that occurs after intestinal I/R is characterized by shortening of the villus length, loss of villus epithelium, necrosis and invasion by inflammatory cells. Pretreatment with PGE1 significantly attenuated the histological damage and PMN infiltration when compared with the control group.
Several therapeutic modalities have been used successfully to attenuate reperfusion injury in animal models of I/R injury of the intestine [1, 14]. Translation of these odds into clinical practice, however, faces numerous obstacles such as the narrow therapeutic window of some compounds and excessive toxicity, or practical impediments (complicated, long procedures), whereas an ideal alternative would be a short course of an established compound using a simple protocol. The present study was conducted to establish if pretreatment of the subject with a single, rapidly administered dose of PGE1 would reduce I/R injury. In practice, this PGE1 administration could not be considered easy, because the SMA approach necessitates a laparotomy or endovascular procedures.
Most reports document the role of PMNs as dominant mediators of ischaemia and reperfusion injury [15]. Neutrophils may contribute to intestinal cell injury by various mechanisms, including free-radical generation, production of vascoconstricting and chemotactic leukotrienes, complement activation, release of proteolytic enzymes, platelet activation and aggregation, and neutrophil-trapping in capillaries with plugging of ischaemic and altered intestinal microvasculature [16]. PGE1 has been shown to inhibit neutrophil chemotaxis, aggregation, lysosomal enzyme release and superoxide anion generation by neutrophils in vitro. An additional potential benefit of reduced neutrophil activation and infiltration of ischaemic intestinal tissue is the preservation of intestinal microvascular patency [17].
Histological damage that occurs after intestinal I/R is characterized by shortening of the villus length, loss of villus epithelium, necrosis and invasion of inflammatory cells [18]. There were no differences in mucosal thickness or in crypt depth; however, there were differences in villi height and villi width. We cannot explain these findings regarding these indicators of mucosal injury. Although results of this study clearly showed the protective effect of PGE1 on I/R intestinal injury, the precise mechanism is still unclear. Various investigations have reported that PGE1 reduces superoxide and peroxynitrite production, thereby reducing I/R injury [5], changing cytokine release [10] and improving collateral flow [19]. The PGE1-induced vasodilatation that occurs in ischaemic limbs appears to have been at the expense of tissue by redistributing microvascular flow to vessels not providing nutritive flow or by limiting cellular oxygen utilization. PGE1 additionally reduces thromboxane A2 synthesis by suppression of platelet activation, limiting increases in pulmonary vascular resistance caused by thromboxane A2 generation and capillary plugging by PMN leucocytes after reperfusion [20, 21]. On the other hand, Gabriel et al. [22] reported that PGE1 had a negative effect on I/R injury during abdominal aortic aneurysm surgery, contrary to Sako et al. [23]. PGE2 stimulates recovery of barrier function in porcine ischaemia-injured ileal mucosa [24] by stimulating closure of interepithelial spaces via cAMP and via a mechanism involving Cl(-) secretion and reductions in paracellular permeability.
The use of mesenteric artery occlusion with reperfusion is a well-established model of intestinal injury resulting from acute vascular occlusion as occurs after embolism or thrombosis. I/R injury has the advantage of being more physiologically relevant than administration of toxic agents, such as thioacetamide, because the major factors causing injury probably internally generated proinflammatory cytokines and free-radical production, rather than resulting from metabolism of an external damaging agent. A considerable number of experimental studies have indicated that I/R injury of the intestine occurs in a biphasic manner characterized by different time frames and mechanisms: an early phase that immediately follows the ischaemia and lasts for 2–3 h; and a late phase that begins 12–24 h from the ischaemia and lasts for ∼3–4 days [1]. Hence, a period of 24 h of reperfusion following ischaemia was chosen to assess the changes in the early phase of reperfusion injury and a period of 7 days for the changes in the late phase. After a period of 7 days, the intestinal mucosa recovers completely independently of the ischaemic period as long as the damage is reversible [25]. Mallick et al. [25] after a 24-h period studied the beginnings of the late phase of reperfusion and demonstrated that ischaemic preconditioning attenuates microvascular disturbances in the mucosal villi of the small bowel following I/R injury.
