Abstract
In the U.S. and worldwide anabolic/androgenic steroid use remains high in the adolescent population. This is concerning given that anabolic/androgenic steroid use is associated with a higher incidence of aggressive behavior during exposure and anxiety during withdrawal. This study uses pubertal Syrian hamsters (Mesocricetus auratus) to investigate the hypothesis that an inverse behavioral relationship exists between anabolic/androgenic steroid-induced aggression and anxiety across adolescent exposure and withdrawal. In the first experiment, we examined aggression and anxiety during adolescent anabolic/androgenic steroid exposure and withdrawal. Adolescent anabolic/androgenic steroid administration produced significant increases in aggression and decreases in anxiety during the exposure period followed by significant decreases in aggression and increases in anxiety during anabolic/androgenic steroid withdrawal. In a second experiment, anabolic/androgenic steroid exposed animals were separated into groups based on their aggressive response during the exposure period and then tested for anxiety during exposure and then for both aggression and anxiety during withdrawal. Data were analyzed using a within subjects repeated measures predictive analysis. Linear regression analysis revealed that the difference in aggressive responding between the anabolic/androgenic steroid exposure and withdrawal periods was a significant predictor of differences in anxiety for both days of testing. Moreover, the combined data suggest that the decrease in aggressive behavior from exposure to withdrawal predicts an increase in anxiety-like responding within these same animals during this time span. Together these findings indicate that early anabolic/androgenic steroid exposure has potent aggression- and anxiety- eliciting effects and that these behavioral changes occur alongside a predictive relationship that exists between these two behaviors over time.
Keywords: Adolescence, Anabolic/androgenic Steroids, Aggression, Anxiety
Introduction
The recreational use of anabolic/androgenic steroids (AAS) by adolescent teens has remained a concern for decades yet its use has risen in recent years worldwide (Harmer, 2010; NIDACapsules, 2007) despite strong evidence for negative acute and long-term physical, psychological and behavioral consequences. While the most common negative behavioral effect of AAS use is increased aggression in adult (Isacsson and Bergman, 1993; Kouri et al., 1995; Kreuz and Rose, 1972; Pope and Katz, 1994; Pope et al., 2000; Pope, 1988; Strauss, 1983; Strauss, 1987; Su et al., 1993) and youth populations alike (Archer, 1991; Beaver et al., 2008; Dabbs et al., 1987; Dabbs et al., 1991; Johnson et al., 1989; Johnson, 1990; Mattsson et al., 1980; Olweus et al., 1980; Olweus, 1987; Scerbo and Kolko, 1994; Schaal et al., 1996; Schalling, 1987), an increased incidence of anxiety-related disorders is being diagnosed in AAS users (Bahrke et al., 1990; Daly et al., 2003; Johnson, 1990; Pagonis et al., 2006a; Pagonis et al., 2006b; Pope and Katz, 1994; Pope, 1988), particularly during withdrawal from AAS use (Bahrke et al., 1990; Brower, 1992; Brower, 2002; Corrigan, 1996; Kashkin and Kleber, 1989; Lindqvist et al., 2007; Malone and Dimeff, 1992; Malone et al., 1995; Perry and Hughes, 1992; Perry et al., 1990; Pope et al., 1996; Su et al., 1993). Interestingly, AAS users in many clinical studies also present with marked increases in both aggression and anxiety (Hall et al., 2005; Pagonis et al., 2006a; Pope et al., 2000; Su et al., 1993), suggesting that AAS exposure may promote the development of both negative behavioral phenotypes simultaneously. Yet, while considerable preclinical study has investigated the link between AAS use and aggression (Lumia et al., 1994; McGinnis et al., 2002a; McGinnis et al., 2002b; McGinnis, 2004; Melloni and Ricci, 2010) or anxiety (Agis-Balboa et al., 2009; Aikey et al., 2002; Ambar and Chiavegatto, 2009; Barreto-Estrada et al., 2004; Bing et al., 1998; Bitran et al., 1993; Costine et al.; Fernandez-Guasti and Martinez-Mota, 2005; Koukoulas et al., 1999; Minkin et al., 1993; Ovsiukova et al., 2003; Parrilla-Carrero et al., 2009; Ricci et al., 2012; Rocha et al., 2007; Rojas-Ortiz et al., 2006), no preclinical studies have investigated the effects of AAS administration on the temporal relationship between the expression of the aggressive- and anxiety- related behavioral phenotypes.
Here we present the first set of preclinical studies that investigate the consequence of adolescent AAS exposure on the relationship between the expression of aggression and anxiety as they present during AAS exposure and withdrawal. We hypothesized that adolescent AAS exposure would produce behavioral alterations in aggression and anxiety during both the exposure and withdrawal time periods, and that the expression of one behavior would predict the expression of the other over time. More specifically, we hypothesized that adolescent AAS-treated animals would present with high levels of aggression and low levels of anxiety during AAS exposure that would predict low levels of aggression and high levels of anxiety in these same animals during AAS withdrawal. To address these hypotheses we first investigated whether adolescent AAS exposure altered anxiety-like responding immediately (i.e., during AAS exposure) or only during withdrawal from AAS as we previously observed (Ricci et al., 2012). Next, adolescent AAS-treated animals were tested for aggression and anxiety during AAS exposure, separated into quantitatively unique groups based on their aggression level during the exposure period, and then tested for both aggression and anxiety during AAS withdrawal. Data from these animals were analyzed using between- and within-subjects statistical procedures, along with simple linear regression analyses to evaluate the predictive relationship between aggression and anxiety during adolescent AAS exposure on aggression and anxiety during withdrawal from adolescent AAS exposure.
Methods
Animals
Intact pubertal male Syrian hamsters (Mesocricetus auratus) postnatal day 21 (P21) were obtained from Charles River Laboratories (Wilmington, MA), individually housed in polycarbonate cages, and maintained at ambient room temperature (22-24°C with 55% relative humidity) on a reverse light/dark cycle (12L:12D; lights off at 7:00). Food and water were provided ad libitum. For aggression testing, stimulus (intruder) males of equal size and weight to the experimental animals were obtained from Charles River one week prior to the behavioral test, group housed at 5 animals/cage in large polycarbonate cages, and maintained as above to acclimate to the animal facility. All intruders were evaluated and prescreened for low aggression (i.e., Disengage and Evade) and submission (i.e., Tail-up Freeze, Flee, and Fly-away) one day prior to the aggression test to control for behavioral differences between stimulus animals, as previously described in a number of our previous studies (Ferris et al., 1997; Melloni et al., 1997; Ricci et al., 2004; Ricci, 2005). Intruders displaying significantly low aggression and/or submissive postures were excluded from use in the behavioral assay. All experimental treatments and behavioral tests described below were administered during the first four hours of the dark cycle under dim-red illumination to control for circadian influences. All studies using live animals were approved by the Northeastern University Institutional Animal Care and Use Committee (NU-IACUC), and all methods used were consistent with guidelines provided by the National Institute of Health for the scientific treatment of animals.
Experimental Treatment
AAS Treatment
Postnatal day 27 (P27) male Syrian hamsters were weighted and received daily subcutaneous (SC) injections (0.1ml - 0.2ml) for 30 consecutive days (P27 - P56) of a mixture (or “stack”) of 3 commonly used AAS (Hall et al., 2005) in doses consistent with repeated, moderate use in humans as described (see (Melloni and Ricci, 2010) for a review). The AAS cocktail was composed of 2 mg/kg testosterone cypionate, 2 mg/kg nortestosterone and 1 mg/kg dihydrotestosterone undecyclate (Steraloids, Newport, RI) dissolved in sesame oil (SO). As control, a separate set of P27 male hamsters received daily subcutaneous (SC) injections (0.1ml - 0.2ml) for 30 consecutive days (P27 - P56) of SO (vehicle control).
Experimental Design
The day following the last AAS injection (P57), AAS-treated animals (n=45) and SO-treated controls (n=22) were randomly assigned to one of two counterbalanced groups and tested for anxiety-like responding using the Elevated Plus Maze (EPM) test and aggressive behavior using the Resident/Intruder (RI) test. At the completion of behavioral testing on P57, animals were placed back into their home cage and withdrawn from AAS (or SO) for 21 days (i.e., until P77) and then again tested for anxiety and aggression.
Following behavioral tests for aggression on P57, AAS-treated animals were re-coded according to their aggressive response as compared to SO-treated control animals and then separated into as a No/Low (NL), Species Normative (SN) or Excessive (E) aggressive responders using inclusion criteria described in the Statistical Analysis section. Animals meeting the criteria for each group of aggressive responders were analyzed using within subjects and linear regression analyses for offensive aggression and anxiety.
In a separate set of AAS-treated animals (n=30) aggression and anxiety tests were performed on P57 using the EPM/RI sequence as there were no notable effects of testing sequence in Experiment 1. At the completion of behavioral testing on P57, animals were withdrawn from AAS for 21 days (i.e., until P77) and then tested again for aggression and anxiety using the same sequence strategy. In this set of animals an array of ancillary behaviors, including social, comfort, and motor behaviors, were measured both during AAS exposure (i.e., on P57) and withdrawal (i.e., on P77) to control for nonspecific behavioral effects of adolescent AAS on behavioral responding at these two time points.
