Abstract
Background:
In this study, the aqueous extract of Anethum graveolens (dill) leaves was studied for its effects on treating convulsions and epilepsy, by using a pentylenetetrazole (PTZ) kindling model. The evaluated plant has a traditional medical reputation for profound anticonvulsant activities, additionally, dill has been claimed to exhibit anti-inflammatory and analgesic properties.
Methods:
For the PTZ kindling induction, mice were given a dose of PTZ (37 mg/kg, intraperitoneally) every other day, and seizure stages were precisely recorded. During and after kindling, the effects of the non-toxic doses of the aqueous extracts (100, 250, and 400 mg/kg) on seizure latency in stage 2 (S2L), seizure latency in stage 4 (S4L), and seizure duration in stage 5 (S5D) were measured.
Results:
The aqueous extract of dill leaves had a noticeable anticonvulsant effect. The 400 mg/kg dose of the extract sample decreased with S5D (P < 0.05), and increased with S2L and S4L significantly (P < 0.05 and P < 0.01, respectively).
Conclusion:
The obtained data shows that the aqueous extract possesses anticonvulsant activity against seizure induced by PTZ. The presence of anticonvulsant compounds in this medicinal herb suggests further activity and guided fractionation studies in order to introduce this plant as a valuable source of anticonvulsant agents.
Keywords: Anethum graveolens, anticonvulsant, kindling, mice, pentylenetetrazole
Introduction
Epilepsy, a common neurological disorder, is characterized by unpredictable and episodic seizure, and researchers from all over the world have been studying many areas of this disorder. Experimental models of epilepsy have been developed to study the basic mechanisms of epileptic seizures and identify new therapeutic approaches. Kindling is one of the most widely used models for the induction of increased convulsions in animals (1). In kindling, the repetitive and intermittent administration of electrical or chemical subconvulsants such as pentylenetetrazol (PTZ) leads to the development of marked tonic-clonic convulsions. Electrical kindling is regarded as a model of complex partial epilepsy (1), and the chemical kindling induced by the convulsant PTZ represents a model of primary generalized epilepsy (2).
Roughly, approximately one percent of the world’s population is afflicted with this neurological disorder (3); however, approximately 40% of all patients with epilepsy can become seizure free by using antiepileptic drugs. On the other hand, the existing antiepileptic drugs are chemical compounds which often have serious side effects, including teratogenicity, chronic toxicity, and adverse effects on cognition and behavior (4). Consequently, it is essential to design and explore novel antiepileptic agents which have the characteristics of being effective as well as safe.
Nowadays, many people living in developing countries are using herbal medicines to treat epilepsy (5). Therefore, the idea of managing epilepsy by using carefully analyzed and wellstudied herbal medications is significant and advisable. Anethum graveolens L. is in the Apiaceae family, and it is found mostly in southwestern Asia (Iran-Khorasan province) or southeastern Europe. It grows to 90–120 cm tall and has slender branched stems, finely divided leaves, small umbels (2–9 cm in diameter) of yellow flowers, and long spindle-shaped roots. In general, dill leaves (dill weeds) and seeds (small fragrant fruits) are used as seasoning (6,7). However, the pharmacological properties, such as antibacterial activity (8), as well as its antihyperlipidemic and antihypercholesterolemic effects (9), have been reported. Moreover, dill seeds have identified pharmacological characteristics, such as promoting appetite and alleviating seizure effects (10). Therefore, it can be proposed that may be these compounds are responsible for the above mentioned pharmacological and biological effects.
Dill leaves have been used as a folk medicine to treat seizure in Iran (11), and the presence of flavonoids in this plant is remarkable (12). Two flavonoids have been isolated from A. graveolens seeds, quercetin 3-O-beta-Dglucuronide and isoharmentin 3-O-beta-Dglucuronide, which have antioxidant activities and could counteract with free radicals (13). The anxiolytic characteristics of flavonoids have been rarely examined, and to date, the anxiolytic-like effects of simple flavones like chrysin have been reported. This flavone behaves as a competitive ligand of benzodiazepine receptors (14); however, it has been shown to prevent the expression of tonic-clonic seizure induced by pentylenetetrazol in mice (15).
