Abstract
The burgeoning obesity and diabetes epidemics threaten health worldwide, yet the molecular mechanisms underlying these phenomena are incompletely understood. Recently, attention has focused on the potential contributions of environmental pollutants that act as endocrine disrupting chemicals (EDCs) in the pathogenesis of metabolic diseases. Because glucocorticoid signaling is central to adipocyte differentiation, the ability of EDCs to stimulate the glucocorticoid receptor (GR) and drive adipogenesis was assessed in the 3T3-L1 cell line. Various EDCs were screened for glucocorticoid-like activity using a luciferase reporter construct, and four (bisphenol A (BPA), dicyclohexyl phthalate (DCHP), endrin, and tolylfluanid (TF)) were shown to significantly stimulate GR without significant activation of the peroxisome proliferator-activated receptor-γ. 3T3-L1 preadipocytes were then treated with EDCs and a weak differentiation cocktail containing dehydrocorticosterone (DHC) in place of the synthetic dexamethasone. The capacity of these compounds to promote adipogenesis was assessed by quantitative oil red O staining and immunoblotting for adipocyte-specific proteins. The four EDCs increased lipid accumulation in the differentiating adipocytes and also upregulated the expression of adipocytic proteins. Interestingly, proadipogenic effects were observed at picomolar concentrations for several of the EDCs. Because there was no detectable adipogenesis when the preadipocytes were treated with compounds alone, the EDCs are likely promoting adipocyte differentiation by synergizing with agents present in the differentiation cocktail. Thus, EDCs are able to promote adipogenesis through the activation of the GR, further implicating these compounds in the rising rates of obesity and diabetes.
INTRODUCTION
The ongoing explosion in obesity and its concomitant metabolic sequela of insulin resistance and diabetes are placing enormous strains on our health-care system. Despite concerted efforts to understand the underlying mechanisms, the causes for the rapidity of this epidemic remain incompletely understood. Given the pace of this change, genetic shifts in the population cannot explain this phenomenon. As such, efforts have focused on identifying environmental factors that tip the balance of energy homeostasis in favor of fat deposition. Declines in physical activity and increases in the caloric density of food certainly contribute to the pathogenesis of obesity; however, these factors likely do not fully account for the magnitude of the epidemic (1,2).
Interestingly, the rise in obesity rates has been preceded by a parallel and exponential increase in synthetic chemical production (3). This correlation led to the articulation of the “environmental obesogen hypothesis” that posits a causative link between these two phenomena (3,4). In support of this concept are epidemiological studies suggesting a link between various synthetic chemicals and the development of obesity (5), insulin resistance (6), and diabetes (7). Although these studies provide tantalizing correlative evidence in support of environmental obesogens, they fail to provide mechanistic details regarding how these compounds may discretely alter biochemical pathways thereby leading to obesity.
Potential mechanisms of adipocyte endocrine disruption are provided by prior work in the fields of sex steroid and thyroid hormone signaling where environmental endocrine disrupting chemicals (EDCs) have been shown to alter nuclear hormone signaling (8,9). Members of the same superfamily of ligand-activated nuclear hormone receptors are critically important for the highly ordered regulation of adipogenesis as well as for energy homeostasis in the mature adipocyte. Two members of this receptor superfamily that are central for adipocyte differentiation are the peroxisome proliferator-activated receptor-γ (PPARγ) and the glucocorticoid receptor (GR) (10,11). Grun et al. have reported that PPARγ is a molecular target for alkylated tin compounds (12). Tributyltin and triphenyltin (TPT) have been shown to be selective and potent agonists of both PPARγ and retinoid X receptors (4,12), and tributyltin has been shown to promote adipogenesis in the murine 3T3-L1 cell line (13). Other compounds implicated in adipogenesis include bisphenol A (BPA) (14,15) and the phthalate metabolite mono-2-ethylhexyl-phthalate (16), the latter of which may operate through stimulation of PPARγ (17,18).
Less is known about potential endocrine disruption of glucocorticoid signaling in preadipocytes despite its critical role in adipogenesis. Previous work has shown that EDCs can compete with ligand binding to GR (19–21), but receptor activity was not reported. Additionally, EDCs modulated enzymatic activities involved in glucocorticoid activation and inactivation (11β-hydroxysteroid dehydrogenase-1 and -2, respectively) (22,23). Thus, alteration of glucocorticoid signaling has been proposed as an important mechanism for environmental endocrine disruption (24). However, the ability of EDCs to directly modulate GR activity in preadipocytes has not been previously demonstrated. In the current work, putative EDCs from various chemical classes were shown to directly activate GR. Further, GR stimulation by these EDCs potentiated adipogenesis in the murine 3T3-L1 cell line, a well-characterized model of adipocyte differentiation (25). Thus, in addition to activation of PPARγ, EDCs may also impact adipocyte formation through modulation of GR, thereby contributing to the environmental factors causally related to the current obesity and diabetes epidemics.
