Abstract
Background
Abnormal glucose metabolism is a central feature of disorders with increased rates of cardio-vascular disease (CVD). Low levels of high density lipoprotein (HDL) are a key predictor for CVD. We used genetic mouse models with increased HDL levels (apoA-I tg) and reduced HDL levels (apoA-I ko) to investigate whether HDL modulates mitochondrial bioenergetics in skeletal muscle.
Methods and Results
ApoA-I ko mice exhibited fasting hyperglycemia and impaired glucose tolerance test (GTT) compared to wild type (wt) mice. Mitochondria isolated from gastrocnemius muscle of apoA-I ko mice displayed markedly blunted ATP synthesis. Endurance capacity (EC) during exercise exhaustion test was impaired in apoA-I ko mice. HDL directly enhanced glucose oxidation by increasing glycolysis and mitochondrial respiration rate (OCR) in C2C12 muscle cells. ApoA-I tg mice exhibited lower fasting glucose levels, improved GTT, increased lactate levels, reduced fat mass, associated with protection against age-induced decline of EC compared to wt mice. Circulating levels of fibroblast growth factor 21 (FGF21), a novel biomarker for mitochondrial respiratory chain deficiencies and inhibitor of white adipose lipolysis, were significantly reduced in apoA-I tg mice. Consistent with an increase in glucose utilization of skeletal muscle, genetically increased HDL and apoA-I levels in mice prevented high fat diet-induced impairment of glucose homeostasis.
Conclusions
In view of impaired mitochondrial function and decreased HDL levels in T2D, our findings indicate that HDL-raising therapies may preserve muscle mitochondrial function and address key aspects of T2D beyond CVD.
Keywords: HDL, obesity, exercise, muscular glucose oxidation, mitochondrial bioenergetics
Introduction
Recent years have seen an alarming rise in the incidence of cardiovascular disease (CVD) linked to obesity-related metabolic factors 1. Epidemiological studies have confirmed a strong association between fat intake, plasma cholesterol levels, and CVD mortality rates 2-5. Of particular concern is the incidence of diabetes in obese patients, since diabetes itself carries a substantially elevated CVD risk 6. One of the strongest independent predictors of CVD is a low level of high-density lipoprotein (HDL) particles and their major protein constituent apolipoprotein A-I (apoA-I) 7,8. Besides its critical role in reverse cholesterol transport and cellular cholesterol efflux, apoAI also has anti-inflammatory, antithrombotic, and antioxidant functions that contribute to its well-known anti-atherogenic function 9-12. Low circulating HDL and apoA-I levels are also a hallmark of insulin-resistance, a pathological precondition leading to type 2 diabetes (T2D) 11,13. It remains unclear, however, whether circulating HDL levels exert an effect on insulin resistance and the development of diabetes.
Several lines of evidence suggest that HDL and apoA-I modulate glucose homeostasis: Infusions of reconstituted HDL particles have been shown to reduce circulating glucose levels and increase insulin levels in patients with T2D through insulin-dependent and -independent mechanisms 14. Cell-based assays have confirmed that both HDL components apoA-I and apoA-II increase beta-cell insulin secretion 15. HDL and apoA-I have been also demonstrated to directly enhance glucose uptake in cultured mouse and human skeletal muscle cells, thus confirming an insulin-independent effect of HDL 14,16. Thus, these results show that HDL and apoA-I are enhancing muscular glucose uptake.
In addition to glucose uptake, intracellular glucose metabolism is known to play a pivotal role in the pathogenesis for insulin resistance and diabetes. After entry into the muscle cell, glucose is oxidized through mitochondrial phosphorylation to ATP as an energy source for cellular metabolism. Glucose is also used to generate glycogen and converted into fat (lipogenesis) for storage and later use as energy 17. All three metabolic routes have been shown to contribute to the development of diabetes. Firstly, oxidative phosphorylation and ATP synthesis are impaired in skeletal muscle from relatives of patients with T2D strongly suggesting that defects in mitochondrial oxidative metabolism are a primary cause of insulin resistance 18. Secondly, dysfunctional muscle glycogen synthesis has been reported to play a dominant role in insulin resistance of diabetic patients 19. Consistently, defects in insulin-mediated glucose oxidation, glycogen synthesis and storage have been already revealed in skeletal muscle from individuals with normal glucose tolerance but with peripheral insulin resistance 20 underscoring the importance of intramyocellular glucose metabolism as crucial player in the development of insulin resistance. Lastly, an excessive conversion of glucose into lipid due to defects in mitochondrial function, leads to accumulation of intramyocellular triglycerides, which also has been implicated in insulin resistance in several population studies 21. Although it has been shown that HDL and apoA-I do increase glucose uptake in muscle cells and thus decrease circulating glucose levels, it has not been determined whether HDL and apoA-I also affect intracellular glucose oxidation, glycogen synthesis, and lipogenesis.
In this study, we investigated the requirement for circulating HDL and apoA-I in normal glucose oxidation of skeletal muscle using a genetic loss- and gain of function mouse model. We show that, in the absence of apoA-I, mitochondrial oxidative phosphorylation is reduced in skeletal muscle resulting in increased circulating glucose levels and impaired muscular function. We provide in vitro and in vivo evidence that HDL and apoA-I enhance glycolysis and mitochondrial oxidative phosphorylation rates of glucose. Overexpression of apoA-I in mice resulted in protection against age-induced decline of endurance capacity, against age-induced fat mass gain and against diet-induced hyperglycemia. Improved mitochondrial function in apoA-I tg mice was further confirmed indirectly by the marked reduction of circulating Fibroblast Growth Factor 21 (FGF-21), a novel biomarker for mitochondrial dysfunction. Our findings point to a key role for circulating HDL and apoA-I in regulating skeletal muscle metabolism and highlight a possible target for the treatment of metabolic diseases such as insulin resistance and T2D.
Methods
An expanded online-only Methods and Results section is available in the online-only Data Supplement.