Behavior Testing
Aggression
Hamsters were tested for offensive aggression using the resident-intruder (RI) paradigm, a well-characterized and ethologically valid model of offensive aggression in Syrian hamsters (Floody and Pfaff, 1977; Lerwill and Makings, 1971). For this measure, a novel intruder of similar size and weight was introduced into the home cage of the experimental animal (resident) and the resident was scored for specific and targeted aggressive responses observed as lateral, flank-directed attacks as previously described (Grimes et al., 2003; Ricci et al., 2006). An attack was scored each time the resident animal would pursue and then either [1] lunge toward and/or [2] confine the intruder by upright and sideways threat; each generally followed by a direct attempt to bite the intruder's dorsal rump and/or flank target area(s). The latency to attack was defined as the period of time between the beginning of the behavioral test and the first attack the residents made toward an intruder. In the case of no attacks, latencies to attack were assigned the maximum latency (i.e., 600s). Each aggression test lasted for 10 minutes and was videotaped and scored manually by two observers unaware of the hamsters' experimental treatment. Inter-rater reliability was set at 95%. No intruder was used for more than one behavioral test, and all subjects were tested during the first 4 hours of the dark cycle under dim red illumination to control for circadian influences on behavioral responding.
Anxiety
Hamsters were tested for anxiety-related behavior using the elevated plus maze (EPM) test as in our previous study (Ricci et al., 2012). The EPM has been used extensively in rodents as a reliable test of anxiety-like responding, with particular use as a sensitive behavioral test to screen for anxiolytic drug effects (Pellow et al., 1985; Pellow and File, 1986). The apparatus consisted of two open arms and two closed arms (30 × 5 cm) elevated to a height of 38.5 cm and intersecting in a central platform (5 × 5 cm). The closed arms had black Plexiglas walls (15 cm high) covered with a black Plexiglas lid on the roof. The apparatus was arranged such that the open arms were opposite to each other. Animals were individually placed in the center of the apparatus facing one of the closed arms. The duration of time (sec) spent beyond a complete body length in the open arms was calculated for each animal over a 5-minute period. An increase in the duration of time spent in the open arms of the EPM was used as an index of anxiolytic behavior (Lister, 1987; Pellow et al., 1985). Each anxiety test was videotaped and coded by two observers unaware of experimental treatment. Animals were tested during the first four hours of the dark cycle under dim-red illumination to control for circadian influences in behavioral responding.
Social Interaction Test
The social interaction test consisted of the introduction of an experimental animal to a novel conspecific in a neutral arena. Social interest in conspecifics was determined by measuring physical contact time and social investigation. Contact time was defined as the period and duration of time during which the AAS-treated animal initiated and maintained contact with the conspecific, while social investigation was defined as the number of times the AAS-treated animal would pursue and initiate an olfactory investigation of the conspecific. Self-grooming was used as a measure of comfort behavior. The neutral arena consisted of a large (L 42cm; W 42cm; H 36cm) black opaque Plexiglas box with white tape on the floor of the box dividing the box into 4 separate quadrants. Animals (experimental and novel) were placed in opposite quadrants to start the beginning of the 10min test. Animals were tested during the first four hours of the dark cycle under dim-red illumination to control for circadian influences in behavioral responding.
Locomotion
The neutral arena apparatus was used separately from the social contact test for measures of locomotion. The apparatus consisted of a large (L 42cm; W 42cm; H 36cm) black opaque Plexiglas box with white tape on the floor of the box dividing the box into 4 equal, separate quadrants. Animals were placed individually in the apparatus and scored for open field matrix crossings (i.e., line crosses) and escape-attempts (i.e., wall climbing) throughout a 10min test period. A ‘line-cross’ was scored when the animal showed four paws over any given line thus belonging within a quadrant. Animals were tested during the first four hours of the dark cycle under dim-red illumination to control for circadian influences in behavioral responding.
Statistical Analyses
Behavioral results from aggression (i.e., attacks) and anxiety (i.e., duration of time spent within the open arms of the EPM) measures were compared between P57 and P77 using paired Student's t-test (two-tailed). Data from the different behavioral response groups (see below) were compared using one–way, analysis of variance (ANOVAs) followed by Fisher's protected least significance difference test post-hoc when appropriate.
Based on the directional hypothesis that aggression and anxiety are inversely related in adolescent AAS-treated animals, animals were divided into three distinct groups of AAS-induced aggressive responders depending on the aggression level displayed during adolescent AAS exposure (i.e., on P57) as compared to SO-treated control animals. Animals were assigned to an AAS “Responder” group, i.e., No/Low (NL), Species Normative (SN), and Excessive (E) Responders depending on where their aggression scores fell in comparison to SO-treated animals. The behavioral response of these groups were consistent with the distribution of aggression scores collected from adolescent AAS-treated animals in greater than two dozen studies performed over the span of the last five years (Carrillo et al., 2009; Carrillo et al., 2011; Grimes et al., 2007; Ricci et al., 2006; Schwartzer and Melloni, 2010). Species Normative responders were those animals in the current study whose level of attack fell within 1 standard deviation (SD) of the mean number of attacks (i.e., Mean = 17.5 +/- 3.7 SD). Animals described as No/Low responders were those animals falling 2 SDs below the distribution mean (i.e., 1.9 +/- 0.4), and Excessive responders as 2 SDs more than the distribution mean (i.e., 32 +/- 4). Analyses within each response group were compared between P57 (i.e., during adolescent AAS exposure) and P77 (i.e., during AAS withdrawal) using repeated measures, one-tailed Student's t tests. Data from NL, SN, and E responders were also analyzed separately using simple linear regression analyses to determine whether attack behavior was predictive of anxiety-like behavior between adolescent AAS exposure (i.e., at P57) and AAS withdrawal (i.e., on P77) for each group.
All behavioral ancillary behaviors were collapsed across No/Low, Normal, and Excessive AAS-induced aggressors in this study, as there were no differences between the groups for any behavior recorded when comparing P57 and P77 data. Thus all behaviors were analyzed using Student's paired t-tests.
The α level for all statistical analyses was set at 0.05.
Results
Aggression and Anxiety During Adolescent AAS Exposure and Withdrawal
Aggressive Behavior
As reported in a number of our prior studies (see (Melloni and Ricci, 2010) for review), adolescent AAS administration results in a significant increase in offensive aggression during the adolescent exposure period (i.e., on P57) compared to SO-treated control animals (t(44)=5.29, p<0.001) (Figure 1). During adolescent exposure, AAS-treated animals exhibited a greater than sixfold increase in number of attacks compared to SO-treated controls. Also, consistent with our previous data (Carrillo et al., 2011; Grimes and Melloni, 2006; Grimes et al., 2006; Ricci et al., 2012), behavioral data from the current study showed that the aggression-stimulating effects of adolescent AAS exposure were no longer present at 3 weeks of AAS withdrawal (i.e., on P77), i.e., a time at which AAS-treated animals were no longer aggressive and displayed a non-aggressive phenotype identical to SO-treated control animals (t(43)=1.98, p>0.1). Further, within treatment group comparisons revealed that AAS-treated animals displayed a significantly lower level of aggression during withdrawal from adolescent AAS exposure (i.e., on P77) as compared to that observed during the AAS exposure period (i.e., on P57) (t(37)=2.471, p<0.05) (Figure 1 upper inset). During withdrawal from adolescent AAS exposure (i.e., on P77), residents executed nearly 3-fold fewer attacks onto intruders placed in their home cage compared to animals tested during adolescent AAS exposure. Conversely however, no significant difference was observed in aggression between the exposure and withdrawal periods in SO-treated control animals. In this case, SO-treated animals directed a nearly identical number of attacks onto intruders (i.e., 2.6±0.1.3 attacks) during the withdrawal period (i.e., on P77) as compared to the exposure period (i.e., 2.67±0.97 attacks on P57) (t(16)=0.076, p>0.1).
Figure 1.