Considering the impact of benzodiazepine receptors and antioxidant constituents (16) in convulsive seizure, we planned to study the anticonvulsant properties of Anethum graveolens. However, according to our literature survey, there have been no reports on the anticonvulsant activity of the aqueous extracts of Anethum graveolens (in vitro/in vivo) in treating these neurological disorders. As a result, we designed the current research to assess the anticonvulsant activities of the aqueous extract of dill leaves on male mice, in order to scientifically justify its use in traditional medicine to treat epilepsy.
Materials and Methods
Animals
Male albino BALB/c mice, weighing 25–35 g, were obtained from the animal house at Sabzevar University of Medical Sciences. After three handling days, eight groups (10 each) were randomly selected. They were placed in polypropylene cages with paddy husk as bedding. The animals were housed at a temperature of 23 ± 2 °C and relative humidity of 30–70%. All procedures involving the care and use of the animals were conducted in accordance with the “Guide to the Care and Use of Experimental Animals” (17) and the Sabzevar University Ethics Committee. All experiments were completed at the same time (8.00 am to 2.00 pm) in the morning to avoid the bias of circadian rhythms (18).
Chemicals
Pentylenetetrazol and sodium valproate were obtained from Sigma-Aldrich Inc, St Louis (MO), United States of America. All solvents used in this study were obtained from local companies in Iran.
Plant material
Anethum graveolens L. was collected from the Khorasan province (northeastern Iran) and authenticated in the Ferdowsi University Herbarium. The plant samples were deposited as plant 293-0107-18 at our own collection (School of Pharmacy, Faculty of Pharmacognosy, Mashhahd University) for future reference.
Preparation of extracts
The freshly collected plant material (leaves) was dried in the shade, and then coarsely powdered. The dried leaves (1.2 kg) were separately and consecutively macerated with solvents (water and ethanol (80 V/V) over a period of three days. The solvents were evaporated off under reduced pressure, and the extracts were stored at –20 °C until tested. The yield (w/w) of the aqueous solution was 2.3%.
Kindling method
In order to perform kindling, an intraperitoneal injection of PTZ (37 mg/kg) was performed every other day. After the PTZ injection, seizure occurred in these animals. These steps were consequently repeated until 13 injections were achieved (19). During the first injections, the primary stages of seizure occurred, and in later injections, upper levels of seizure can occur. These stages included: stage 0 = absence of response; stage 1 = facial automatism, with twitching of the ears and whiskers; stage 2 = convulsive waves propagating throughout the body; stage 3 = forelimb clonus with rearing; stage 4 = tonic-clonic convulsions with loss of posture; and stage 5 = fully generalized tonic-clonic seizure with falling (20). In this study, the measureable and comparable statistical parameters (in seconds) are shown as the following. Stage 2 latency (S2L) was measured from the beginning of the stimulation until the beginning of stage 2 seizure. Stage 4 latency (S4L) was measured from the beginning of the stimulation until the beginning of stage 4 seizure. The seizure duration of stage 5 (S5D) was measured from the beginning of stage 5 until the end of this stage. For the S2L and S4L parameters, we used the cumulative S2L (the sum of the latency of the stage 2 durations recorded after 13 daily injections) and cumulative S4L in the first part of experiments (groups 1–5). The animals received daily PTZ and dill injections for 13 days, therefore the convulsive parameters were statistically and cumulatively analyzed.
Experimental groups
According to the minimum toxic dose (500 mg/kg), the dosages were selected for the three kindling acquisition groups in this order: 100, 250, and 400 mg/kg. After obtaining the dill extract, the required amount was weighed. Then it was dissolved in normal saline to reach the required dose and the solution (10 mL/kg) was injected intraperitoneally. After 30 minutes, the PTZ (37 mg/kg) was injected into the animals and the seizure parameters were immediately documented. The first group of animals was injected with normal saline (10 mL/kg;ip), 30 minutes before injecting the PTZ, and this schedule was repeated 13 times, every other day. The second group of animals received sodium valproate (100 mg/kg) thirty minutes before injecting the PTZ. This group was considered to be the positive control. The third, fourth, and fifth groups of animals received 100, 250, and 400 mg/kg of the aqueous extract, respectively, 30 minutes before the PTZ injection. In the sixth to eighth groups, the aqueous extract was injected one time in dosages of 100, 250, and 400 mg/kg, thirty minutes before the PTZ injection in the kindled animals. The animals should have demonstrated stage 5 or stage 4 for three consecutive PTZ injections before they were said to be kindled (1). The seizure parameters (S2L, S4L, S5D) were monitored for precisely 20 minutes after each PTZ injection in all groups.