METHODS AND PROCEDURES
3T3-L1 cell culture and differentiation
3T3-L1 preadipocytes were cultured as previously described (26). Two days after reaching confluency, differentiation was initiated by the addition of Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Manassas, VA) containing 10% fetal bovine serum (FBS; Aleken Biologicals, Nash, TX), 167 nmol/l porcine insulin, 0–100 nmol/l dehydrocorticosterone (DHC) and 0.5 mmol/l isobutylmethylxanthine (all from Sigma, St Louis, MO). After 3 days, the medium was removed, and the cells were cultured for two additional days in DMEM plus 10% FBS and 167 nmol/l insulin. The cells were then maintained in DMEM plus 10% FBS medium until use, usually 1–3 days after completion of the differentiation protocol. The effects of EDCs on 3T3-L1 differentiation were determined by incorporating the EDCs into the first 3 days of the differentiation protocol. EDCs under study included BPA, dicyclohexyl phthalate (DCHP), endrin, TPT, and tolylfluanid (TF) (all from Sigma).
Preparation and analysis of cell lysates
Whole-cell lysates were prepared by first washing the cells three times with ice-cold PBS followed by scraping into homogenization buffer (50 mmol/l HEPES, pH 7.4, 150 mmol/l NaCl, 10 mmol/l NaF, 10 mmol/l EDTA, 10% glycerol, 0.5% Triton X-100, and protease inhibitors, aprotinen and benzamidine, added immediately prior to use). For immunoblots, whole-cell lysates were resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose (Whatman, Maidstone, UK). Western blots were probed with anti-adiponectin (Chemicon, Temecula, CA); anti-CCAAT/enhancer binding protein α, anti-GR, anti-insulin receptor subunit β, and antiprotein phosphatase 1 (Santa Cruz Biotechnology, Santa Cruz, CA); and anti-β-actin (Sigma) antibodies. Immunoblots were then incubated with horseradish peroxidase-conjugated goat anti- rabbit or goat antimouse immunoglobulin G (Bio-Rad, Hercules, CA) followed by autoradiography using ECL reagent (GE Health Care, Little Chalfont, UK).
Luciferase assays
3T3-L1 preadipocytes were transiently transfected in six-well plates using Lipofectamine Plus (Invitrogen, Carlsbad, CA) with a luciferase reporter construct. To assess GR activity, the promoter for the mouse mammary tumor virus, containing a glucocorticoid response element, was cloned into a vector containing the luciferase reporter (generous gift of F. Wondisford, Johns Hopkins). PPARγ activity was determined using a luciferase construct containing two copies of the phosphoenolpyruvate carboxykinase PPARγ response element into the pGL2-Promoter vector (Promega, Madison, WI) (27). Subconfluent preadipocytes in six-well plates were transiently transfected with 2 µg of glucocorticoid response element-Luc or 2 µg of PPARγ response element-Luc plus 2 µg of PPARγ using Lipofectamine Plus over 16– 18 h. Transfection media was removed, and the cells were washed with PBS prior to 24-h treatment with EDCs or controls in DMEM plus 10% calf serum. Mock-treated cells were incubated with an equivalent amount of vehicle. Cells were harvested and lysed, and luciferase activity determined as previously described (27).
Assessment of 3T3-L1 lipid accumulation
Lipid accumulation of differentiated 3T3-L1 adipocytes was determined by quantitative oil red O staining. Briefly, oil red O (Sigma) was dissolved in isopropanol overnight at a concentration of 0.35% followed by 0.2 µm filtration, dilution in water to a final concentration of 0.2%, and refiltration. Adipocytes were washed with PBS and fixed in 10% Formalin for 60 min. Cells were washed with 60% isopropanol, allowed to dry, and stained with oil red O for 10 min. Following multiple water washes, the plates were dried at room temperature. Oil red O was eluted using 100% isopropanol, and absorbance at 500 nm was measured.
Calculations and statistics
Relative luciferase activity was calculated as the percentage of mock for three experiments performed in triplicate. Oil red O staining was calculated as the average of three experiments performed in quadruplicate relative to unsupplemented differentiation cocktail. Statistical significance was determined using two-tailed, paired Student’s t-test.