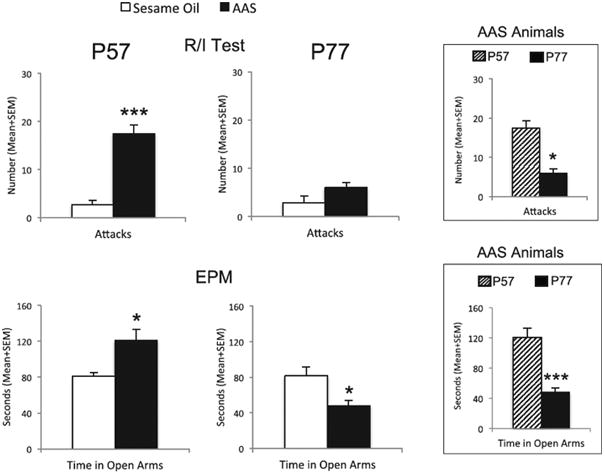
Adolescent AAS exposure alters aggression and anxiety during adolescent AAS exposure and withdrawal. Top Graphs. The number of attacks of control- (SO; white bars) and AAS- (black bars) treated hamsters in a resident/intruder (R/I) test during adolescent exposure (i.e., on P57) and withdrawal (i.e., on P77). Top Inset. The number of attacks of AAS-treated hamsters during adolescent AAS exposure (i.e., on P57, striped bars) and withdrawal (i.e., on P77, black bars). Bottom Graphs. The duration of time control- (SO; white bars) and AAS- (black bars) treated hamsters spent in the open arm of the elevated plus maze (EPM) during adolescent exposure (i.e., on P57) and withdrawal (i.e., on P77). Bottom Inset. The duration of time spent in the open arm of the EPM of AAS-treated hamsters during adolescent AAS exposure (i.e., on P57, striped bars) and withdrawal (i.e., on P77, black bars). Bars denote SEM; *p<0.05, **p<0.01, ***p<0.001,
Anxiety
Adolescent AAS treated-animals displayed a significantly lower level of anxiety-like behavior during adolescent AAS exposure (i.e., on P57) compared to SO-treated control animals as measured by the duration of time spent in the open arms of the EPM (t(40)=2.22, p<0.05) (Figure 1). Indeed, during adolescent AAS exposure, AAS-treated animals spent significantly more time (>30% more time) in the open arms of the EPM compared to SO-treated control animals. However, behavioral data from the current study showed that the anxiolytic effects of adolescent AAS exposure were no longer present at 3 weeks of AAS withdrawal (i.e., on P77). Rather, consistent with our recent findings (Ricci et al., 2012), during withdrawal from adolescent AAS administration (i.e., on P77), AAS-treated animals displayed a higher level of anxiety-like responding in the EPM compared to SO-treated controls. Specifically, during withdrawal from adolescent AAS exposure, AAS-treated animals spent significantly less time (<20% less time) in the open arms of the EPM compared to SO-treated control animals (t(45)=2.05, p<0.05) (Figure 1). Within treatment group comparisons revealed that AAS treated-animals displayed a significantly higher level of anxiety-like behavior in the EPM test during withdrawal from adolescent AAS exposure (i.e., on P77) as compared to that observed during AAS exposure (i.e., on P57) (Figure 1 lower inset). During withdrawal from adolescent AAS exposure, animals spent significantly less time in the open arms of the EPM compared to the duration of time spent in the open arms of the EPM during the AAS exposure period (t(41)=4.65, p<0.001). During AAS withdrawal, animals spent more than 50% of the time in the open arms of the EPM compared to animals tested during adolescent AAS exposure. Conversely however, no significant difference was observed in anxiety-like behavior between the exposure and withdrawal periods in SO-treated control animals. In this case, SO-treated animals spent an average of 81.52±9.8 seconds in the open arms of the EPM during the withdrawal period (i.e., on P77) as compared to 80.79±4.3 seconds in the open arms of the EPM during the exposure period (i.e., on P57) (t(19)=0.081, p>0.1).
Adolescent AAS-Induced Aggression: Quantitative Separation of Responders
Based on our hypothesis that aggression and anxiety are inversely related in adolescent AAS-treated animals, animals were divided into adolescent AAS-induced No/Low (NL), Species Normative (SN) and Excessive (E) Responder groups as defined by their aggression level displayed during the adolescent AAS exposure period (i.e., on P57) as compared to SO-treated littermates.
No/Low Aggressive Responders
Adolescent AAS-treated animals were characterized as No/Low (NL) responders display very little, if any, aggression during adolescent exposure (i.e., on P57) compared to SO-treated control animals, displaying a non-aggressive phenotype identical to SO-treated animals. On average NL responders executed fewer than 2 attacks on an opponent during the RI test. When the number of attacks in NL responders was compared between AAS- and SO- treatment groups, there was no significant difference in the number of attacks directed towards intruders between groups (t(28)=0.71, p>0.1) (Figure 2).
Figure 2.
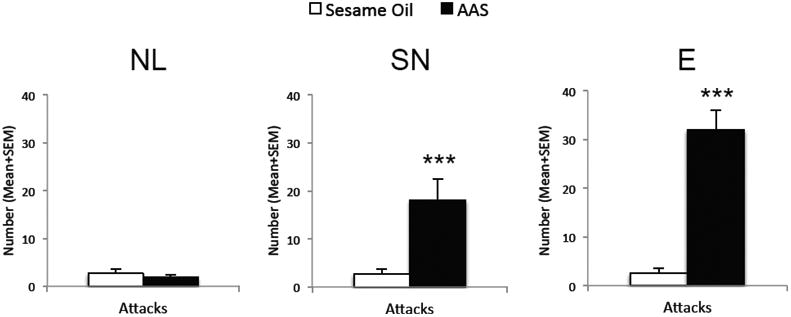
Adolescent AAS-induced No/Low (NL), Species Normative (SN) and Excessive (E) Responders as defined by the aggression level displayed during adolescent AAS exposure. The number of attacks of control- (SO; white bars) and AAS- (black bars) treated hamsters in a resident/intruder test during adolescent exposure (i.e., on P57). Bars denote SEM; ***p<0.001,
Species Normative Aggressive Responders
As observed in a number of our previous studies (see (Melloni and Ricci, 2010) for a comprehensive list of studies), Species Normative (SN) responders display a high level of offensive aggression during the AAS exposure period (i.e., on P57) compared to SO-treated littermates. On average SN responders direct in excess of 18 attacks on an opponent during the RI test. When the number of attacks in SN responders was compared between AAS- and SO- treatment groups, SN responders showed a significant increase in the number of attacks directed onto intruders (t(33)=6.25, p<0.001) (Figure 2).
Excessive Aggressive Responders
As observed in a very few of our AAS-treated animals (n=5), Excessive (E) responders display an exceedingly high level of offensive aggression during the adolescent AAS exposure period (i.e., on P57) compared to SO-treated controls. On average E responders execute in excess of 32 attacks on an opponent during the aggression test. As seen with the SN responders, the high number of attacks on intruders executed by E responders during AAS exposure was significantly greater than the number of attacks directed onto intruders by SO-treated control animals (t(18)=10.83, p<0.001) (Figure 2).
Aggression and Anxiety: A Comparative Assessment of Behavioral Response Patterns Across Adolescent AAS Exposure and Withdrawal
Based on our hypothesis that aggression and anxiety are inversely related in adolescent AAS-treated animals, animals were divided into adolescent AAS-induced No/Low (NL), Species Normative (SN) and Excessive (E) Responders (as defined by the aggression level displayed during adolescent AAS exposure on P57) and compared for their aggressive and anxiety-like responses during AAS exposure (i.e., on P57) and during AAS withdrawal (i.e., on P77).
No/Low Responders
NL responders display very little, if any, aggression during the adolescent AAS exposure period (i.e., on P57), on average executing less than 2 attacks on an opponent during the RI test. When the number of attacks in NL responders was compared across adolescent AAS exposure and AAS withdrawal (i.e., on P77), there was a significant increase in the number of attacks executed on intruders during the AAS withdrawal period (t(19)=3.38, p<0.01) (Figure 3), indicating that AAS-treated NL responders are significantly more aggressive during AAS withdrawal. On average the NL groups' attack frequency increased nearly five fold during withdrawal from AAS. In terms of anxiety-like behavior, as evidenced by the duration of time spent in the open arms of the EPM, NL responders show a trend towards a decrease in anxiety during AAS withdrawal compared to during the adolescent AAS exposure period (t(13)=1.9, p=0.067) (Figure 3). Here, NL responders spent more time (i.e., ∼50%) more time in the open arms of the EPM during AAS withdrawal compared to the exposure period, indicating that AAS-treated NL responders are slightly less anxious during AAS withdrawal. Interestingly, during adolescent AAS exposure (i.e., on P57) NL responders very spent little time (i.e., approximately 20% of the test period on average) in the open arms of the EPM.
Figure 3.
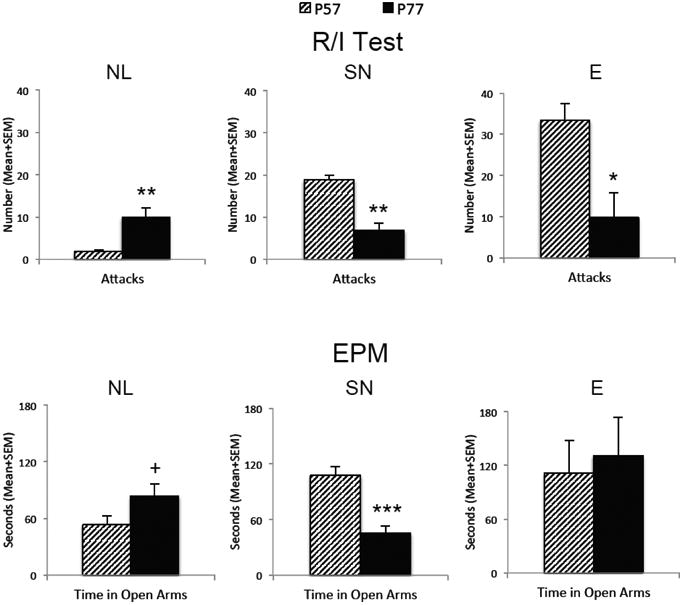
Adolescent AAS exposure differentially alters aggression and anxiety during AAS exposure (i.e., at P57, striped bars) and withdrawal (i.e., at P77, black bars) across three subpopulations of aggressive responders. The number of attacks in a resident/intruder test (R/I Test; top graphs) and the duration of time spent in the open arm of the elevated plus maze (EPM; bottom graphs) were measured for animals categorized as No/Low (NL; left), Species Normative (SN; middle), or Excessive (E; right) Responders. Bars denote SEM; *p<0.05, **p<0.01, ***p<0.001, +p=0.067.