Mice
Age-matched male apoA-I deficient (apoA-I ko), human apoA-I transgenic (apoA-I tg), and control (wt) C57/Bl6J mice (The Jackson Laboratories, Bar Harbor, ME) were housed in specific pathogen–free facilities with a 12-hour light/12-hour dark cycle and were fed basal rodent chow 5058 PicoLab Mouse Diet 20 (LabDiet, Richmond, IN). Mice that underwent the diet-induced obesity study were fed a low fat diet containing 4.8% fat by weight (D12328; Research Diets, New Brunswick, NJ) or a high-fat diet containing 35.8% fat by weight (D12330; Research Diets) for 12 weeks. All experimental procedures conformed to institutional guidelines for animal experiments and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Cincinnati.
Respiration studies in isolated skeletal muscle mitochondria and cultured muscle cells
After sacrifice, gastrocnemius muscles were harvested and mitochondria were isolated immediately as described 22. Muscle mitochondria respiration measurements were made in triplicate by the Seahorse 24XF analyzer (Seahorse Biosciences Inc., North Billerica, MA). For the determination of extracellular acidification rate (ECAR) and mitochondrial oxygen consumption rate (OCR) in the murine skeletal muscle C2C12 cell line (ATCC, Manassas, Virginia), cells were incubated for 4 hours with increasing amounts of human HDL and 4,5 mg/ml glucose utilizing the Seahorse XF24 analyzer as published 23.
Results
Circulating HDL is required for normal glucose homeostasis
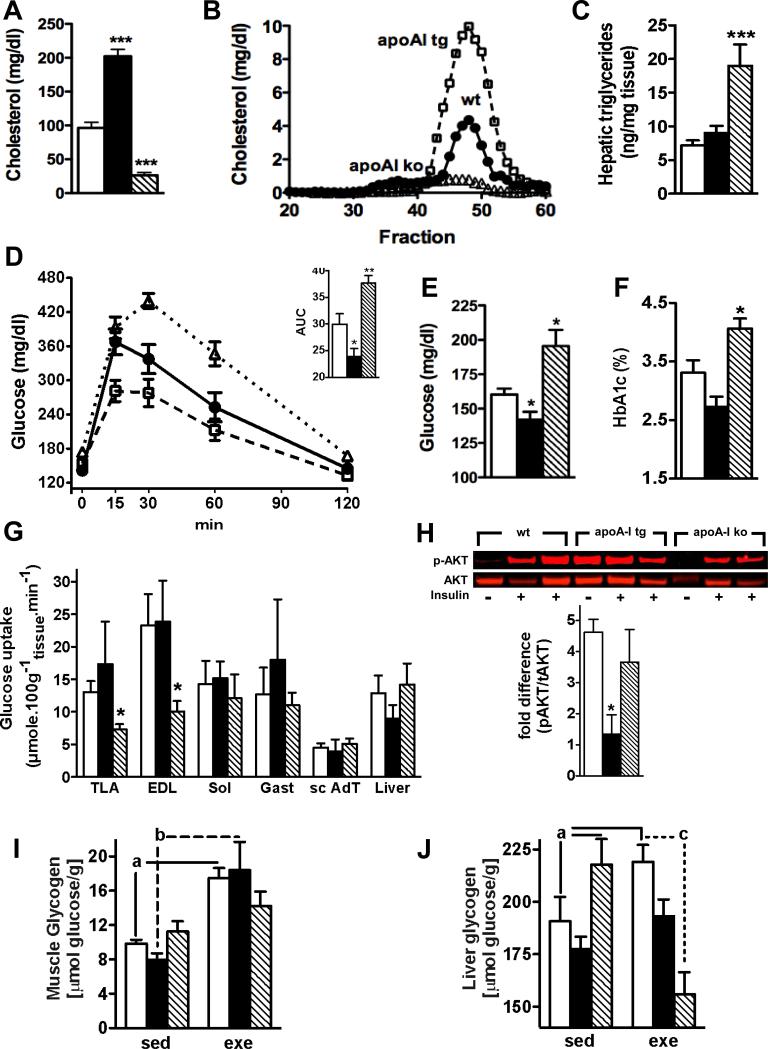
To understand the role of circulating HDL levels in glucose homeostasis, we used a genetic loss- and gain-of-function mouse model for apoA-I, the main protein component of HDL. FPLC analysis of apolipoprotein profiles revealed a severely reduced HDL particle concentration in apoA-I deficient mice (apoA-I ko) and a markedly increased HDL particle concentration in human apoA-I overexpressing (apoA-I tg) mice compared to wild-type (wt) controls (Figure 1A & B). ApoA-I ko mice exhibited increased hepatic triglyceride content compared to wt and apoA-I tg mice (Figure 1C). We detected markedly higher fasting glucose and HbA1c levels in chow-fed apoA-I ko mice compared to wt littermates (Figure 1E and F). In contrast, apoA-I tg mice had significantly lower fasting glucose levels compared to wt littermates (Figure 1E). Response to intraperitoneal glucose tolerance test (GTT) was impaired in apoA-I ko and improved in apoA-I tg mice compared to wt littermates (Figure 1D). These results indicate that circulating HDL levels are important for the efficient clearance of glucose from the circulation.
Figure 1.
HDL modulates whole body glucose homeostasis through an direct effect on glycolytic muscle fibers: Fasting cholesterol (A) levels, lipoprotein profiles (B, HDL = fraction 40-53), hepatic triglyceride levels (C), glucose tolerance tests (D), fasting glucose levels (E) and HbA1c levels (F) (n=6-15 per goup); deoxyglucose uptake (G) in tibialis lateralis anterior (TLA) muscle, extensor digitorum longus (EDL) muscle, soleus (SOL) muscle , gastrocnemius (GAST) muscle , subcutaneous adipose tissue (ScAT), and liver under hyperinsulinemic-euglycemic clamp conditions (n = 6-8 per group); basal and insulin-induced AKT phosphorylation in quadriceps muscle (H, n = 2-3 per group), glycogen content in quadriceps (I) and liver (J) of chow fed and age-matched wt (open bars, filled circles), apoA-I tg (filled bars; open squares) and apoA-I ko mice (hatched bars; open triangles) (n=6-14 per group). AUC = area under the curve, sed = sedentary, exe = exercised. Data are expressed as means ± SEM. *P < 0.05; **P < 0.005; ***P < 0.0005 vs. wt mice and a = P < 0.05 vs. sedentary wt mice, b = P < 0.05 vs. sedentary apoA-I tg mice, c = P < 0.001 vs. exercised apoA-I ko mice.