RESULTS
Endocrine disruption of nuclear hormone activity in the preadipocyte
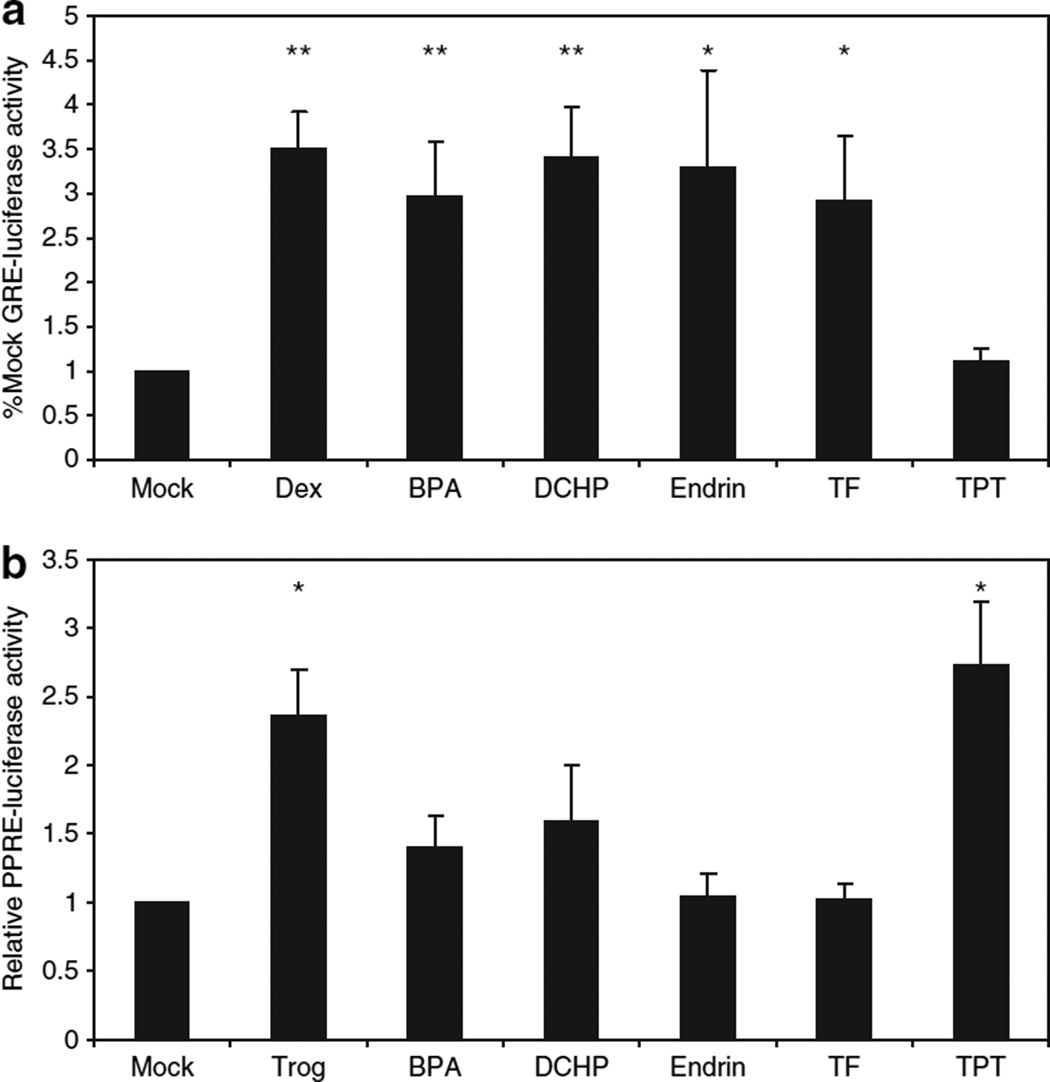
Activation of the GR plays a critical function in adipocyte differentiation (11,28), yet the potential role of this pathway in EDC action remains unclear. To determine which EDCs may have instrinsic glucocorticoid-like activity, we screened 13 compounds from various chemical classes (alkylated tin compounds (tributyltin, TPT), fungicides (TF), insecticides (aldicarb, endrin, and 1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene), polychlorinated biphenyls (Aroclor 1254, PCB 77, PCB 126, PCB 154), phthalates (benzylbutyl phthalate, DCHP), and plasticizers (BPA)) using a GR-dependent luciferase reporter assay. The effect of EDCs on GR-mediated luciferase expression was assessed after exposure to 1 µmol/l EDC for 24 h. This concentration was chosen because some putative EDCs had been shown to inhibit dexamethasone binding to the GR with an IC50 of ~1 µmol/l (20). Under these conditions, four EDCs were shown to significantly stimulate GR-mediated luciferase expression (BPA, DCHP, endrin, and TF) (Figure 1a). Interestingly, the extent of stimulation was comparable to that achieved by the synthetic glucocorticoid dexamethasone. In contrast, the EDC TPT that has previously been reported to act as a PPARγ ligand did not display any significant activation of GR (Figure 1a).
Figure 1.
Endocrine disrupting chemical-induced luciferase expression. 3T3-L1 preadipocytes were transfected with either (a) a luciferase reporter containing a glucocorticoid response element or (b) a luciferase reporter containing a peroxisome proliferator-activated receptor-γ (PPARγ) response element and a second plasmid expressing PPARγ. The cells were subsequently treated with vehicle (Mock), 1 µmol/l dexamethasone (Dex), bisphenol A (BPA), dicyclohexyl phthalate (DCHP), endrin, tolylfluanid (TF), or troglitazone (Trog); or 100 nmol/l triphenyltin (TPT) for 24 h followed by measurement of luminescence. Results are expressed relative to vehicle-treated (mock) as means ± s.e.m. for three experiments performed in triplicate. Using paired Student’s t-test on raw luminescence data, statistical significance is represented as **(P < 0.01) or *(P < 0.05).