Species Normative Responders
As in a number of our previous studies (see (Melloni and Ricci, 2010) for review), SN responders display a high level of offensive aggression during the AAS exposure period (i.e., on P57), on average executing approximately 18 attacks on an opponent during the RI test. Similarly, as we have described previously (Carrillo et al., 2011; Grimes and Melloni, 2006; Grimes et al., 2006), SN responders direct a significantly decreased number of attacks on intruders during AAS withdrawal (i.e., on P77) as compared to during the adolescent AAS exposure period (t(19)=3.38, p<0.01) (Figure 3), indicating that AAS-treated SN responders are significantly less aggressive during AAS withdrawal. On average, the aggressive response of SN responders to adolescent AAS exposure decreased by more than half over the course of AAS withdrawal. In terms of anxiety-like behavior, as evidenced by the duration of time spent in the open arms of the EPM, SN responders display little anxiety-like responding when tested during the adolescent AAS exposure period, on average spending nearly half of the test period in the open arms of the EPM test (Figure 3). However, when tested again during AAS withdrawal, an increase in anxiety-like responding emerges in SN responders (Figure 3). Here, significant differences in the duration of time spent in the open arms of the EPM emerge between the adolescent AAS exposure and withdrawal periods such that SN responders spent significantly less time in the open arms during AAS withdrawal (i.e., nearly 40% less) as they did during AAS exposure (t(17)=5.13, p<0.001) (Figure 3), indicating that AAS-treated SN responders are significantly more anxious during AAS withdrawal. In this instance, SN responders spent very little time (i.e., only about 18% of the test period) in the open arms of the EPM during AAS withdrawal.
Excessive Responders
As observed in few of our AAS-treated animals (n=5), E responders display an exceedingly high level of offensive aggression during the AAS exposure period (i.e., on P57), on average executing in excess of 30 attacks on an opponent during the aggression test. However, as seen with the SN responders, the high number of attacks on intruders executed by E responders during AAS exposure drops significantly during AAS withdrawal (i.e., P77) t(4)=3.03, p<0.05) (Figure 3), indicating that AAS-treated E responders are significantly less aggressive during AAS withdrawal. Indeed, E responders execute 2 fold fewer attacks on opponents during AAS withdrawal than during the adolescent AAS exposure period. In terms of anxiety-like behavior, as evidenced by the duration of time spent in the open arms of the EPM, E responders failed to show a significant difference in anxiety-like responding during AAS exposure compared to during AAS withdrawal (t(4)=0.62, p>0.1) (Figure 3). Here, both during AAS exposure and withdrawal, E responders spent a large amount of time (i.e., >40% of the test period on average) in the open arms of the EPM.
Between Responder Group Comparisons
During adolescent AAS exposure (i.e., on P57), animals across NL, SN, and E responder groups show significant increases in offensive aggression (F(2,37)=86.47, p<0.0001) concomitant with significant decreases in anxiety-like behavior (F(2,34)=5.62, p<0.01). A priori planned comparisons between groups that responded reliably to adolescent AAS treatment revealed a significant increase in offensive aggression (t(33)=7.89, p<0.0001) and significant decrease in anxiety (t(30)=3.59, p<0.01), i.e., specifically between the NL and SN responder groups. For instance, during adolescent AAS exposure (i.e., on P57), SN responders directed a nearly 10 fold increase in the number of attacks onto opponents in the RI test and spent greater than twice as much time in the open arms of the EPM as compared to NL counterparts. During AAS withdrawal however, (i.e., on P77), while there were no significant differences in aggression identified between NL, SN and E responder groups (F(2,37)=1.31, p>0.1), there were significant increases in anxiety (F(2,34)=5.52, p<0.01) observed between groups. In particular, as above, a priori planned comparisons revealed a significant increase in anxiety (t(30)=2.14, p<0.05) specifically between the NL and SN responder groups. For instance, during AAS withdrawal (i.e., on P77), SN responders spent less than half the time in the open arms of the EPM as compared to NL counterparts.
Linear Regression Analyses
Figure 4 summarizes the results of the simple linear regression analyses for the differences of aggression and anxiety scores during adolescent AAS exposure (i.e., P57) and withdrawal (i.e., P77) for each responder distribution.
Figure 4.
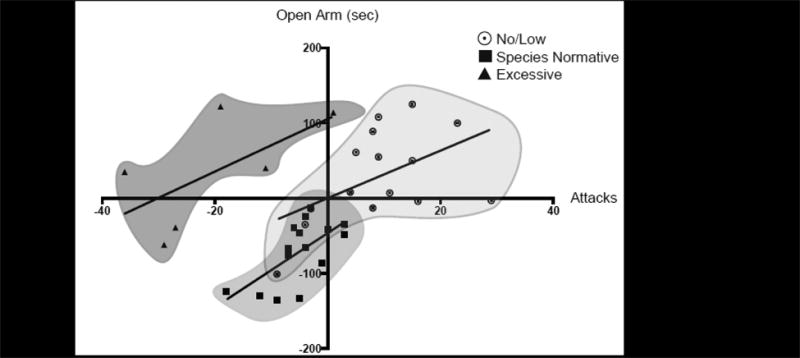
Linear correlation plots for the simple linear regression analyses of each individual subpopulation of AAS-induced aggressive responders for the difference (i.e., P77 minus P57) in the number of attacks and the difference (i.e., P77 minus P57) in time spent in the open arms of the elevated plus maze during adolescent AAS exposure (i.e., at P57) and AAS withdrawal (i.e., at P77). ¤ = No/Low Responders, o = Species Normative Responders, and ▲ = Excessive Responders.
NL responders primarily fell into the upper right quadrant of the graph (Quadrant 1) and thus were characterized as having fewer attacks in the RI test during adolescent AAS exposure (i.e., on P57) than during AAS withdrawal (i.e., on P77), while also spending less time in the open arms of the EPM during adolescent AAS exposure than during AAS withdrawal. The amount of difference in aggression between P57 and P77 for these animals was found to be a significant predictor of change in anxiety-like responding (β=3.16, p=0.05), where in all probability it would seem that as the level of aggression becomes greater from P57 to P77, so does anxiety-like behavior.
Data for animals designated as SN responders primarily fell in the lower left quadrant of the graph (Quadrant 3) of Figure 4 and thus were more likely than the other two groups of responders to execute more attacks and spend more time in the open arms of the EPM during adolescent AAS exposure than during AAS withdrawal. Similar to the NL responders, the difference in aggression of SN animals between P57 and P77 was a significant predictor of change in anxiety-like responding (β=4.93, p<0.01), where, as the level of aggression decreases from P57 to P77, the level of anxiety increases. For both the NL and SN animals, aggression change was found to explain a significant portion of the variance in EPM duration difference between AAS exposure and AAS withdrawal (NL: R2=0.26; SN: R2=0.41, p≤0.05 each).
Aggression and anxiety difference measures for E responders are present in the upper left portion of the graph (Quadrant 2) in Figure 4, indicating that these animals exhibited high levels of aggression along with high levels of anxiety-like responding during the adolescent AAS exposure period, but had lower aggression scores and longer durations in the open arms of the EPM during AAS withdrawal. However, the regression analysis for behavior testing differences in E responders failed to reveal a predictive relationship between the difference scores for the two behavioral tests across days (β=3.50, p>0.1).
To further examine the predictive relationship of aggression and anxiety-like behavior between adolescent AAS exposure and AAS withdrawal, we pooled the behavioral data for each day for both NL and SN responders. A linear regression analysis revealed that the difference in aggressive responding from P57 to P77 was a significant predictor of differences in anxiety for both days of testing (β=3.04, p<0.01), where, the decrease in aggressive attacks from P57 to P77 occurs alongside an increase in anxiety-like responding within these same animals from P57 to P77 (Figure 5). Accordingly, aggression difference accounts for a significant portion of the variability observed in anxiety difference between testing days (i.e., P77-P57; R2=0.23, p<0.01).
Figure 5.
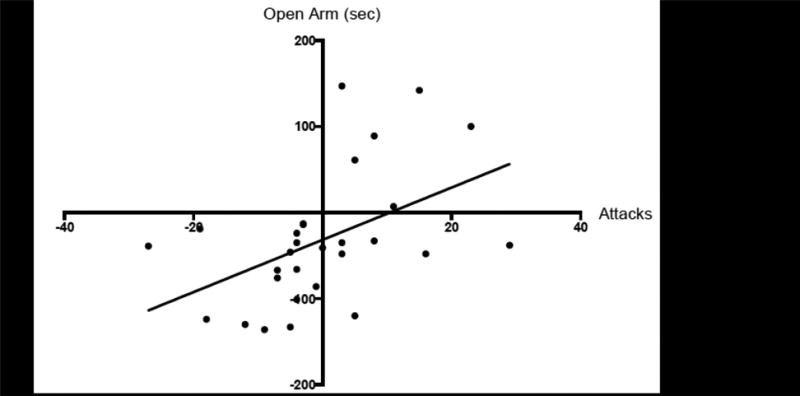
Linear correlation plot for the simple linear regression analysis of pooled data for No/Low and Species Normative Responders for the difference (i.e., P77 minus P57) in the number of attacks and the difference (i.e., P77 minus P57) in time spent in the open arms of the elevated plus maze during adolescent AAS exposure (i.e., at P57) and AAS withdrawal (i.e., at P77).