To find out which tissue is responsible for HDL-mediated improvement of glucose tolerance and whether HDL modulates basal or insulin-mediated uptake of glucose, we determined deoxyglucose uptake under euglycemic-hyperinsulinemic clamp (HEC) conditions (Figure 1G and supplemental Figure 1S). This analysis revealed a significant decrease of glucose transport in muscles with a high content of glycolytic (white) fibers IIB like the tibialis lateralis anterior (TLA) and the extensor digitorum longus (EDL) muscle of apoA-I ko mice compared to wt and apoA-I tg mice. However, no difference was observed in muscles with a high content of oxidative (red) fibers I and IIA, like the soleus (SOL) or in muscles with a mixed content of IIA and IIB fibers like the gastrocnemius muscle. Since white fibers are far less responsive to insulin than red fibers due to markedly lower protein levels of glucose transporter 4 (GLUT4), the rate-limiting transporter for insulin-mediated glucose uptake24-26, we conclude that apoA-I is modulating glucose transport in muscle directly and independently of insulin. Similar glucose transport rates in fat and liver of wt, apoA-I tg and ko mice rule out these tissues as potential mediators for the HDL-mediated effect on glucose metabolism and point to a muscle-specific effect of HDL (Figure 1G). Assessment of AKT activation after acute insulin injection as a downstream target for insulin signaling revealed that Insulin increased AKT phosphorylation in quadriceps muscle of wt and apoA-I ko mice to the same extent (Figure 1H). In contrast, apoA-I tg mice showed markedly enhanced basal AKT phosphorylation (2.7 ± 1 fold over wt and 2.4 ± 0.2 fold over apoA-I ko mice) with no further increase after insulin injection (Figure 1H). These results also point to an insulin-independent effect of HDL on skeletal muscle.
Glycogen levels are an important determinant of exercise capacity 27 and defects in glycogen synthesis have been shown to play a dominant role in the development of insulin resistance 19. To understand whether circulating HDL levels are also modulating intracellular glycogen storage, we assessed glycogen levels in skeletal muscle of sedentary and exercised wt, apoA-I tg and apoA-I ko mice. Although muscle glycogen levels were similar between sedentary groups, exercised apoA-I ko mice failed to increase glycogen levels to the same extent as wt and apoA-I tg mice (Figure 1I). These results suggest that in absence of apoA-I metabolic adaptations to exercise are hampered in skeletal muscle. Since liver glycogen is quantitatively more important than mucle glycogen for endurance capacity in rodents27-30 we also determined hepatic glycogen content in our mouse groups: Sedentary apoA-I ko mice exhibited markedly increased glycogen levels compared to wt mice. However, upon training glycogen was severely reduced in apoA-I ko mice while it increased in exercised wt and apoA-I tg mice (Figure 1J). Our results indicate that glycogen metabolism is dysfunctional in the absence of apoA-I.
HDL modulates muscle function through an effect on mitochondrial bioenergetics
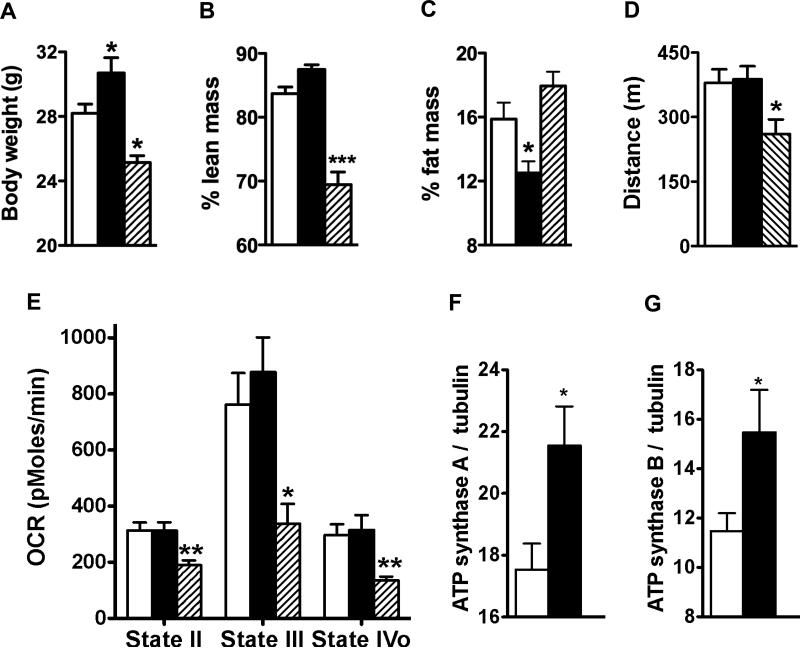
We characterized body composition and muscle function in our gain- and loss-of-function models. Lean mass was significantly decreased in apoA-I ko mice (Figure 2 A & B) compared to wt littermates and, conversely, slightly increased in apoA-I tg mice. Since loss of lean mass typically reflects altered muscle biology, we next tested for differences in muscular function by determining oxidative capacity of skeletal muscle in endurance tests using mouse treadmills. We found that reduced circulating HDL in apoA-I ko mice correlated with considerably decreased endurance capacity (Figure 2D). Our results suggest that circulating HDL and apoA-I play an important role in normal skeletal muscle metabolism and function.
Figure 2.
Normal HDL levels are required for proper function of skeletal mitochondria: (A-C) Body composition, (D) distance covered during treadmill exercise to exhaustion test (n = 15 per group); (E) oxygen consumption rate (OCR) in mitochondria isolated from gastrocnemius muscle in basal state (State II), after addition of ADP (State III), and after addition of of the ATP synthase inhibitor oligomycin (State IVo); and protein expression of ATP synthase subunit A and B (F,G) in gastrocnemius muscle homogenates of chow fed wt (open bars), apoA-I tg (filled bars) and apoA-I ko mice (hatched bars) (n = 4 per group). Data are expressed as means ± SEM *P < 0.05; **P < 0.005, ***P < 0.0005 vs. wt mice.