Prior studies have demonstrated PPARγ-agonist activity for some EDCs (12,16). To test whether the four identified GR-agonists had significant PPARγ activity, we used a PPARγ-luciferase reporter assay; however, because 3T3-L1 preadipocytes do not express appreciable PPARγ, we cotransfected the cells with a PPARγ plasmid as well as the reporter construct. Consistent with published data (12,13), the alkylated tin compound TPT stimulated PPARγ-mediated luciferase expression to an extent comparable to that of 1 µmol/l of the synthetic PPARγ agonist troglitazone (Figure 1b). Of note, cell toxicity was observed in preliminary experiments with TPT at 1 µmol/l so a dose of 100 nmol/l was used. In contrast, the four EDCs with GR-agonist properties did not significantly stimulate PPARγ activity at 1 µmol/l (Figure 1b).
EDC induction of adipogenesis: protein expression
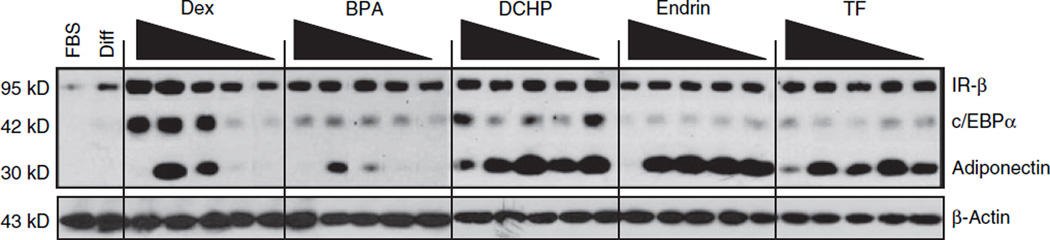
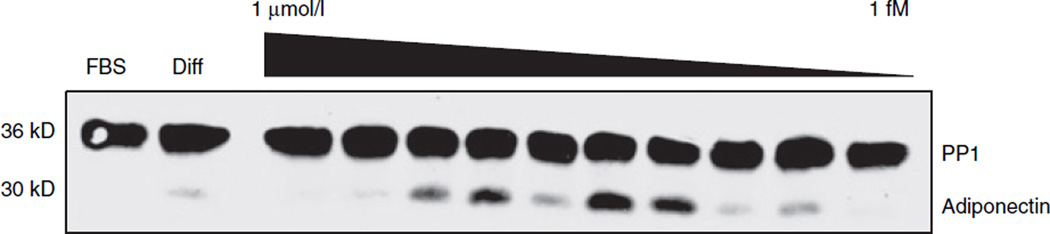
Next, to test the physiological significance of GR activation by EDCs in the 3T3-L1 preadipocytes, the effects of EDCs on adipogenesis were determined. First, the ability of the EDCs to induce adipocyte differentiation was assessed. Confluent 3T3-L1 cells were differentiated by a standard protocol in which cells were incubated with a differentiation cocktail for three days followed by 2 days in insulin-containing media (26). In parallel, replicate wells were incubated with 100 nmol/l BPA, DCHP, endrin, or TF, either alone or in combination with insulin and isobutylmethylxanthine, during the first 3 days of the protocol. Using oil red O staining and immunoblotting for proteins induced during adipocyte differentiation (e.g., adiponectin), the four EDCs did not promote differentiation under these two conditions (data not shown). The inability of the EDCs to fully substitute for dexamethasone in the differentiation protocol suggests that they were not able to sufficiently activate GR on their own to initiate the adipogenic program. So next, the ability of the EDCs to potentiate differentiation was investigated. When EDCs were included in the standard differentiation cocktail for the first 3 days of the protocol, no discernable increase in adipocyte differentiation was obtained as assessed by immunoblotting or oil red O staining (data not shown). However, the inclusion of the superactive synthetic glucocorticoid dexamethasone in the cocktail could cause maximal GR activation and thus mask any EDC-induced effects. Therefore, 3T3-L1 cells were differentiated using the inactive murine glucocorticoid DHC in place of the dexamethasone, an approach that has previously been shown to induce submaximal adipogenesis in these cells (29). Each EDC or the positive control dexamethasone was included for the first 3 days of the protocol and then all wells were incubated for 2 days in media containing 167 nmol/l insulin. Cell lysates were prepared and simultaneously analyzed by antiinsulin receptor subunit β, anti-CCAAT/enhancer binding protein α, and anti-adiponectin immunoblotting. In parallel, a second set of gels were run and analyzed by anti-β-actin to serve as a protein loading control due to comigration of actin with CCAAT/enhancer binding protein α. Addition of each of the EDCs in this protocol enhanced 3T3-L1 differentiation as determined by immunoblotting (Figure 2), although BPA was the least efficacious agent. Interestingly, at the highest concentration of dexamethasone and the EDCs, adiponectin expression was largely reduced whereas expression of the three other proteins was not affected. The precise reason for this finding is unclear although dexamethasone treatment of mature 3T3-L1 adipocytes has been shown to reduce adiponectin expression (30). Additionally, GR levels were not altered either by differentiation as previously described (31) or by incubation with the EDCs (data not shown). Startlingly, DCHP, endrin, and TF were found to enhance adipogenesis at concentrations as low as 100 pmol/l. Using DCHP as a representative of this group, a more complete dose curve revealed stimulation of adipogenesis into the low picomolar range (Figure 3). The physiologic significance of this finding is supported by epidemiologic data that have demonstrated an average serum endrin concentration of 5.9 nmol/l in a population of Spanish women (32), average urine BPA concentrations of 11.4 nmol/l in a US cohort (33), and blood levels of BPA in pregnant Korean woman as high as 290 nmol/l (34).