Ancillary Behaviors During Adolescent AAS Exposure and Withdrawal
There were no significant differences observed in measures of social, comfort, or motor behaviors scored during adolescent AAS exposure (i.e., on P57) and during AAS withdrawal (i.e., on P77): p>0.05 for all behaviors analyzed (Table 1). Briefly, there were no differences in the amount of time AAS-treated animals spent in contact with conspecifics either in the open arena or during an aggressive encounter between adolescent AAS exposure and AAS withdrawal groups (t(32)=0.95), nor were there differences in olfactory investigation (t(32)=0.57). There were also no differences in the frequency of grooming, line crosses or cage climbing behaviors displayed during adolescent AAS exposure and during AAS withdrawal (t(32)=0.58, 0.85, and 0.46 respectively).
Table 1.
Effects of AAS during the adolescent AAS exposure period (i.e., at P57) and AAS withdrawal (i.e., at P77) on general measures of behavioral activation.
| Behavior | Testing Period | |||
|---|---|---|---|---|
|
| ||||
| P57 | P77 | t-value | Probability | |
|
|
||||
| Social Behavior (±SD) | ||||
| Contact Time (s) | 384.1±124.8 | 387.8±96.1 | t(32)=0.95 | > 0.05 |
| Olfactory Investigation | 1.6±1.4 | 1.4±1.2 | t(32)=0.57 | > 0.05 |
| Comfort Behavior (±SD) | ||||
| Self Grooming | 2.2±1.1 | 2.4±1.6 | t(32)=0.58 | > 0.05 |
| Locomotion Behavior (±SD) | ||||
| Line Crosses | 7.1±4.1 | 6.9±3.5 | t(32)=0.85 | > 0.05 |
| Wall Climbing | 1.7±1.3 | 1.5±1.1 | t(32)=0.46 | > 0.05 |
Discussion
The range and extent of aggression and anxiety have been shown to correlate with the severity of AAS abuse in human populations, with longer abuse times correlating with greater levels of anxiety (Pagonis et al., 2006a; Pagonis et al., 2006b). Yet, while a considerable number of clinical and preclinical studies have investigated the link between AAS use and aggression or anxiety, no studies have investigated the relationship between AAS use and aggression and anxiety. And, importantly, those studies investigating the relationship between AAS use and aggression or anxiety have produced mixed results that vary with the animal species, AAS, dose, duration, and the experimental paradigm used. For example, in laboratory studies using adult and adolescent male rats and hamsters, chronic AAS exposure has consistently been shown to produce elevated levels of aggressive responding (Lumia et al., 1994; McGinnis et al., 2002a; McGinnis et al., 2002b; McGinnis, 2004; Melloni and Ricci, 2010). By comparison, both anxiolytic-like (Aikey et al., 2002; Bing et al., 1998; Bitran et al., 1993; Fernandez-Guasti and Martinez-Mota, 2005; Koukoulas et al., 1999; Ovsiukova et al., 2003) and anxiogenic-like (Agis-Balboa et al., 2009; Ambar and Chiavegatto, 2009; Minkin et al., 1993; Parrilla-Carrero et al., 2009; Rocha et al., 2007; Rojas-Ortiz et al., 2006) actions of AAS have been observed following acute and chronic exposure to AAS in adult male rats and mice. Similarly, in adolescent female mice, repeated exposure to AAS significantly increases anxiety-like responding (Barreto-Estrada et al., 2004; Costine et al.). Together, these data indicate that adolescent and adult AAS exposure markedly increases both aggression and anxiety in preclinical animal models. While informative, these studies fail to provide definitive answers regarding the influence of AAS exposure on the relationship between aggression and anxiety.
Here we present the first set of preclinical studies that investigate the consequence of adolescent AAS exposure on the relationship between aggression and anxiety. In these studies pubertal male hamsters were used as an adolescent animal model to investigate the consequences of adolescent AAS exposure on the expression of aggression and anxiety during adolescent AAS exposure and withdrawal. In these studies, we found that adolescent AAS administration had potent aggression-stimulating effects during the adolescent exposure period that diminished during AAS withdrawal (Carrillo et al., 2011; Grimes and Melloni, 2006; Grimes et al., 2006) - a time when adolescent AAS-treated animals present with a significant increase in anxiety (Ricci et al., 2012). Given the findings that adolescent AAS exposure promotes elevated levels of anxiety at a time when AAS-induced increases in aggression subside, we hypothesized that adolescent AAS exposure would produce behavioral alterations along a continuum in both aggression and anxiety, and that the expression of one behavior would predict the expression of the other over time. In accord with this hypothesis, we found in our first set of studies that the immediate behavioral effects of adolescent exposure to AAS (i.e., those present during adolescent AAS exposure) are largely anxiolytic and exist at a time when increased aggressive behavior is present. Similarly we also found that the prolonged effects of adolescent AAS exposure (i.e., those present during AAS withdrawal) are largely anxiogenic and exist at a time when the aggressive phenotype has diminished to non-aggressive, control levels. These findings are important and may explain, at least in part, why adolescent AAS-treated animals no longer respond aggressively during AAS withdrawal (Carrillo et al., 2011; Grimes and Melloni, 2006; Grimes et al., 2006); i.e., they become anxious – supporting the notion that aggression and anxiety are linked. Indeed, there is support for an intimate behavioral relationship between anxiety and aggression in humans (Apter et al., 1990; Fehon et al., 2001; Guillot and Chapouthier, 1996; Veenema and Neumann, 2007; Veenema et al., 2007) and in animals (Apter et al., 1990; Fehon et al., 2001; Guillot and Chapouthier, 1996; Veenema and Neumann, 2007; Veenema et al., 2007). For instance, in mice selectively bred for High (HAB) and Low (LAB) Anxiety Behavior, aggression and anxiety are negatively correlated, such that LAB animals reliably display higher levels of aggressive behavior compared to HAB animals (Veenema et al., 2007). Similarly, in recent studies we showed that during withdrawal from adolescent AAS exposure, hamsters display heightened levels of anxiety in the notable absence of aggression (Ricci et al., 2012), supporting the notion that aggression and anxiety are negatively correlated. In contrast however, mice selected for short attack latencies exhibit higher levels of anxiety compared to those selected for longer attack latencies (Hogg et al., 2000). Similarly, highly aggressive animals from different inbred mice strains, also display heightened levels of anxiety (Apter et al., 1990; Fehon et al., 2001; Guillot and Chapouthier, 1996; Veenema and Neumann, 2007; Veenema et al., 2007). While there is little doubt that a behavioral relationship between aggression and anxiety exists, there remains a paucity of preclinical research investigating the developmental nature of this interaction, particularly as it pertains to AAS abuse, as it exists as a precipitating factor in the generation of these two behaviors. Thus, in this study we questioned whether there was a functional and/or predictive relationship between the display of these two AAS-induced behavioral phenotypes across AAS exposure and withdrawal.
We found that the relationship between aggression and anxiety during AAS exposure and withdrawal could be largely explained by the aggressive response profile produced during the adolescent AAS exposure period. When animals were separated into No/Low (NL), Species Normative (SN) and Excessive (E) response groups based upon their level of aggression during adolescent AAS exposure, an inverse behavioral relationship between aggression and anxiety during AAS exposure and withdrawal was found, particularly in the SN and NL responder groups. For instance, during adolescent AAS exposure, SN animals respond aggressively to intruders (nearly 20 attacks) but display no appreciable anxiety (∼120 seconds in the open arms of the EPM), while during AAS withdrawal these same animals display less aggression towards intruders (∼6 attacks) in the presence of higher levels of anxiety (∼45 seconds in the open arms of the EPM). So, during adolescent AAS exposure, SN animals are aggressive but not anxious, but then during AAS withdrawal these animals become anxious and not aggressive. Conversely, during adolescent AAS exposure, NL animals respond with no noticeable levels of aggression (<2 attacks) but do so in the presence of relatively high levels anxiety (<50 seconds in the open arms of the EPM), while during AAS withdrawal these same animals respond more aggressively to intruders (10 attacks) in the presence of less anxiety (∼80 seconds in the open arms of the EPM). So, during adolescent AAS exposure, NL animals are anxious but not aggressive, but then during AAS withdrawal these same animals become more aggressive and less anxious. Together, these data suggest that, with the exception of the E responders, adolescent exposure to moderate doses of AAS affects both anxiety and aggression simultaneously and in a direct inverse manner over time.