To understand whether HDL and apoA-I can alter mitochondrial function in skeletal muscle in vivo, we analyzed mitochondrial bioenergetics using Seahorse analyzer. In the absence of apoA-I, oxygen consumption rate (OCR) was markedly reduced and ATP synthesis was clearly blunted in mitochondria isolated from gastrocnemius muscle (Figure 2E). Elevated HDL was associated with a moderate increase in ATP synthesis (Figure 2E). This small enhancement of mitochondrial ATP synthesis was associated with increases in expression of the ATP synthase alfa and beta subunits in muscle homogenate of apoA-I tg mice compared to wt mice (Figure 2F & G). In contrast, no differences in OCR were detected in mitochondria isolated from liver between the different genotypes (supplemental Figure 2S). These results provide evidence that circulating HDL is required for normal mitochondrial function in skeletal muscle.
HDL and apoA-I directly enhance cellular respiration of glucose in skeletal muscle and prevent age-induced decline of endurance capacity
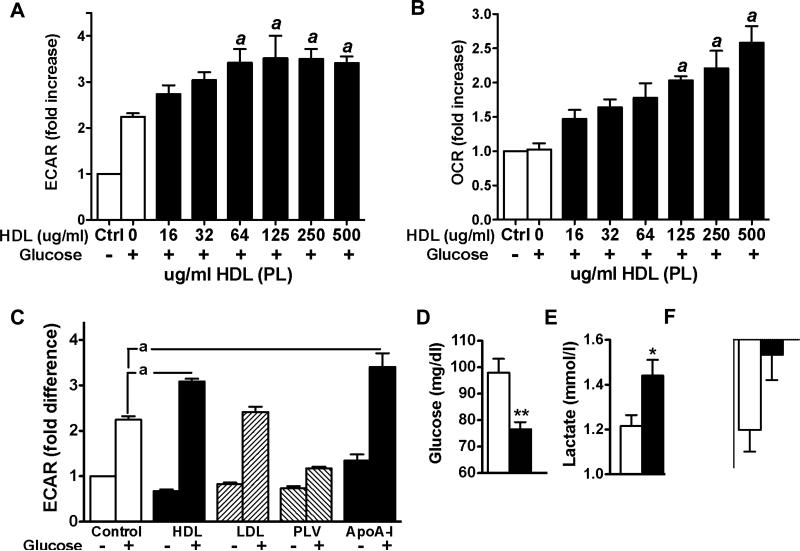
To address whether the observed effect of HDL on mitochondrial bioenergetics occurs cell autonomously in skeletal muscle, we applied cell-based in vitro assays. Using Seahorse XF24 analyzer we detected that HDL isolated from human subjects increases glycolysis and OCR in murine C2C12 myoblasts in a dose dependent manner (Figure 3A and B). Subsequently we determined that HDL and apoA-I, but not LDL or phospholipid vesicles, enhanced glycolysis considerably (Figure 3C). Our results indicate that HDL and apoA-I directly affect the breakdown of glucose by increasing both components of cellular respiration, glycolysis and mitochondrial oxidative phosphorylation. Thus, the observed effects on cellular respiration are direct and cell autonomous, and are specific to HDL and apoA-I.
Figure 3.
HDL and apoA-I enhance cellular respiration of glucose in skeletal muscle and prevent age-induced decline of endurance capacity: (A and B) Extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) in C2C12 myoblasts incubated with glucose (5 mmol/L; open bars) and increasing amounts of HDL (closed bars). (C) ECAR in C2C12 myoblasts incubated with glucose (5 mmol/L, open bar), glucose and HDL (100 ug/ml; closed bars), LDL (100 ug/ml; hatched bars), phospholipid vesicles (100 ug/ml; hatched bars), and apoA-I (same protein concentration as HDL) (n= 7-12 per group). Data in A, B, and C are expressed as means ± SEM. a = P < 0.05, vs. cells incubated with glucose only. (D) fasting glucose, (E) fasting lactate, and (F) change of distance covered during treadmill exercise exhaustion tests performed 8 weeks apart in aging wt (open bars) and apoA-I transgenic mice (closed bars) (n = 15 per group). Data in D, E, and F are expressed as means ± SEM *P < 0.05; **P < 0.005 vs. wt mice.
Our observations that normal circulating HDL levels are required for proper skeletal muscle function raised the question whether increasing HDL levels above normal circulating HDL concentrations further improve skeletal muscle glucose utilization and muscle function in vivo. We therefore analyzed fasting glucose and lactate levels in mice with normal (wt) and genetically raised HDL levels (apoA-I tg). Consistent with an increase in glucose utilization, we found that physiologically relevant increases in HDL levels correlated strongly with reduced fasting glucose levels compared to wt mice (Fig. 3D). HDL-induced increases in glycolysis were reflected by higher circulating lactate levels (Fig. 3E). To address the question whether raising HDL levels may further improve muscular function we investigated whether age-induced decline in muscle performance is prevented in apoA-I tg mice. Endurance capacity was better maintained in aging apoA-I tg mice than wt mice (Figure 3F). Thus, HDL and apoA-I appear to play a role in the preservation of muscle function during aging by directly enhancing glucose utilization in skeletal muscle.
Raising HDL levels decreases fat mass in association with reduced circulating FGF21 levels and enhanced FFA release from white adipose tissue
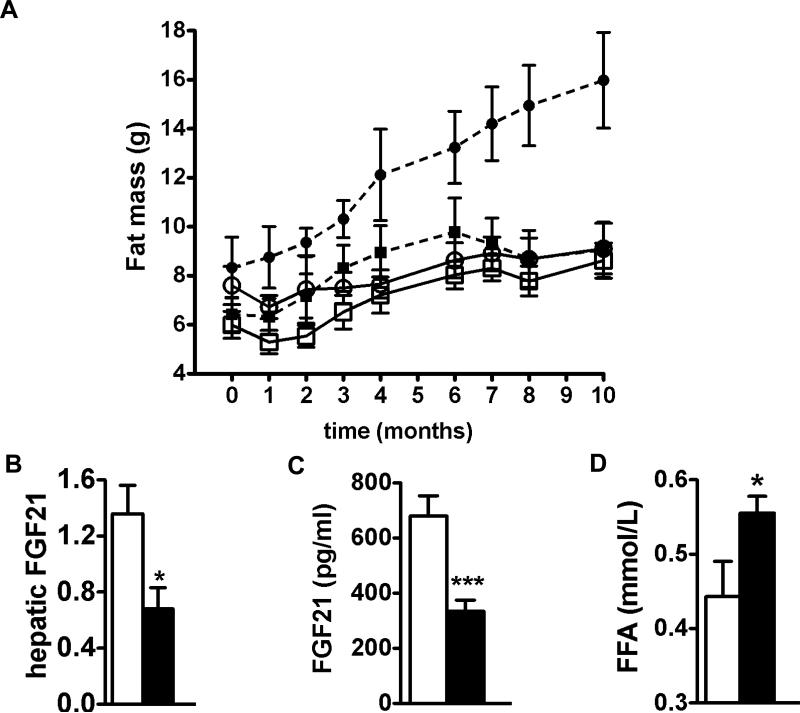
To address whether HDL-enhanced muscular metabolism also results in a leaner phenotype, we monitored body composition of apoA-I tg and wt mice for 10 months: Age-induced increases in fat mass did not occur in apoA-I tg mice. Sedentary apoA-I tg mice retained a remarkably stable fat mass throughout their life span (Figure 4A). To understand whether the observed difference in fat mass is similar to that induced by chronic physical activity, we compared fat mass of sedentary apoA-I tg mice to that of apoA-I tg and wt mice subjected to daily aerobic exercise training throughout the study. As shown in Figure 4A, sedentary apoAI tg mice exhibit fat mass values close to the levels of running mice. These results suggest that raising HDL levels under sedentary conditions may lead to a leaner phenotype that seems metabolically more similar to a physical active condition.