Figure 2.
Endocrine disrupting chemical-induction of adipocyte protein expression. 3T3-L1 differentiation was induced by incubation of confluent preadipocytes for 3 days in 10% fetal bovine serum (FBS) in Dulbecco’s modified Eagle’s medium (DMEM) containing 100 nmol/l dehydrocorticosterone, 167 nmol/l insulin, and 0.5 mmol/l isobutylmethylxanthine alone (Diff) or supplemented with dexamethasone (Dex), bisphenol A (BPA), dicyclohexyl phthalate (DCHP), endrin, or tolylfluanid (TF) at concentrations ranging from left to right from 1 µmol/l to 100 pmol/l, with each well representing a progressive tenfold dilution. The cells were subsequently treated for an additional 2 days in 10% FBS in DMEM containing 167 nmol/l insulin. Whole-cell lysates were prepared, and the protein expression pattern was analyzed by immunoblotting for insulin receptor subunit α (IR-α), CCAAT/enhancer binding protein α (c/EBPα), adiponectin, and β-actin after resolution by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Protein expression was also compared to undifferentiated preadipocytes maintained in 10% FBS in DMEM (FBS). Results shown are representative of three independent experiments.
Figure 3.
Dose-dependence of endocrine disrupting chemical-induced adipogenesis. 3T3-L1 differentiation was induced by incubation of confluent preadipocytes for 3 days in 10% fetal bovine serum (FBS) in Dulbecco’s modified Eagle’s medium (DMEM) containing 100 nmol/l dehydrocorticosterone, 167 nmol/l insulin, and 0.5 mmol/l isobutylmethylxanthine alone (Diff) or supplemented with 1 µmol/l to 1 fmol/l dicyclohexyl phthalate. The cells were subsequently treated for an additional 2 days in 10% FBS in DMEM containing 167 nmol/l insulin. Whole-cell lysates were prepared, and the protein expression pattern was analyzed by immunoblotting for adiponectin, and protein phosphatase 1 (PP1) after resolution by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Protein expression was also compared to undifferentiated preadipocytes maintained in 10% FBS in DMEM (FBS). Results shown are representative of three independent experiments.
EDC induction of adipogenesis: lipid accumulation
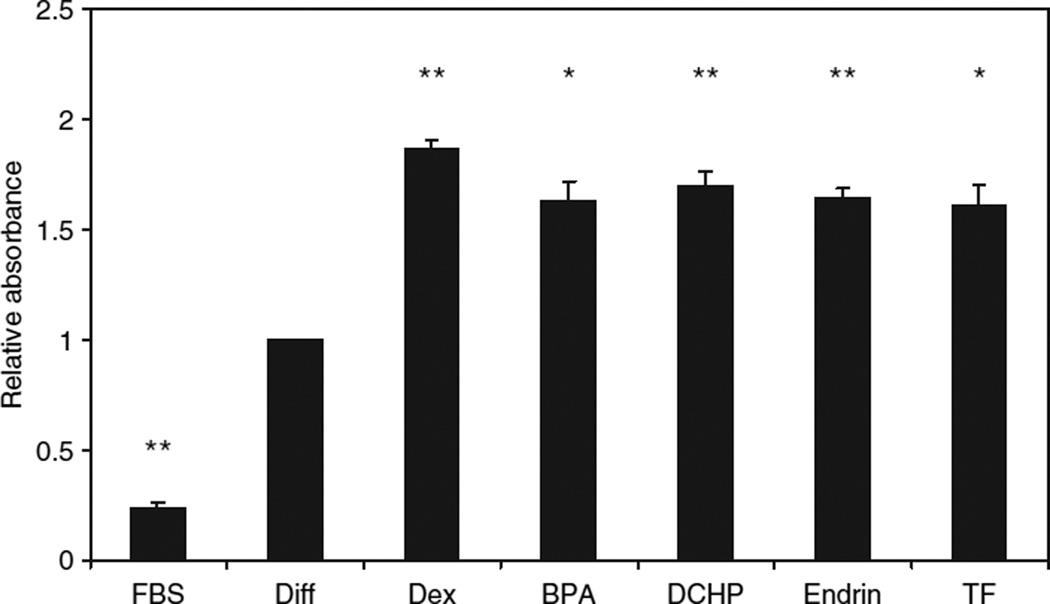
To further characterize the proadipogenic properties of these EDCs, lipid accumulation was measured in parallel. Cells were differentiated as above except that 50 nmol/l DHC was used for the first 3 days, and cells were maintained in FBS-containing media for 3 days following completion of the differentiation protocol because lipid accumulation continues to increase during this period. Cells were then fixed and stained with oil red O with staining quantified using a spectrophotometer. The DHC containing differentiation cocktail induced a fourfold increase in oil red O staining over undifferentiated cells treated with 10% FBS media throughout. Lipid accumulation was increased a further 87% by addition of 100 nmol/l dexamethasone. All four GR-active EDCs (BPA, DCHP, endrin, and TF) used at 100 nmol/l significantly stimulated adipocyte lipid accumulation compared to the differentiation cocktail alone (Figure 4), although they were not able to fully recapitulate the effects of dexamethasone (range: 61–70% increase over differentiation cocktail alone).