Acute toxicity study
Different doses of the extract were injected intraperitoneally into the separated groups of six mice. The number of deaths was determined at 24 hours after treatment, and the Lethal Dosage (LD50) values and corresponding confidence limits were determined using the Litchfield and Wilcoxon method.
Statistical analysis
The results are expressed as the means, accompanied by the number of observations. A repeated measures ANOVA was used to determine changes in the cumulative S2L, (the sum of the latency of the stage 2 durations recorded after 13 daily injections), cumulative S4L during the first part of the experiments (groups 1–5), and a P value < 0.05 was considered to represent a significant difference.
Results
Effect of aqueous extract of dill leaves on kindling acquisition
For the first part of this study, the effect of the aqueous dill extract (100, 250, and 400 mg/kg) was used to investigate PTZ kindling acquisition. The injection of the aqueous dill extract (100 mg/kg) every 48 hours had no significant effects on the seizure parameters. The repeated measures ANOVA showed that in the S2L group receiving the extract sample, seizure was reduced from 120.7 seconds (SD 21.4) to 99.5 seconds (SD 20) seconds, which had no significant difference (F12,36 = 1.83, P = 0.079) when compared with the group receiving normal saline 90.7 seconds (SD 16.2) to 72.8 seconds (SD 6.2) seconds. The S4L group showed no significant changes after 13 dill extract injections. The S4L in the group receiving the extract was reduced from 118.7 seconds (SD 6.5) to 112.5 seconds (SD 29.2), which had no significance (F12,156 = 1.53, P = 0.16) when compared with the normal saline group from 138.1 seconds (SD 13.7) to 97.4 seconds (SD 10). Additionally, the S5D groups had no significant changes after 13 extract injections, compared to the normal saline group. The S5D was increased from 4 to 10.2 seconds (SD 1.5) in the extract group, which showed no significant differences (F12,36 = 0.51, P = 0.88) when compared to the saline group from 2.3 seconds (SD 0.5) to 7.1 (SD 1) seconds.
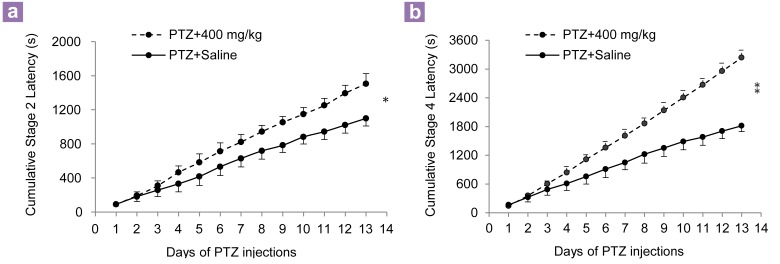
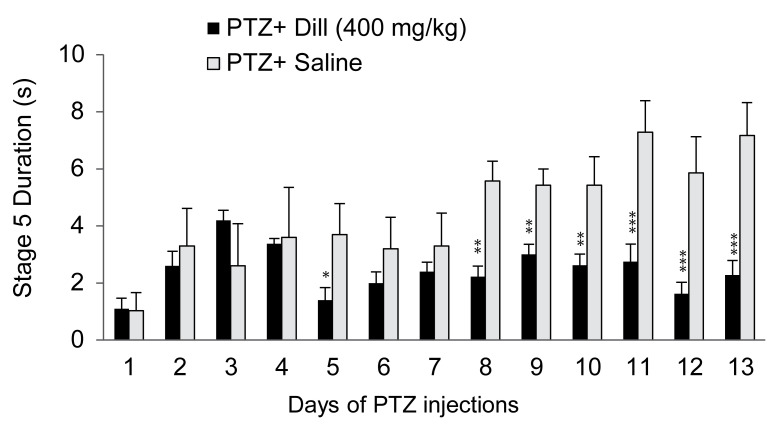
Similar to the 100 mg/kg of dill extract injection, the injection of 250 mg/kg of the same sample every 48 hours had no effects on the seizure parameters (Table 1). The injection of 400 mg/kg aqueous dill extract every 48 hours had significant effects on the seizure parameters. The repeated measures ANOVA showed that the S2L in the group receiving the extract was increased from 89.6 seconds (SD 6.8) to 111.4 seconds (SD 7.4), which showed significant changes compared to the saline group from 90.7 seconds (SD 16.2) to 72.8 seconds (SD 6.2) seconds (F12,132 = 2.1; P < 0.05) (Figure 1a). The repeated measures ANOVA showed that the S4L in the extract injected group was increased from 143.6 seconds (SD 26.5) to 297.2 seconds (SD 17.3) and had significant differences (F12,168 = 2.53, P < 0.01) compared to the group receiving saline from 138.1 seconds (SD 13.7) to 136.7 seconds (SD 4.3) (Figure 1b). The S5D showed significant changes after 13 treatments of the dill extract (400 mg/kg) injection, compared to the saline group. The S5D showed an increase of 1.1 seconds (SD 0.3) to 2.2 seconds (SD 0.5) in the extract receiving group, which was significant when compared to the saline group from 1.3 seconds (SD 0.6) to 7.1 seconds (SD 1) (F12,72 = 2.1, P = 0.02) (Figure 2).