Figure 4.
Raising HDL levels decreases body fat mass in association with reduced circulating FGF21 levels and enhanced FFA release from white adipose tissue. (A) Fat mass of sedentary (dotted lines) and treadmill exercised (continuous lines) wt (circles) and apoA-I tg mice (squares) on chow. (B), Fasting hepatic FGF21 expression levels, (C) fasting circulating FGF21 levels, and (D) fasting FFA levels of sedentary wt (open bars) and apoA-I tg mice (closed bars) (n = 6-7 per group). Data are expressed as means ± SEM *P < 0.05; ***P < 0.0005 vs. wt mice.
Fibroblast growth factor 21 (FGF21), a novel biomarker for mitochondrial deficiencies 31, is increased in patients with the metabolic syndrome and correlates inversely with circulating HDL levels in humans and monkeys 32,33. We measured FGF21 levels in sedentary apoA-I tg and wt mice as an additional indicator of improved mitochondrial function. Both, hepatic expression and circulating levels of FGF21, were significantly lower in apoA-I tg mice compared to wt mice (Figure 4B & C). We propose that enhanced muscular mitochondrial function in apoA-I tg mice is reflected by lower circulating FGF21 levels. FGF21 is also known to inhibit lipolysis in white adipose tissue 34,35. To understand whether the reduction of fat mass may be partly due to an increase in fatty acid release from white adipose tissue we analyzed fasting free fatty acid (FFA). As shown in Figure 4D, we found that FFA levels were increased in apoA-I tg mice compared to wt mice, indicating that lipolysis in white adipose tissue is enhanced. Consequently, FGF21-mediated inhibition of lipolysis may be reduced in apoA-I tg mice.
Raising HDL protects against diet-induced hyperglycemia through increased glucose utilization
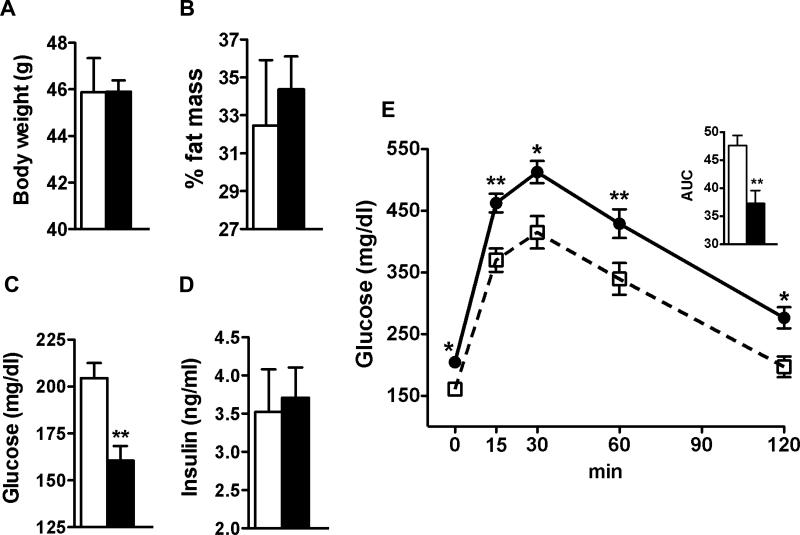
To investigate the therapeutic potential of HDL in metabolic disease, we fed age-matched male apoA-I tg and wt mice HFD for 12 weeks and analyzed body composition and whole body glucose homeostasis. Although raising circulating HDL levels did not protect against diet-induced obesity (Figure 5A & B), fasting glucose levels were significantly lower in apoA-I tg mice compared to wt mice throughout the HFD feeding study (Figure 5C). This difference was even increased compared to the difference observed when mice were fed chow diet (data not shown). HbA1c levels were slightly reduced in apoA-I tg mice compared to wt mice (3.8 ± 0.2 vs 4.2 ± 0.08 %, n = 8). Mice of either group developed fasting hyperinsulinemia to the same extent (Figure 5D). However, GTT revealed that raising HDL levels improves the development of diet-induced impairment of glucose homeostasis (Figure 5E) independently of body-weight gain and diet-induced hyperinsulinemia.
Figure 5.
Raising HDL protects against diet-induced hyperglycemia through increased glucose utilization. (A &B) body composition, fasting glucose (C) and fasting insulin (D) levels of diet-induced obese wt (open bars) and apoA-I tg (closed bars) mice. (E) glucose tolerance test in diet-induced obese wt (closed circles) and apoA-I tg mice (open quadrants) (n = 7-8 per group). AUC = area under the curve. Data are expressed as means ± SEM *P < 0.05; **P < 0.005, ***P < 0.0005 vs. wt mice.
Discussion
The present studies establish that circulating HDL is required for normal glucose homeostasis in skeletal muscle. We show that apoA-I directly increases glucose utilization by enhancing cellular respiration in skeletal muscle cells. Furthermore, increased apoA-I, and presumably increased HDL levels lead to reduced body fat mass and enhanced FFA release by white adipose tissue via a mechanism that involves reduction of circulating FGF21 levels. Taken together, these findings establish a key role for circulating apoA-I in maintaining muscle metabolism and function and suggest a novel role for HDL or some of its subspecies in the prevention and treatment of metabolic diseases.