Figure 4.
Endocrine disrupting chemical-induced adipocyte lipid accumulation. Differentiation of confluent 3T3-L1 preadipocytes was induced as described in Figure 2 with the exception that dehydrocorticosterone was used at 50 nmol/l, and the cells were incubated for an additional 3 days in unsupplemented 10% fetal bovine serum (FBS) in Dulbecco’s modified Eagle’s medium (DMEM) after the insulin step because lipid accumulation increases during this period. The cells were then stained with oil red O, and the extent of lipid accumulation was determined by absorbance at 500 nm of the isopropanol-extracted oil red O in a spectrophotometer. Data are compared to preadipocytes maintained in 10% FBS in DMEM (FBS). Results are expressed relative to unsupplemented differentiation cocktail (Diff) as means ± s.e.m. for three experiments performed in quadruplicate. Using paired Student’s t-test, statistical significance is represented as **(P < 0.01) or *(P < 0.05).
DISCUSSION
Modulation of sex steroid and thyroid hormone action through disruption of nuclear hormone receptors has been extensively studied as a means by which EDCs can alter sexual development and cellular physiology (8,9,35). Recent work has extended endocrine disruption to cellular processes involved in energy homeostasis. Several groups have shown that alkylated tin compounds (12,13) and phthalate metabolites (16) can promote adipogenesis through stimulation of the nuclear hormone receptor PPARγ. Activation of GR is a critical regulator of adipocyte differentiation, yet the effects of EDCs on GR in preadipocytes had not been determined.
Using a glucocorticoid response element incorporated upstream of a luciferase reporter, four EDCs (BPA, DCHP, endrin, and TF) were found to stimulate GR activity in 3T3-L1 preadipocytes. In contrast to the known PPARγ agonist TPT, these four compounds did not significantly stimulate PPARγ activity in parallel reporter assays. These findings suggest that BPA, DCHP, endrin, and TF would preferentially activate GR over PPARγ in preadipocytes.
To determine whether activity in our reporter assay correlated with biological effects, 3T3-1 preadipocytes were exposed to the four GR-activating EDCs. Despite potent effects in the GR reporter assay, EDCs were insufficient to substitute for dexamethasone in the differentiation protocol (data not shown), suggesting GR activation by EDCs alone was not sufficient to initiate differentation. To more accurately reflect the physiological milieu of adipocyte differentiation in vivo, a low-potency differentiation cocktail containing DHC was utilized in combination with the various EDCs. Under these conditions, the four EDCs promoted adipogenesis as reflected by expression of several proteins upregulated during adipocyte differentiation and lipid accumulation as assessed by quantitative oil red O staining. Because the ability of EDCs to promote adipogenesis occurred under submaximal conditions, these studies suggest that the effect of EDCs is likely mediated through synergism with endogenous adipogenic signals.
In early work focused on endocrine disruption of adipogenesis, Masuno et al. reported an acceleration of 3T3-L1 differentiation in the presence of 10–80 µmol/l BPA (14,15). More recently, Grun et al. showed that tributyltin promotes 3T3-L1 adipogenesis at 10–100 nmol/l (12). In contrast to these studies, some EDCs promoted adipogenesis using a physiological differentiation cocktail well into the picomolar range. Although any individual’s body burden of an EDC is highly variable, ranges reported in population studies for some EDCs cover the concentrations that showed biological activity in these studies (32–34). For some individuals endocrine disruption of glucocorticoid signaling may be an important component in the constellation of factors mediating the development of metabolically significant obesity.
The structural diversity of the four compounds studied (BPA, DCHP, endrin, and TF) coupled with their structural dissimilarity from endogenous glucocorticoids raise interesting questions about the specific molecular mechanisms by which these compounds promote glucocorticoid activity. In contrast to studies showing alterations in GR expression induced by exposure to hexachlorobenzene (36), no differences in GR expression upon treatment with EDCs were observed. Prereceptor modulation of glucocorticoid activation (mediated through activation and inactivation of glucocorticoids by 11β-hydroxysteroid dehydrogenase-1 and -2) has become an important area of study for understanding GR signaling (37). In addition to its direct effects on GR (38), Thiram has been shown to inhibit 11β-hydroxysteroid dehydrogenase-2 activity (23) as have di- and tri-alklyated tin compounds (22), which would be predicted to potentiate glucocorticoid action in vivo. The marked activation of GR by the EDCs in the current study in the absence of DHC (Figure 1a) suggest a direct effect of the EDCs on this transcription factor. However, the potential regulation of 11β-hydroxysteroid dehydrogenase-1 by EDCs in the differentiation experiments cannot be excluded at this time.