The majority of previous works on intestinal ischaemia presented agents administered to subjects at varying time points before ischaemia. In the review article by Mallick et al. [1], it was suggested that the previously used settings were not satisfactorily proportional to real clinical conditions. Therefore, there is a place for studies where any substance with possible anti-PMN infiltration effects should be administered before ischaemia has been established. There are some surgical conditions in which one can find a window of opportunity to manage I/R injury before ischaemia, such as vascular operations (i.e. aortic aneurysm repair, embolectomy for an acute mesenteric occlusion or repair of traumatic vascular lacerations), or organ transplantations (i.e. solid organ or hollow viscus). Another population that might benefit from prophylactic treatment of I/R injury is the premature and very low birth-weight newborn infants that are at high risk of developing necrotizing enterocolitis. Thromboembolic acute occlusion of the SMA is usually unpredictable, and so pretreatment with PGE1 in these patients is not possible, nor is the application of the results of this study. Moreover, PGE1 treatment after intestinal ischaemia instauration could be a necessary study in the future.
This study presents several limitations. First, because therapy was administered before arterial occlusion, our experimental design tested whether infarct salvage could be achieved by the prevention of ischaemic and reperfusion injury, but could not test specifically whether treatment might have limited ischaemic or reperfusion injury. Furthermore, the separation of anatomic improvement from functional improvement with PGE1 is difficult to understand, and hypothetically, structural and functional changes should be correlated. Finally, a direct comparison of PGE1 effects on intestinal mucosa achieved in vivo would have been desirable; future studies will be performed to determine optimal human dosage and route of PGE1 administration.
In conclusion, this study demonstrated the efficacy of PGE1 to protect intestinal mucosa from I/R injury, when administered before ischaemia. We concluded that PGE1 treatment before intestinal ischaemia attenuated or prevented histological damage from I/R injury by inhibiting neutrophil infiltration. We believe that PGE1 may prove to be a useful technique against intestinal mucosa injury in a variety of clinical conditions and surgical operations. As a future consideration, further studies are required to elucidate the specific mechanisms involved in the mucoprotective action of this drug.
Conflicts of interest: none declared.
REFERENCES
- 1.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–77. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 2.Linfert D, Chowdhry T, Rabb H. Lymphocites and ischemia-reperfusion injury. Transplant Rev (Orlando) 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milano PM, Douillet CD, Riesenman PJ, Robinson WP, 3rd, Beidler SK, Zarzaur BL, et al. Intestinal ischemia-reperfusion injury alters purinergic receptor expression in clinically relevant extraintestinal organs. J Surg Res. 2008;145:272–8. doi: 10.1016/j.jss.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Scheidt W, Costard-Jaeckle A, Stempfle HU, Deng MC, Schwaab B, Haaff B, et al. Prostagladin E1 testing in heart failure-associated pulmonary hypertension enables transplantation: the PROPHET study. J Heart Lung Transplant. 2006;25:1070–6. doi: 10.1016/j.healun.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Huk I, Brovkovych V, Nanobashvili J, Neumayer C, Polterauer P, Prager M, et al. Prostaglandin E1 reduces ischemia/reperfusion injury by normalizing nitric oxide and superoxide release. Shock. 2000;14:234–42. doi: 10.1097/00024382-200014020-00026. [DOI] [PubMed] [Google Scholar]
- 6.Blikslager AT, Pell SM, Young KM. PGE2 triggers recovery of transmucosal resistance via EP receptor cross talk in porcine ischemia-injured ileum. Am J Physiol Gastrointest Liver Physiol. 2001;281:G375–81. doi: 10.1152/ajpgi.2001.281.2.G375. [DOI] [PubMed] [Google Scholar]