Our finding that HDL and apoA-I directly enhance glucose oxidation in the skeletal muscle cell provides additional support for the hypothesis that HDL and apoA-I exert intracellular functions independent of their lipid transport function. Previously, Drew et al 14 reported that the actions of HDL and apoA-I on glucose uptake occur through a receptor-mediated event involving ABCA1, an ABC class of transporter. The authors suggested that, in addition to mediating reverse cholesterol transport, HDL also initiates a calcium-sensitive signaling cascade through ABCA1. Consistent with those findings, Han et al. 16 showed that selectively extracting cholesterol from the plasma membrane using methyl beta-cyclodextrin had no effect on AMPK phosphorylation in C2C12 myotubes, indicating that cholesterol efflux itself does not affect AMPK phosphorylation in skeletal muscle. However, HDL and apoA-I may also mediate their effect on glucose uptake through alternative pathways to ABCA1 signaling. Accordingly, Han and colleagues provided in vitro evidence that endocytosis of apoA-I through the clathrin-dependent pathway is required for apoA-I-mediated AMPK and ACC activation 16. HDL has been also shown to transport and deliver endogenous microRNAs to recipient cells with functional gene regulatory consequences 36. ApoA-I may also co-localize with lipid droplets and mitochondria within the skeletal muscle cell 37, further strengthening the hypothesis that apoA-I acts intracellularly besides through its known surface receptors. Thus, although there is some evidence that HDL may mediate its effects on glucose uptake through one of its known receptors, it seems obvious that alternative and not yet determined underlying mechanisms are involved.
Our present study provides in vivo and in vitro evidence that HDL and apoA-I increase glucose oxidation, thus giving insight into how the cell handles HDL-enhanced glucose entry downstream of AMPK activation. Our finding that skeletal muscle ATP synthesis is severely blunted in the absence of apoA-I ex vivo in association with an impairment of endurance capacity highlights for the first time the importance of circulating HDL in normal muscle cell metabolism. Drew and colleagues previously showed that palmitate oxidation is increased in human skeletal muscle cells incubated with HDL or apoA-I 14. Their results complement ours by determining the effect of HDL-mediated AMPK activation on fatty acid utilization and, taken together, give a more detailed view of HDL-induced intracellular metabolic pathways. There is recent evidence that HDL particles also increase glycogen synthesis in the rat skeletal muscle cell line L6 38 after an overnight starvation period. In contrast, we did not detect differences in glycogen content in muscle of apoA-I ko or apoA-I tg mice compared to wt mice. We think that this discrepancy may arise due to different experimental conditions. Our animals experiments did not include a fasting period and may therefore better portray the state of normal intracellular glycogen content.
Our in vitro results determining that glycolysis rates in skeletal muscle are markedly increased by HDL and apoA-I were confirmed by our in vivo findings that fasting lactate levels were significantly higher in mice with genetically raised HDL and apoA-I levels. One explanation for this observation is that at the point when glycogen stores in skeletal muscle have been replenished, the glucose taken up is converted into lactate to maintain enhanced glucose utilization. The lactate released by skeletal muscle is taken up by the liver and converted into glycogen by the so-called “indirect pathway of glycogen synthesis” to preserve this energy for the future 20. Our findings that lactate levels are increased in apoA-I tg mice suggest that this may enhance the Cori cycle and allow lactate released by skeletal muscle to be used for oxidation but also for anabolic purposes by all tissues. According to this hypothesis, the lactate taken up would then be converted to pyruvate, a precursor for acetyl-CoA, which has many important anabolic functions 20. In light of our observation that apoA-I tg mice exhibit a lower age-induced decline in muscle performance compared to age-matched wt mice, we conclude that the endurance capacity may be better preserved partially through an increased flux through the Cori cycle in addition to the enhancement in glucose oxidation. Simultaneously, HDL particles also increase glucose uptake in cultured adipocytes via a mechanism involving GLUT 4 translocation and AMPK activation 38. Since adipose tissue releases significant amounts of lactate 39, our observations that fasting lactate levels were increased and fat mass was reduced in apoA-I tg mice may be in part due to adipocytes not completely utilizing the glucose taken up, but instead releasing it as lactate which then serves as a substrate in the Cori cycle for anabolic processes in other tissues. This conclusion is supported by a leaner body composition of apoA-I tg mice. In contrast to data provided by Ruan et al 40, we show that apoA-I tg mice are not protected against diet-induced obesity. Although the initial difference in fat mass disappeared after 12 weeks on HFD, apoA-I tg mice exhibited markedly lower glucose levels and better glucose tolerance than wt mice, indicating that raising HDL levels may offer a potential therapeutic option for obese and insulin-resistant patients who do not respond to weight-reducing measures.
Our studies furthermore may provide some intriguing insights into the inverse relationship of circulating HDL and FGF21 levels. A recent multi-center study showed that measuring circulating FGF21 concentrations reliably identified primary respiratory chain deficiencies in skeletal muscle of humans. The authors report that circulating FGF21 levels are about 10-fold increased in patients with mitochondrial disorders compared to healthy individuals and their data suggest that FGF21 induction is triggered by primary respiratory chain deficiencies 31. Based on our findings presented herein we propose that FGF21 expression has been down-regulated by the improved mitochondrial function in apoA-I tg mice. FGF21 is also known to function as an inhibitor of lipolysis in mice, monkeys and human subjects 32-35,41. FGF21 is mainly expressed in the liver and thymus, and once secreted into circulation, it exerts its effects on adipose tissue like an endocrine hormone by binding its receptor β-klotho 34. Thus, our finding that FGF21 levels are reduced in apoA-I tg mice may point to an additional novel mechanism by which genetically raised HDL modulates lipolysis.