Data from other groups provide support for the hypothesis that EDCs can modulate GR activity by direct binding to the receptor. Johansson et al. reported that TF and methylsulfonyl PCBs compete with glucocorticoids for binding to murine GR (21), suggesting TF has affinity for the ligand-binding domain of GR. In addition to direct binding to the ligand-binding domain, EDCs may alter GR ligand affinity through allosteric effects or modulation of the ligand-binding domain. Gumy et al. have reported that dibutyltin can inhibit GR activation through insertion at an allosteric site near the steroid-binding pocket (19). The dithiocarbamate pesticide Thiram was also found to reduce dexamethasone binding to the GR, possibly through oxidation of thiol-containing amino acid residues in the ligand-binding domain (38). Although these studies demonstrated inhibition at the level of GR, similar sites of regulation can be invoked to explain the EDC-mediated GR activation seen in our studies.
Obesity and its metabolic consequences exert a costly toll in both morbidity and mortality, and emerging data suggest that EDCs may be an important contributing factor to this epidemic. The current work shows that four structurally distinct EDCs promote adipogenesis through the potentiation of GR activity. Interestingly, the differential activation of PPARγ and GR in the maturing adipocyte raises profound questions about the contribution of various EDCs to the development of metabolic diseases. Although PPARγ stimulation promotes adipogenesis, stimulation of this receptor in mature adipocytes promotes an insulin-sensitive phenotype (39). In contrast, the proadipogenic effects of glucocorticoids are followed by the induction of insulin resistance in mature adipocytes, as is seen in the development of Cushing’s Syndrome (40). Further work will be needed to fully elucidate the deleterious effects of these potential environmental glucocorticoids on preadipocyte and adipocyte physiology.
ACKNOWLEDGMENTS
The research was supported in part by an individual Ruth L. Kirschstein National Research Service Award from the National Institutes of Health (F32ES017391) (to R.M.S.).
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Qi L, Cho YA. Gene-environment interaction and obesity. Nutr Rev. 2008;66:684–694. doi: 10.1111/j.1753-4887.2008.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessesen DH. Update on obesity. J Clin Endocrinol Metab. 2008;93:2027–2034. doi: 10.1210/jc.2008-0520. [DOI] [PubMed] [Google Scholar]
- 3.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8:185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- 4.Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 5.Hatch EE, Nelson JW, Qureshi MM, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data 1999–2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR., Jr Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007;30:622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- 7.Lee DH, Lee IK, Song K, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 8.Hodges LC, Bergerson JS, Hunter DS, Walker CL. Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicol Sci. 2000;54:355–364. doi: 10.1093/toxsci/54.2.355. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann PJ, Schomburg L, Köhrle J. Interference of endocrine disrupters with thyroid hormone receptor-dependent transactivation. Toxicol Sci. 2009;110:125–137. doi: 10.1093/toxsci/kfp086. [DOI] [PubMed] [Google Scholar]
- 10.Gregoire FM. Adipocyte differentiation: from fibroblast to endocrine cell. Exp Biol Med (Maywood) 2001;226:997–1002. doi: 10.1177/153537020122601106. [DOI] [PubMed] [Google Scholar]
- 11.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 12.Grün F, Watanabe H, Zamanian Z, et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006;20:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 13.Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol. 2005;67:766–774. doi: 10.1124/mol.104.008409. [DOI] [PubMed] [Google Scholar]
- 14.Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci. 2005;84:319–327. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- 15.Masuno H, Kidani T, Sekiya K, et al. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J Lipid Res. 2002;43:676–684. [PubMed] [Google Scholar]