Table 1.
Effect of aqueous extract of Dill (250 mg/kg) on stage 2 latency, stage 4 latency, and stage 5 duration
| Days of PTZ injections | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seizure Parameters | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Stage 2 latency | 113.7 | 74.1 | 90 | 90 | 96 | 100.2 | 101.5 | 103.2 | 108.6 | 112.8 | 114.7 | 118.7 | 118.8 |
| Stage 4 latency | 89 | 73.5 | 61.4 | 62.9 | 76.1 | 76.8 | 83.2 | 87.5 | 90.8 | 90.5 | 92.9 | 95.5 | 97.6 |
| Seizure duration | 180 | 175.7 | 219.4 | 156.6 | 133.4 | 146 | 133.9 | 125.6 | 118.3 | 117.6 | 104.7 | 100.9 | 96.4 |
Figure 1:
(a) Effect of aqueous extract of dill (400 mg/kg) on cumulative seizure, stage 2 latency and (b) cumulative seizure, stage 4 latency during pentylenetetrazol kindling. Values are means (SD) (cumulative). *P < 0.05, and **P < 0.01 when comparing the pentylenetetrazole (PTZ) + 400 mg/kg and PTZ + saline groups (n = 10).
Figure 2:
Effect of aqueous extract of dill (400 mg/kg) on seizure stage 5 duration during pentylenetetrazol (PTZ) kindling. Values are means (SD) *P < 0.05, and **P < 0.01 and ***P < 001 when compared to the PTZ + saline group (n = 10).
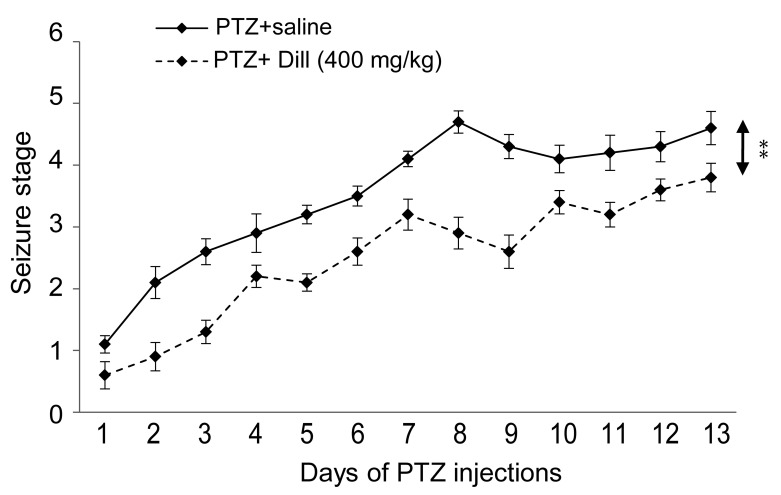
The injection of 400 mg/kg of the aqueous dill extract every 48 hours resulted in a significant retardation of kindling acquisition. A two-way ANOVA and post hoc Tukey’s test showed that the average number of injections required to reach the fully kindled state (stage 5) or other seizure stages was significantly increased in the PTZ + dill (400 mg/kg) group, when compared to the PTZ +saline group (P < 0.01) (Figure 3).