A number of intervention studies have shown that acute and long-term physical exercise have a clear beneficial effect on circulating HDL levels and on glucose tolerance in healthy subjects and in elderly, overweight and dyslipidemic patients 42-44. Putting our findings into perspective, we propose that, besides its direct effects on glucose tolerance, physical activity may also enhance glucose oxidation indirectly by increasing HDL levels. Our rationale is supported by the finding that physical activity-induced increases in HDL levels correlate strongly with the up-regulation of gene expression sets for glycolysis and oxidative phosphorylation in physical active vs. inactive co-twins in a recently published Gene Set Enrichment Analysis 45. Although the underlying mechanisms for the association between up-regulated skeletal muscle metabolic pathways and high circulating HDL levels in physical active subjects are largely unknown, it seems plausible that current therapeutic approaches to raise HDL levels may partly mimic exercise-mediated effects on glucose homeostasis.
In conclusion, our studies show strong evidence that HDL and apoA-I are required for normal glucose homeostasis and muscle mitochondrial function. We provide evidence that the key HDL component apoA-I directly increases glucose utilization by enhancing cellular respiration in skeletal muscle cells. Furthermore, we demonstrate that genetically raising HDL levels leads to a reduction in fat mass in association with a decrease in circulating FGF21 levels. Since low and dysfunctional HDL might contribute to the exacerbation of insulin resistance, therapeutic approaches to raise HDL levels and improve HDL function may not only benefit patients with cardiovascular disease but also improve metabolic diseases such as insulin resistance and T2D.
Supplementary Material
Acknowledgements
We thank the Cincinnati Mouse Metabolic Phenotyping Center NIH U24DK59630 for providing the treadmill equipment in our endurance exercise experiments. We are grateful for the statistical advice provided by Gabriele Kastenmüller at the Helmholtz Zentrum München.
Funding Sources: This work was supported by the basic science grant 1-10-BS-72 from the American Diabetes Association, the National Institutes of Health Grant 2K12HD051953-06 granted to Susanna M. Hofmann, and in part by the Helmholtz Alliance ICEMED - Imaging and Curing Environmental Metabolic Diseases, through the Initiative and Networking Fund of the Helmholtz Association. Maarit Lehti has been supported by the Academy of Finland.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Samper-Ternent R, Snih Al S. Obesity in Older Adults: Epidemiology and Implications for Disability and Disease. Rev Clin Gerontol. 2012;22:10–34. doi: 10.1017/s0959259811000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rees K, Dyakova M, Ward K, Thorogood M, Brunner E. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst Rev. 2013;3:CD002128. doi: 10.1002/14651858.CD002128.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frohlich J, Al-Sarraf A. Cardiovascular risk and atherosclerosis prevention. Cardiovasc. Pathol. 2013;22:16–18. doi: 10.1016/j.carpath.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore HJ, Davey Smith G. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev. 2012;5:CD002137. doi: 10.1002/14651858.CD002137.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ooi EMM, Ng TWK, Watts GF, Barrett PHR. Dietary fatty acids and lipoprotein metabolism: new insights and updates. Curr Opin Lipidol. 2013;24:192–197. doi: 10.1097/MOL.0b013e3283613ba2. [DOI] [PubMed] [Google Scholar]
- 6.Howard BV, Taskinen M-R. CVD in women. Nutr Metab Cardiovasc Dis. 2010;20:377–378. doi: 10.1016/j.numecd.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Boden WE. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High--Density Lipoprotein Intervention Trial. Am J Cardiol. 2000;86:19L–22L. doi: 10.1016/s0002-9149(00)01464-8. [DOI] [PubMed] [Google Scholar]
- 8.McNeill AM, Katz R, Girman CJ, Rosamond WD, Wagenknecht LE, Barzilay JI, Tracy RP, Savage PJ, Jackson SA. Metabolic syndrome and cardiovascular disease in older people: The cardiovascular health study. J Am Geriatr Soc. 2006;54:1317–1324. doi: 10.1111/j.1532-5415.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 9.Gordon SM, Hofmann S, Askew DS, Davidson WS. High density lipoprotein: it's not just about lipid transport anymore. Trends Endocrinol Metab. 2011;22:9–15. doi: 10.1016/j.tem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rader DJ, Tall AR. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat Med. 2012;18:1344–1346. doi: 10.1038/nm.2937. [DOI] [PubMed] [Google Scholar]
- 11.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 12.Larach DB, deGoma EM, Rader DJ. Targeting high density lipoproteins in the prevention of cardiovascular disease? Curr Cardiol Rep. 2012;14:684–691. doi: 10.1007/s11886-012-0317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 14.Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, Thomas WG, Mukhamedova N, de Courten B, Forbes JM, Yap FY, Kaye DM, van Hall G, Febbraio MA, Kemp BE, Sviridov D, Steinberg GR, Kingwell BA. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119:2103–2111. doi: 10.1161/CIRCULATIONAHA.108.843219. [DOI] [PubMed] [Google Scholar]
- 15.Fryirs MA, Barter PJ, Appavoo M, Tuch BE, Tabet F, Heather AK, Rye K-A. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler Thromb Vasc Biol. 2010;30:1642–1648. doi: 10.1161/ATVBAHA.110.207373. [DOI] [PubMed] [Google Scholar]
- 16.Han R, Lai R, Ding Q, Wang Z, Luo X, Zhang Y, Cui G, He J, Liu W, Chen Y. Apolipoprotein A-I stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia. 2007;50:1960–1968. doi: 10.1007/s00125-007-0752-7. [DOI] [PubMed] [Google Scholar]
- 17.McArdle WD, Katch FI, Katch VL. Exercise physiology: Nutrition, energy, and human performance. Wolters Kluwer Health. 2009 [Google Scholar]
- 18.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 20.LeRoith D, Olefsky JM, Taylor SI. Diabetes Mellitus: A Fundamental and Clinical Text. Google Books; 2003. [Google Scholar]