- 16.Feige JN, Gelman L, Rossi D, et al. The endocrine disruptor monoethylhexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem. 2007;282:19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- 17.Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 18.Venkata NG, Robinson JA, Cabot PJ, et al. Mono(2-ethylhexyl) phthalate and mono-n-butyl phthalate activation of peroxisome proliferator activated-receptors alpha and gamma in breast. Toxicol Lett. 2006;163:224–234. doi: 10.1016/j.toxlet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Gumy C, Chandsawangbhuwana C, Dzyakanchuk AA, et al. Dibutyltin disrupts glucocorticoid receptor function and impairs glucocorticoid-induced suppression of cytokine production. PLoS ONE. 2008;3:e3545. doi: 10.1371/journal.pone.0003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson M, Nilsson S, Lund BO. Interactions between methylsulfonyl PCBs and the glucocorticoid receptor. Environ Health Perspect. 1998;106:769–772. doi: 10.1289/ehp.98106769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson M, Johansson N, Lund BO. Xenobiotics and the glucocorticoid receptor: additive antagonistic effects on tyrosine aminotransferase activity in rat hepatoma cells. Basic Clin Pharmacol Toxicol. 2005;96:309–315. doi: 10.1111/j.1742-7843.2005.pto960406.x. [DOI] [PubMed] [Google Scholar]
- 22.Atanasov AG, Nashev LG, Tam S, Baker ME, Odermatt A. Organotins disrupt the 11beta-hydroxysteroid dehydrogenase type 2-dependent local inactivation of glucocorticoids. Environ Health Perspect. 2005;113:1600–1606. doi: 10.1289/ehp.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atanasov AG, Tam S, Röcken JM, Baker ME, Odermatt A. Inhibition of 11 beta-hydroxysteroid dehydrogenase type 2 by dithiocarbamates. Biochem Biophys Res Commun. 2003;308:257–262. doi: 10.1016/s0006-291x(03)01359-7. [DOI] [PubMed] [Google Scholar]
- 24.Odermatt A, Gumy C, Atanasov AG, Dzyakanchuk AA. Disruption of glucocorticoid action by environmental chemicals: potential mechanisms and relevance. J Steroid Biochem Mol Biol. 2006;102:222–231. doi: 10.1016/j.jsbmb.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Cowherd RM, Lyle RE, McGehee RE., Jr Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10:3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- 26.Brady MJ, Kartha PM, Aysola AA, Saltiel AR. The role of glucose metabolites in the activation and translocation of glycogen synthase by insulin in 3T3-L1 adipocytes. J Biol Chem. 1999;274:27497–27504. doi: 10.1074/jbc.274.39.27497. [DOI] [PubMed] [Google Scholar]
- 27.Temple KA, Cohen RN, Wondisford SR, et al. An intact DNA-binding domain is not required for peroxisome proliferator-activated receptor gamma (PPARgamma) binding and activation on some PPAR response elements. J Biol Chem. 2005;280:3529–3540. doi: 10.1074/jbc.M411422200. [DOI] [PubMed] [Google Scholar]
- 28.Bujalska IJ, Kumar S, Hewison M, Stewart PM. Differentiation of adipose stromal cells: the roles of glucocorticoids and 11beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140:3188–3196. doi: 10.1210/endo.140.7.6868. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Temple KA, Jones SA, et al. Differential modulation of 3T3-L1 adipogenesis mediated by 11beta-hydroxysteroid dehydrogenase-1 levels. J Biol Chem. 2007;282:11038–11046. doi: 10.1074/jbc.M606197200. [DOI] [PubMed] [Google Scholar]
- 30.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 31.Baugh JE, Jr, Floyd ZE, Stephens JM. The modulation of STAT5A/GR complexes during fat cell differentiation and in mature adipocytes. Obesity (Silver Spring) 2007;15:583–590. doi: 10.1038/oby.2007.500. [DOI] [PubMed] [Google Scholar]
- 32.Botella B, Crespo J, Rivas A, et al. Exposure of women to organochlorine pesticides in Southern Spain. Environ Res. 2004;96:34–40. doi: 10.1016/j.envres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U. S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YJ, Ryu HY, Kim HK, et al. Maternal and fetal exposure to bisphenol A in Korea. Reprod Toxicol. 2008;25:413–419. doi: 10.1016/j.reprotox.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 35.Colborn TDD, Meyers JP. Our Stolen Future. New York: Plume; 1996. [Google Scholar]
- 36.Lelli SM, Ceballos NR, Mazzetti MB, Aldonatti CA, San Martín de Viale LC. Hexachlorobenzene as hormonal disruptor–studies about glucocorticoids: their hepatic receptors, adrenal synthesis and plasma levels in relation to impaired gluconeogenesis. Biochem Pharmacol. 2007;73:873–879. doi: 10.1016/j.bcp.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Draper N, Stewart PM. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J Endocrinol. 2005;186:251–271. doi: 10.1677/joe.1.06019. [DOI] [PubMed] [Google Scholar]
- 38.Garbrecht MR, Krozowski ZS, Snyder JM, Schmidt TJ. Reduction of glucocorticoid receptor ligand binding by the 11-beta hydroxysteroid dehydrogenase type 2 inhibitor, Thiram. Steroids. 2006;71:895–901. doi: 10.1016/j.steroids.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Wilding J. Thiazolidinediones, insulin resistance and obesity: Finding a balance. Int J Clin Pract. 2006;60:1272–1280. doi: 10.1111/j.1742-1241.2006.01128.x. [DOI] [PubMed] [Google Scholar]
- 40.Rebuffé-Scrive M, Krotkiewski M, Elfverson J, Björntorp P. Muscle and adipose tissue morphology and metabolism in Cushing’s syndrome. J Clin Endocrinol Metab. 1988;67:1122–1128. doi: 10.1210/jcem-67-6-1122. [DOI] [PubMed] [Google Scholar]