The data from linear regression modeling supports the existence of multiple populations that are differentially responsive to AAS regarding the display of anxiety and aggression. Data for SN and NL responder populations suggest that aggression and anxiety share a predictive relationship such that behavioral changes that occur between AAS exposure and withdrawal for one behavior predict the magnitude of change in the other. Combined with the within-subject data, these models also suggest that the direction of the behavioral changes that occur between exposure and withdrawal are predictable based on the initial behavioral state (i.e., the ‘level’ of anxiety or aggression) during the exposure period. Additionally, our data suggest that as the difference in one behavior (i.e., aggression or anxiety) during exposure and withdrawal becomes more prominent, the change in the other behavior becomes less prominent supporting a role for a modulatory (or feedback) mechanism implicated in the expression of aggression and anxiety. For instance, in this study the relationship between the measures of change in aggression and anxiety for NL responders was found to be predictive where it can be assumed that low aggression during exposure ostensibly becomes dis-inhibited during AAS withdrawal – leading to an increase in aggressive responding. Similarly, for anxiety, the level of anxiety-like behavior becomes slightly greater during AAS withdrawal. In terms of aggression, the change in aggressive behavior was found to predict the change in anxiety, suggesting that behaviorally, in NL responders, the degree to which aggression is suppressed during adolescent AAS exposure can be used to estimate how anxious an animal will be during the AAS withdrawal phase. The same type of predictive relationship was present in SN responders, except, in their case, the degree to which aggression was heightened during AAS exposure provided predictive information related to the amount of anxiety expressed during withdrawal. Simply stated, the increase in aggression observed during adolescent AAS exposure can likely be used to predict increased anxiety-like responding during AAS withdrawal. Interestingly, the predictive relationship between aggression and anxiety changes when the data for SN and NL responders are combined, producing a predictive relationship that more closely resembles the SN responders. In this case, linear regression analysis revealed that aggression difference observed between adolescent AAS exposure and AAS withdrawal was a significant predictor of differences in anxiety for both days of testing, such that the increase in aggression observed during the adolescent AAS exposure period can likely be used to predict the increase in anxiety-like behavior during AAS withdrawal. Together these data provide important information regarding the predictive relationship between aggression and anxiety in adolescent AAS treated animals across the term of their use, showing an inverse behavioral relationship between aggression and anxiety during adolescent AAS exposure and withdrawal.
The similarity between SN and NL responders in terms of their predictive relationship may suggest some level of shared neural circuitry mediating the aggressive and anxious responses brought on by adolescent AAS exposure and during AAS withdrawal, where alterations in behavior may be a function of the expression or release of neurochemical signal(s) involved in both behavioral responses. There is support for a complex neurobiological relationship between aggressive behavior and anxiety. For instance, close examination of the neural circuits regulating aggressive behavior and anxiety reveal overlapping neural loci, supporting the notion that aggression and anxiety share a common neuroanatomy (Delville et al., 2000; Ernst and Fudge, 2009; Ferris, 2000; Newman et al., 1999; Pratt, 1992). In particular, the anterior hypothalamus (AH), central amygdala (CeA), frontal cortex (FC), lateral septum (LS), and medial amygdala (MeA) appear to be sites of overlap for neural inputs that influence both aggressive behavior (Delville et al., 2000; Nelson and Trainor, 2007) and anxiety (Pratt, 1992). Prior studies have shown altered anxiety-like behavior in response to LS kindling in rats (Thomas and Gunton, 2011) as well as alterations in neurosteroid concentrations within the FC of mice (Maayan et al., 2006). Further, in recent studies we showed that the heightened level of anxiety observed during AAS withdrawal was correlated with a significant decrease in serotonin (5HT) afferent innervation to several of these brain sites in hamsters, most notably the AH, CeA, and MeA, but not the LS or FC (Ricci et al., 2012). Along these lines, in the current study we showed that adolescent hamsters exposed to AAS displayed significantly more aggression and were significantly less anxious during the AAS exposure period (i.e., on P57) compared to AAS withdrawal (i.e., on P77). The timeframe over which these behavioral changes take place mirror the timeframe over which AAS-mediated alterations in neural circuitry and neurochemistry occur in select brain regions (e.g., AH, MeA, CeA) and neural systems (e.g., 5HT) associated with aggression and anxiety (Carrillo et al., 2011; Grimes and Melloni, 2006; Grimes et al., 2006), suggesting that perhaps the functioning of the 5HT neural system within these brain sites may modulate the behavioral change from aggression during adolescent AAS exposure to anxiety during AAS withdrawal observed in the current study. Indeed, 5HT has been shown to modulate both anxiety and aggression (Bouwknecht et al., 2007; Cheeta et al., 2001; Costall and Naylor, 1992; Hammack et al., 2009; Higgins et al., 1991; Ishiwata et al., 2005; Levita et al., 2004; Lowry et al., 2008; Morrison et al., 2013; Sakaue et al., 2003; van der Vegt et al., 2003), and in recent studies we have shown that the aggression-eliciting effects of AAS exposure during adolescent development are modulated, in part, by 5HT neural signaling (Grimes and Melloni, 2002; Grimes and Melloni, 2005; Ricci et al., 2006), as are the anxiogenic effects that appear during AAS withdrawal (Ricci et al., 2012). These data support the notion of a specific “gating” mechanism (perhaps modulated by 5HT) whereby the expression of the “aggressive” brain state can permit or preclude the expression of the “anxious” brain state and vise versa, an investigation currently ongoing in our laboratory.
In summary, these studies provide data that identify a predictive, inverse behavioral relationship between adolescent AAS-induced alterations in aggression and anxiety between adolescent AAS exposure and withdrawal. Specifically, these data show that increases in aggressive behavior observed during adolescent AAS exposure are followed by increases in anxiety during AAS-withdrawal, and that these behavioral changes occur alongside a predictive relationship between aggression and anxiety that takes place between AAS exposure and withdrawal. Together, these data provide important information regarding the behavioral relationship between aggression and anxiety in adolescent AAS treated animals during AAS exposure and withdrawal, showing an inverse behavioral relationship between these two behaviors over time.
Highlights.
Anabolic steroids elicit aggression during exposure and anxiety during withdrawal.
Difference in aggression from exposure to withdrawal predict difference in anxiety
Decrease in aggression from exposure to withdrawal predicts increase in anxiety.
Acknowledgments
This work was supported by research grant (R01) DA10547 from the National Institutes on Drug Abuse (NIDA) to R.H.M. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agis-Balboa RC, Pibiri F, Nelson M, Pinna G. Enhanced fear responses in mice treated with anabolic androgenic steroids. Neuroreport. 2009;20:617–21. doi: 10.1097/WNR.0b013e32832a2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42:448–60. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- Ambar G, Chiavegatto S. Anabolic-androgenic steroid treatment induces behavioral disinhibition and downregulation of serotonin receptor messenger RNA in the prefrontal cortex and amygdala of male mice. Genes Brain Behav. 2009;8:161–73. doi: 10.1111/j.1601-183X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- Apter A, van Praag HM, Plutchik R, Sevy S, Korn M, Brown SL. Interrelationships among anxiety, aggression, impulsivity, and mood: a serotonergically linked cluster? Psychiatry Res. 1990;32:191–9. doi: 10.1016/0165-1781(90)90086-k. [DOI] [PubMed] [Google Scholar]
- Archer J. The influence of testosterone on human aggression. Br J Psychol. 1991;82:1–28. doi: 10.1111/j.2044-8295.1991.tb02379.x. [DOI] [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE, 3rd, Wright JE. Psychological and behavioural effects of endogenous testosterone levels and anabolic-androgenic steroids among males. A review Sports Med. 1990;10:303–37. doi: 10.2165/00007256-199010050-00003. [DOI] [PubMed] [Google Scholar]
- Barreto-Estrada JL, Barreto J, Fortis-Santiago Y, Rivera-Ramos I, Fortis-Santiago A, Jorge JC. Modulation of affect after chronic exposure to the anabolic steroid 17alpha-methyltestosterone in adult mice. Behav Neurosci. 2004;118:1071–9. doi: 10.1037/0735-7044.118.5.1071. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Vaughn MG, Delisi M, Wright JP. Anabolic-androgenic steroid use and involvement in violent behavior in a nationally representative sample of young adult males in the United States. Am J Public Health. 2008;98:2185–7. doi: 10.2105/AJPH.2008.137018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing O, Heilig M, Kakoulidis P, Sundblad C, Wiklund L, Eriksson E. High doses of testosterone increase anticonflict behaviour in rat. Eur Neuropsychopharmacol. 1998;8:321–3. doi: 10.1016/s0924-977x(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav. 1993;27:568–83. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res Bull. 2007;72:32–43. doi: 10.1016/j.brainresbull.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroids; addictive, psychiatric and medical consequences. Am J Addic. 1992;1:100–14. [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence. Curr Psychiatry Rep. 2002;4:377–87. doi: 10.1007/s11920-002-0086-6. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Melloni RH., Jr Adolescent anabolic androgenic steroids reorganize the glutamatergic neural circuitry in the hypothalamus. Brain Res. 2009;1249:118–27. doi: 10.1016/j.brainres.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Melloni RH. Developmental and withdrawal effects of adolescent AAS exposure on the glutamatergic system in hamsters. Behav Neurosci. 2011;125:452–64. doi: 10.1037/a0023475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeta S, Irvine EE, Kenny PJ, File SE. The dorsal raphe nucleus is a crucial structure mediating nicotine's anxiolytic effects and the development of tolerance and withdrawal responses. Psychopharmacology (Berl) 2001;155:78–85. doi: 10.1007/s002130100681. [DOI] [PubMed] [Google Scholar]