- 7.Blikslager AT, Roberts MC, Rhoads JM, Argenzio RA. Prostaglandins I2 and E2 have a synergistic role in rescuing epithelial barrier function in porcine ileum. J Clin Invest. 1997;100:1928–33. doi: 10.1172/JCI119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kan T, Mitomi H, Saigenji K, Atari E. The effect of prostaglandin E1 and prostaglandin F2 alpha in the ischemic small intestine of dogs. Nihon Shokakibyo Gakkai Zasshi. 1993;90:114–23. [PubMed] [Google Scholar]
- 9.Conde C, Herreros V, Rodríguez-Toves LA, Vaquero C. Effects of PGE1 on preservation of renal tissue after warm ischemia. Morphometric and histologic study . Spanish J Surg Res. 2001;4:61–9. [Google Scholar]
- 10.Sketch MH, Whelton A, Schollmayer E, Koch JA, Bernink PJ, Woltering F, et al. Prostaglandin E1 Study Group. Prevention of contrast media-induced renal dysfunction with prostaglandin E1 a randomized, double-blind, placebo-controlled study. Am J Ther. 2001;8:155–62. doi: 10.1097/00045391-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states: a morphological, hemodynamic and metabolic reappraisal. Arch Surg. 1970;101:478–83. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 12.Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567–74. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- 13.Naito Y, Takagi T, Yoshikawa T. Molecular fingerprints of neutrophil-dependent oxidative stress in inflammatory bowel disease. J Gastroenterol. 2007;42:787–98. doi: 10.1007/s00535-007-2096-y. [DOI] [PubMed] [Google Scholar]
- 14.Oltean M, Pullerits R, Zhu C, Blomgren K, Hallberg EC, Olausson M. Donor pretreatment with FK506 reduces reperfusion injury and accelerates intestinal graft recovery in rats. Surgery. 2007;141:667–77. doi: 10.1016/j.surg.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin Immunol. 2011;141:3–14. doi: 10.1016/j.clim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Vollmar B, Menger MD. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbeks Arch Surg. 2011;396:13–29. doi: 10.1007/s00423-010-0727-x. [DOI] [PubMed] [Google Scholar]
- 17.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frishman WH, Novak S, Brandt LJ, Spiegel A, Gutwein A, Kohi M, et al. Pharmacologic management of mesenteric occlusive disease. Cardiol Rev. 2008;16:59–68. doi: 10.1097/CRD.0b013e31815a6600. [DOI] [PubMed] [Google Scholar]
- 19.Milio G, Cospite V, Cospite M. Effects of PGE1 in patients suffering from peripheral arterial occlusive disease. Minerva Cardioangiol. 2003;51:311–6. [PubMed] [Google Scholar]
- 20.Ney P, Braun M, Szymanski C, Bruch L, Schrör K. Antiplatelet, antineutrophil and vasodilating properties of 13,14_dihydro-PGE1 (PGE0)—n in vivo metabolite of PGE1 in man. Eicosanoids. 1991;4:177–84. [PubMed] [Google Scholar]
- 21.Krueger U, Scholz H, Heise M, Adeberg P, Petzold M, Zanow J, et al. Effect of intravenous iloprost and alprostadil (PGE1) on peripheral resistance during femoro-distal reconstructions. Int Angiol. 2000;19:358–65. [PubMed] [Google Scholar]
- 22.Gabriel A, Werba A, Mares P, Grubhofer G, Hrska F, Griesmacher A, et al. Influence of prostaglandin E1 on tissue ischemia during surgical repair of the abdominal aorta. J Cardiothorac Vasc Anesth. 1996;10:201–6. doi: 10.1016/s1053-0770(96)80237-0. [DOI] [PubMed] [Google Scholar]
- 23.Sako H, Hadama T, Miyamoto S, Anai H, Wada T, Iwata E, et al. Effect of prostaglandin E1 on ischemia-reperfusion injury during abdominal aortic aneurysm surgery. Surg Today. 2006;36:140–6. doi: 10.1007/s00595-005-3116-2. [DOI] [PubMed] [Google Scholar]
- 24.Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel antagonist of the CIC-2 chloride channel, lubiprostone. Am J Physiol Gastrointest Liver Physiol. 2007;292:G647–56. doi: 10.1152/ajpgi.00183.2006. [DOI] [PubMed] [Google Scholar]
- 25.Mallick IH, Winslet MC, Seifalian AM. Ischemic preconditioning of small bowel mitigates the late phase of reperfusion injury: heme oxygenase mediates cytoprotection. Am J Surg. 2010;199:223–31. doi: 10.1016/j.amjsurg.2009.01.011. [DOI] [PubMed] [Google Scholar]