- 21.Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrinol Metab. 2012;23:391–398. doi: 10.1016/j.tem.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messer JI, Jackman MR, Willis WT. Pyruvate and citric acid cycle carbon requirements in isolated skeletal muscle mitochondria. Am. J. Physiol., Cell Physiol. 2004;286:C565–72. doi: 10.1152/ajpcell.00146.2003. [DOI] [PubMed] [Google Scholar]
- 23.Costanzo-Garvey DL, Pfluger PT, Dougherty MK, Stock JL, Boehm M, Chaika O, Fernandez MR, Fisher K, Kortum RL, Hong E-G, Jun JY, Ko HJ, Schreiner A, Volle DJ, Treece T, Swift AL, Winer M, Chen D, Wu M, Leon LR, Shaw AS, McNeish J, Kim JK, Morrison DK, Tschöp MH, Lewis RE. KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity. Cell Metab. 2009;10:366–378. doi: 10.1016/j.cmet.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kern M, Wells JA, Stephens JM, Elton CW, Friedman JE, Tapscott EB, Pekala PH, Dohm GL. Insulin responsiveness in skeletal muscle is determined by glucose transporter (Glut4) protein level. Biochem J. 1990;270:397–400. doi: 10.1042/bj2700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James DE, Jenkins AB, Kraegen EW. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol. 1985;248:E567–74. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- 26.Shortreed KE, Krause MP, Huang JH, Dhanani D, Moradi J, Ceddia RB, Hawke TJ. Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS ONE. 2009;4:e7293. doi: 10.1371/journal.pone.0007293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pederson BA. Exercise Capacity of Mice Genetically Lacking Muscle Glycogen Synthase: IN MICE, MUSCLE GLYCOGEN IS NOT ESSENTIAL FOR EXERCISE. J Biol Chem. 2005;280:17260–17265. doi: 10.1074/jbc.M410448200. [DOI] [PubMed] [Google Scholar]
- 28.Terjung RL, Baldwin KM, Molé PA, Klinkerfuss GH, Holloszy JO. Effect of running to exhaustion on skeletal muscle mitochondria: a biochemical study. Am J Physiol. 1972;223:549–554. doi: 10.1152/ajplegacy.1972.223.3.549. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin KM, Reitman JS, Terjung RL, Winder WW, Holloszy JO. Substrate depletion in different types of muscle and in liver during prolonged running. Am J Physiol. 1973;225:1045–1050. doi: 10.1152/ajplegacy.1973.225.5.1045. [DOI] [PubMed] [Google Scholar]
- 30.Reitman J, Baldwin KM, Holloszy JO. Intramuscular triglyceride utilization by red, white, and intermediate skeletal muscle and heart during exhausting exercise. Proc Soc Exp Biol Med. 1973;142:628–631. doi: 10.3181/00379727-142-37081. [DOI] [PubMed] [Google Scholar]
- 31.Suomalainen A, Elo JM, Pietiläinen KH, Hakonen AH, Sevastianova K, Korpela M, Isohanni P, Marjavaara SK, Tyni T, Kiuru-Enari S, Pihko H, Darin N, Õunap K, Kluijtmans LAJ, Paetau A, Buzkova J, Bindoff LA, Annunen-Rasila J, Uusimaa J, Rissanen A, Yki-Järvinen H, Hirano M, Tulinius M, Smeitink J, Tyynismaa H. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 2011;10:806–818. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hojman P, Pedersen M, Nielsen AR, Krogh-Madsen R, Yfanti C, Akerstrom T, Nielsen S, Pedersen BK. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009;58:2797–2801. doi: 10.2337/db09-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kharitonenkov A, Wroblewski VJ, Koester A, Chen Y-F, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 34.Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M. FGF21 attenuates lipolysis in human adipocytes - a possible link to improved insulin sensitivity. FEBS Lett. 2008;582:1725–1730. doi: 10.1016/j.febslet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 35.Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150:4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- 36.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Wang Y, Li J, Yu J, Pu J, Li L, Zhang H, Zhang S, Peng G, Yang F, Liu P. Proteome of skeletal muscle lipid droplet reveals association with mitochondria and apolipoprotein a-I. J Proteome Res. 2011;10:4757–4768. doi: 10.1021/pr200553c. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Zhang Y, Feng H, Guo R, Jin L, Wan R, Wang L, Chen C, Li S. High density lipoprotein (HDL) promotes glucose uptake in adipocytes and glycogen synthesis in muscle cells. PLoS ONE. 2011;6:e23556. doi: 10.1371/journal.pone.0023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thacker SV, Nickel M, DiGirolamo M. Effects of food restriction on lactate production from glucose by rat adipocytes. Am J Physiol. 1987;253:E336–42. doi: 10.1152/ajpendo.1987.253.4.E336. [DOI] [PubMed] [Google Scholar]
- 40.Ruan X, Li Z, Zhang Y, Yang L, Pan Y, Wang Z, Feng G-S, Chen Y. Apolipoprotein A-I possesses an anti-obesity effect associated with increase of energy expenditure and up-regulation of UCP1 in brown fat. J Cell Mol Med. 2011;15:763–772. doi: 10.1111/j.1582-4934.2010.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Ge H, Weiszmann J, Hecht R, Li Y-S, Véniant MM, Xu J, Wu X, Lindberg R, Li Y. Inhibition of lipolysis may contribute to the acute regulation of plasma FFA and glucose by FGF21 in ob/ob mice. FEBS Lett. 2009;583:3230–3234. doi: 10.1016/j.febslet.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Nestel PJ, Podkolinski M, Fidge NH. Marked increase in high density lipoproteins in mountaineers. Atherosclerosis. 1979;34:193–196. doi: 10.1016/0021-9150(79)90139-4. [DOI] [PubMed] [Google Scholar]
- 43.Olchawa B, Kingwell BA, Hoang A, Schneider L, Miyazaki O, Nestel P, Sviridov D. Physical fitness and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2004;24:1087–1091. doi: 10.1161/01.ATV.0000128124.72935.0f. [DOI] [PubMed] [Google Scholar]
- 44.Sviridov D, Kingwell B, Hoang A, Dart A, Nestel P. Single session exercise stimulates formation of pre beta 1-HDL in leg muscle. J Lipid Res. 2003;44:522–526. doi: 10.1194/jlr.M200436-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Kujala UM, Mäkinen V-P, Heinonen I, Soininen P, Kangas AJ, Leskinen TH, Rahkila P, Würtz P, Kovanen V, Cheng S, Sipilä S, Hirvensalo M, Telama R, Tammelin T, Savolainen MJ, Pouta A, O'Reilly PF, Mäntyselkä P, Viikari J, Kähönen M, Lehtimäki T, Elliott P, Vanhala MJ, Raitakari OT, Järvelin M-R, Kaprio J, Kainulainen H, Ala-Korpela M. Long-Term Leisure-Time Physical Activity and Serum Metabolome. Circulation. 2013;127:340–348. doi: 10.1161/CIRCULATIONAHA.112.105551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.