- Corrigan B. Anabolic steroids and the mind. Medical J of Australia. 1996;165:222–26. doi: 10.5694/j.1326-5377.1996.tb124932.x. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. Anxiolytic potential of 5-HT3 receptor antagonists. Pharmacol Toxicol. 1992;70:157–62. doi: 10.1111/j.1600-0773.1992.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Costine BA, Oberlander JG, Davis MC, Penatti CA, Porter DM, Leaton RN, Henderson LP. Chronic anabolic androgenic steroid exposure alters corticotropin releasing factor expression and anxiety-like behaviors in the female mouse. Psychoneuroendocrinology. 35:1473–85. doi: 10.1016/j.psyneuen.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs JM, Jr, Frady RL, Carr TS, Besch NF. Saliva testosterone and criminal violence in young adult prison inmates. Psychosom Med. 1987;49:174–82. doi: 10.1097/00006842-198703000-00007. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Jr, Jurkovic GJ, Frady RL. Salivary testosterone and cortisol among late adolescent male offenders. J Abnorm Child Psychol. 1991;19:469–78. doi: 10.1007/BF00919089. [DOI] [PubMed] [Google Scholar]
- Daly RC, Su TP, Schmidt PJ, Pagliaro M, Pickar D, Rubinow DR. Neuroendocrine and behavioral effects of high-dose anabolic steroid administration in male normal volunteers. Psychoneuroendocrinology. 2003;28:317–31. doi: 10.1016/s0306-4530(02)00025-2. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–82. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon DC, Grilo CM, Lipschitz DS. Correlates of community violence exposure in hospitalized adolescents. Compr Psychiatry. 2001;42:283–90. doi: 10.1053/comp.2001.24580. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30:762–70. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–40. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF. Adolescent stress and neural plasticity in hamsters: a vasopressin-serotonin model of inappropriate aggressive behaviour. Exp Physiol. 2000;85 Spec No:85S–90S. doi: 10.1111/j.1469-445x.2000.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Floody OR, Pfaff DW. Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J Comp Physiol Psychol. 1977;91:443–64. doi: 10.1037/h0077341. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Melloni RH., Jr Serotonin modulates offensive attack in adolescent anabolic steroid-treated hamsters. Pharmacol Biochem Behav. 2002;73:713–21. doi: 10.1016/s0091-3057(02)00880-8. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH., Jr Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Horm Behav. 2003;44:271–80. doi: 10.1016/s0018-506x(03)00138-7. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Melloni RH. Serotonin 1B receptor activity and expression modulate the aggression-stimulating effects of adolescent anabolic steroid exposure in hamsters. Behavioral Neuroscience. 2005;119:1184–94. doi: 10.1037/0735-7044.119.5.1184. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Melloni RHJ. Prolonged alterations in the serotonin neural system following the cessation of adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behavioral Neuroscience. 2006;120:1242–51. doi: 10.1037/0735-7044.120.6.1242. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH. Plasticity in anterior hypothalamic vasopressin correlates with aggression during anabolic/androgenic steroid withdrawal. Behav Neurosci. 2006;120:115–24. doi: 10.1037/0735-7044.120.1.115. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH. Alterations in anterior hypothalamic vasopressin, but not serotonin, correlate with teh temporal onset of aggressive behavior during adolescent anabolic-steroid exposure in hamsters. Behav Neurosci. 2007;121:941–948. doi: 10.1037/0735-7044.121.5.941. [DOI] [PubMed] [Google Scholar]
- Guillot PV, Chapouthier G. Intermale aggression and dark/light preference in ten inbred mouse strains. Behav Brain Res. 1996;77:211–3. doi: 10.1016/0166-4328(95)00163-8. [DOI] [PubMed] [Google Scholar]
- Hall RCW, Hall RCW, Chapman MJ. Psychiatric complications of anabolic steroid abuse. Psychosomatics. 2005;46:285–90. doi: 10.1176/appi.psy.46.4.285. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1309–20. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer PA. Anabolic-androgenic steroid use among young male and female athletes: is the game to blame? Br J Sports Med. 2010;44:26–31. doi: 10.1136/bjsm.2009.068924. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Jones BJ, Oakley NR, Tyers MB. Evidence that the amygdala is involved in the disinhibitory effects of 5-HT3 receptor antagonists. Psychopharmacology (Berl) 1991;104:545–51. doi: 10.1007/BF02245664. [DOI] [PubMed] [Google Scholar]
- Hogg S, Hof M, Wurbel H, Steimer T, de Ruiter A, Koolhaas J, Sluyter F. Behavioral profiles of genetically selected aggressive and nonaggressive male wild house mice in two anxiety tests. Behav Genet. 2000;30:439–46. doi: 10.1023/a:1010246717180. [DOI] [PubMed] [Google Scholar]
- Isacsson G, Bergman U. Can anabolic steroids cause personality changes? Nord Med. 1993;108:180–1. [PubMed] [Google Scholar]
- Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133:893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Jay MS, Shoup B, Rickert VI. Anabolic steroid use by male adolescents. Pediatrics. 1989;83:921–4. [PubMed] [Google Scholar]
- Johnson MD. Anabolic steroid use in adolescent athletes. Pediatr Clin North Am. 1990;37:1111–23. doi: 10.1016/s0031-3955(16)36977-2. [DOI] [PubMed] [Google Scholar]
- Kashkin KB, Kleber HD. Hooked on hormones? An anabolic steroid addiction hypothesis. Jama. 1989;262:3166–70. doi: 10.1001/jama.262.22.3166. [DOI] [PubMed] [Google Scholar]
- Koukoulas I, Webb GC, Bottema CD, Gill C, Johnston CI, Aldred GP. Genomic characterization of the sheep vasopressin V1a receptor gene and promoter, with assignment to bands q23-24 of sheep chromosome 3 and cattle chromosome 5. Gene. 1999;240:183–92. doi: 10.1016/s0378-1119(99)00407-2. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Lukas SE, Pope HG, Jr, Oliva PS. Increased aggressive responding in male volunteers following the administration of gradually increasing doses of testosterone cypionate. Drug Alcohol Depend. 1995;40:73–9. doi: 10.1016/0376-8716(95)01192-7. [DOI] [PubMed] [Google Scholar]
- Kreuz LE, Rose RM. Assessment of aggressive behavior and plasma testosterone in a young criminal population. Psychosom Med. 1972;34:321–32. doi: 10.1097/00006842-197207000-00006. [DOI] [PubMed] [Google Scholar]
- Lerwill CJ, Makings P. The agonistic behavior of the golden hamster. Animal Behavior. 1971;19:714–721. [Google Scholar]
- Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG. 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience. 2004;128:583–96. doi: 10.1016/j.neuroscience.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Lindqvist AS, Eriksson B, Ehrnborg C, Fahlke C, Moberg T, Rosen T. First Nordic Conference on Abuse of Anabolic Steroids and Anti-dping Work. Uppsala; Sweden: 2007. AAS abuse in former competitive sport athletes. [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lumia AR, Thorner KM, McGinnis MY. Effects of chronically high doses of the anabolic androgenic steroid, testosterone, on intermale aggression and sexual behavior in male rats. Physiol Behav. 1994;55:331–5. doi: 10.1016/0031-9384(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Maayan R, Touati-Werner D, Ram E, Strous R, Keren O, Weizman A. The protective effect of frontal cortex dehydroepiandrosterone in anxiety and depressive models in mice. Pharmacol Biochem Behav. 2006;85:415–21. doi: 10.1016/j.pbb.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Malone DA, Jr, Dimeff RJ. The use of fluoxetine in depression associated with anabolic steroid withdrawal: a case series. J Clin Psychiatry. 1992;53:130–2. [PubMed] [Google Scholar]
- Malone DA, Jr, Dimeff RJ, Lombardo JA, Sample RH. Psychiatric effects and psychoactive substance use in anabolic-androgenic steroid users. Clin J Sport Med. 1995;5:25–31. doi: 10.1097/00042752-199501000-00005. [DOI] [PubMed] [Google Scholar]
- Mattsson A, Schalling D, Olweus D, Low H, Svensson J. Plasma testosterone, aggressive behavior, and personality dimensions in young male delinquents. J Am Acad Child Adolesc Psychiatry. 1980;19:476–490. doi: 10.1016/s0002-7138(09)61065-7. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Breuer ME, Possidente B. Physical provocation potentiates aggression in male rats receiving anabolic androgenic steroids. Horm Behav. 2002a;41:101–10. doi: 10.1006/hbeh.2001.1742. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Possidente BP. Effects of withdrawal from anabolic androgenic steroids on aggression in adult male rats. Physiol Behav. 2002b;75:541–9. doi: 10.1016/s0031-9384(02)00657-1. [DOI] [PubMed] [Google Scholar]
- McGinnis MY. Anabolic androgenic steroids and aggression: studies using animal models. Ann N Y Acad Sci. 2004;1036:399–415. doi: 10.1196/annals.1330.024. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr, Connor DF, Hang PT, Harrison RJ, Ferris CF. Anabolic-androgenic steroid exposure during adolescence and aggressive behavior in golden hamsters. Physiol Behav. 1997;61:359–64. doi: 10.1016/s0031-9384(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr, Ricci LA. Adolescent exposure to anabolic/androgenic steroids and the neurobiology of offensive aggression: A hypothalamic neural model based on findings in pubertal Syrian hamsters. Horm Behav. 2010;58:177–191. doi: 10.1016/j.yhbeh.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Minkin DM, Meyer ME, van Haaren F. Behavioral effects of long-term administration of an anabolic steroid in intact and castrated male Wistar rats. Pharmacol Biochem Behav. 1993;44:959–63. doi: 10.1016/0091-3057(93)90031-n. [DOI] [PubMed] [Google Scholar]
- Morrison TR, Ricci LA, Melloni RH. The role of vasopressin-serotonin interactions in aggressive behavior. In: Miczek KA, Meyer-Lindenberg A, editors. Current Topics in Behavioral Neurosciences: The Neuroscience of Aggression. Springer Press; 2013. In Press. [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–46. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Newman MG, Borkovec TD, Hope DA, Kozak MJ, McNally RJ, Taylor CB. Future directions in the treatment of anxiety disorders: an examination of theory, basic science, public policy, psychotherapy research, clinical training, and practice. J Clin Psychol. 1999;55:1325–45. doi: 10.1002/(sici)1097-4679(199911)55:11<1325::aid-jclp2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- NIDACapsules. 2007 http//www.nida.nih.gov/NIDACapsules/NCIndex.html.
- Olweus D, Mattsson A, Schalling D, Low H. Testosterone, aggression, physical, and personality dimensions in normal adolescent males. Psychosom Med. 1980;42:253–69. doi: 10.1097/00006842-198003000-00003. [DOI] [PubMed] [Google Scholar]
- Olweus D. Testosterone and adrenaline: aggressive antisocial behavior in normal adolescent males. In: Mednick SA, Moffitt TE, Stack SA, editors. The Causes of Crime: New Biological Approaches. Cambridge University Press; Cambridge, England: 1987. pp. 239–262. [Google Scholar]
- Ovsiukova MV, Amikishieva AV, Kaudriavtseva NN, Obut TA. Anxiolytic effect of dehydroepiandrosterone sulfate in male mice with high anxiety. Zh Vyssh Nerv Deiat Im I P Pavlova. 2003;53:789–93. [PubMed] [Google Scholar]
- Pagonis TA, Angelopoulos NV, Koukoulis GN, Hadjichristodoulou CS. Psychiatric side effects induced by supraphysiological doses of combinations of anabolic steroids correlate to the severity of abuse. Eur Psychiatry. 2006a;21:551–62. doi: 10.1016/j.eurpsy.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Pagonis TA, Angelopoulos NV, Koukoulis GN, Hadjichristodoulou CS, Toli PN. Psychiatric and hostility factors related to use of anabolic steroids in monozygotic twins. Eur Psychiatry. 2006b;21:563–9. doi: 10.1016/j.eurpsy.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Parrilla-Carrero J, Figueroa O, Lugo A, Garcia-Sosa R, Brito-Vargas P, Cruz B, Rivera M, Barreto-Estrada JL. The anabolic steroids testosterone propionate and nandrolone, but not 17alpha-methyltestosterone, induce conditioned place preference in adult mice. Drug Alcohol Depend. 2009;100:122–7. doi: 10.1016/j.drugalcdep.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–9. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Perry HM, Hughes GW. A case of affective disorder associated with the misuse of ‘anabolic steroids’. Br J Sports Med. 1992;26:219–20. doi: 10.1136/bjsm.26.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PJ, Andersen KH, Yates WR. Illicit anabolic steroid use in athletes. A case series analysis. Am J Sports Med. 1990;18:422–8. doi: 10.1177/036354659001800416. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51:375–82. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Kouri EM, Powell KF, Campbell C, Katz DL. Anabolic-androgenic steroid use among 133 prisoners. Compr Psychiatry. 1996;37:322–7. doi: 10.1016/s0010-440x(96)90013-9. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch Gen Psychiatry. 2000;57:133–40. doi: 10.1001/archpsyc.57.2.133. discussion 155-6. [DOI] [PubMed] [Google Scholar]
- Pope HG, Katz DL, Champoux R. Anabolic-androgenic steroid use among 1010 college men. Physician Sports Med. 1988;16:75–81. doi: 10.1080/00913847.1988.11709554. [DOI] [PubMed] [Google Scholar]
- Pratt JA. The neuroanatomical basis of anxiety. Pharmacol Ther. 1992;55:149–81. doi: 10.1016/0163-7258(92)90014-q. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Grimes JM, Melloni RH., Jr Serotonin type-3 receptors modulate the aggression-stimulating effects of adolescent cocaine exposure. Behav Neurosci. 2004;118:1097–1110. doi: 10.1037/0735-7044.118.5.1097. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Rasakham S, Grimes JM, Melloni RH. Serotonin 1A receptor activity and expression modulate adolescent anabolic/androgenic steroid induced aggression in hamsters. Pharmacol Biochem Behav. 2006;85:1–11. doi: 10.1016/j.pbb.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Morrison TR, Melloni RH., Jr Serotonin modulates anxiety-like behaviors during withdrawal from adolescent anabolic steroid exposure in Syrian hamsters. Horm Behav. 2012;62:569–578. doi: 10.1016/j.yhbeh.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci LA, Knyshevski I, Melloni RH., Jr Serotonin Type-3 Receptors Stimulate Offensive Aggression in Syrian Hamsters. Behavioral Brain Research. 2005;156:19–29. doi: 10.1016/j.bbr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rocha VM, Calil CM, Ferreira R, Moura MJ, Marcondes FK. Influence of anabolic steroid on anxiety levels in sedentary male rats. Stress. 2007;10:326–31. doi: 10.1080/10253890701281344. [DOI] [PubMed] [Google Scholar]
- Rojas-Ortiz YA, Rundle-Gonzalez V, Rivera-Ramos I, Jorge JC. Modulation of elevated plus maze behavior after chronic exposure to the anabolic steroid 17alpha-methyltestosterone in adult mice. Horm Behav. 2006;49:123–8. doi: 10.1016/j.yhbeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Baba A, Matsuda T. The 5-HT1A receptor agonist MKC-242 reverses isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology (Berl) 2003;170:73–9. doi: 10.1007/s00213-003-1515-x. [DOI] [PubMed] [Google Scholar]
- Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: relationship to aggressive, hyperactive, and internalizing behaviors. J Am Acad Child Adolesc Psychiatry. 1994;33:1174–84. doi: 10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Schaal B, Tremblay RE, Soussignan R, Susman EJ. Male testosterone linked to high social dominance but low physical aggression in early adolescence. J Am Acad Child Adolesc Psychiatry. 1996;35:1322–30. doi: 10.1097/00004583-199610000-00019. [DOI] [PubMed] [Google Scholar]
- Schalling D. personality correlates of plasma testosterone levels in young delinquants: an example of person-situation interaction? In: Mednick SA, Moffitt TE, Stack SA, editors. The Causes of Crime: New Biological Approaches. Cambridge University Press; Cambridge, England: 1987. pp. 283–291. [Google Scholar]
- Schwartzer JJ, Melloni RH., Jr Anterior hypothalamic dopamine D2 receptors modulate adolescent anabolic/androgenic steroid-induced offensive aggression the Syrian hamsterr. Behav Pharm. 2010;21:314–322. doi: 10.1097/FBP.0b013e32833b10f1. [DOI] [PubMed] [Google Scholar]
- Strauss RH, Wright JE, Finerman GAM. Side-effects of anabolic steroids in weight trained men. Physician and Sprrtsmedicine. 1983;11:87–96. doi: 10.1080/00913847.1983.11708706. [DOI] [PubMed] [Google Scholar]
- Strauss RH, Wright JE, Finerman GAM. Anabolic Steroids. In: Strauss RH, editor. Drugs and Performance in Sports. WB Saunders; Philidelphia: 1987. pp. 59–67. [Google Scholar]
- Su TP, Pagliaro M, Schmidt PJ, Pickar D, Wolkowitz O, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. Jama. 1993;269:2760–4. [PubMed] [Google Scholar]
- Thomas E, Gunton DJ. Kindling of the lateral septum and the amygdala: effects on anxiety in rats. Physiol Behav. 2011;104:653–8. doi: 10.1016/j.physbeh.2011.07.005. [DOI] [PubMed] [Google Scholar]
- van der Vegt BJ, Lieuwes N, van de Wall EH, Kato K, Moya-Albiol L, Martinez-Sanchis S, de Boer SF, Koolhaas JM. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav Neurosci. 2003;117:667–74. doi: 10.1037/0735-7044.117.4.667. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Neurobiological mechanisms of aggression and stress coping: a comparative study in mouse and rat selection lines. Brain Behav Evol. 2007;70:274–85. doi: 10.1159/000105491. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Torner L, Blume A, Beiderbeck DI, Neumann ID. Low inborn anxiety correlates with high intermale aggression: link to ACTH response and neuronal activation of the hypothalamic paraventricular nucleus. Horm Behav. 2007;51:11–9. doi: 10.1016/j.yhbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]