Figure 3:
Effect of aqueous extract of dill (400 mg/kg) on number of stimulations needed to achieve different seizure stages. There is a significant increase in the number of stimulations in all seizure stages. Values are means (SD) **P < 0.01 (n = 10).
During this part of the study, five mice died in the three groups: two animals in group three (100 mg/kg) died during the 11th injection and two animals in group four (250 mg/kg) died during the 10 and 11th injections. Ultimately, one mouse in group 5 (400 mg/kg) died during the 12th injection.
Effect of the aqueous extract of dill leaves on PTZ- Kindled mice
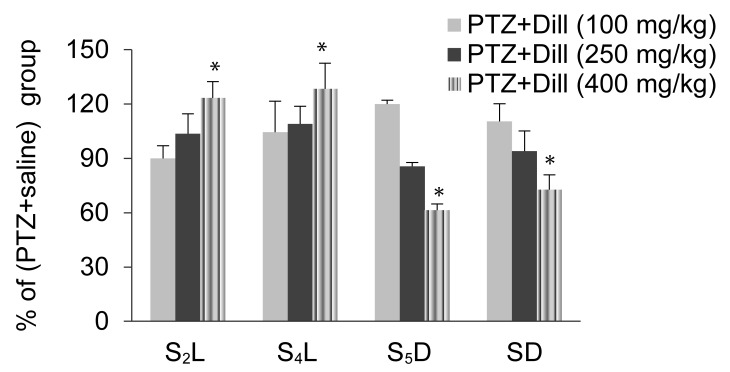
During the second part of this study, the effects of the dill extract (100, 250, and 400 mg/kg) were studied on the seizure parameters in the kindled animals. As shown in Figure 4, only 400 mg/kg of the dill extract manipulated the seizure parameters. The S2L in the dose of 400 mg/kg exhibited a 22.1% increase when compared with the saline group from 47.5 seconds (SD 8.3) in the saline group to 58 seconds (SD 9.1) in the 400 mg/kg extract sample (P < 0.05). The S4L showed a 28.8% increase in the amount of 400 mg/kg when compared with the saline group from 95.1 seconds (SD 9.9) in the saline group to 122.5 seconds (SD 14.2) in the 400 mg/kg extract sample] (P < 0.05). S5D at 400 mg/kg showed a 35.6% reduction when compared to the saline group from 7 seconds (SD 1.3) in the saline group to 4.3 seconds (SD 3.5) in the 400 mg/kg extract sample (P < 0.05).
Figure 4:
Effect of intraperitoneal injection of aqueous extract of dill on seizure stage 2 latency (S2L), seizure stage 4 latency (S4L), seizure stage 5 duration (S5D), and seizure duration (SD) in pentylenetetrazol (PTZ) kindled animals. Data are expressed as the base percentage of the PTZ+saline group. The aqueous extract of dill decreases the SD and S5D. Increase in S2L and S4L relative to the PTZ + saline group (n = 10). *P < 0.05.
Discussion
The results of the present study showed that the dill extract has anticonvulsant effects, and the injection of the dill extract sample at the dosage of 400 mg/kg, thirty minutes before injecting the PTZ could noticeably increase the S2L and S4L. Furthermore, the dose of 400 mg/kg reduced the S5D, while the doses of 100 mg/kg and 250 mg/kg showed no significant changes in the measured seizure parameters.
Medicinal herbs have fewer side effects than chemically synthesized drugs; therefore, they are considered to be good alternatives. The anticonvulsant effects of herbal medicines have been reported in various studies (21–24). Any possible antiepileptic effects have not been reported for dill extract, thus far, except in the cases of acute seizure in our group (25). Moreover, studies have been done in dill extract effects on blood sugar, lipids, and cholesterol (26,27), and dill extract has been investigated for its antibacterial activity (28,29). There have been reports about the antispasmodic activity of dill on the mouse ileum (30). In addition, it has been shown, that dill can block the entrance of calcium into gastrointestinal smooth muscles, and therefore reduce spasms and contractions. Because calcium entry is involved in cell (neuron) excitability, it can be suggested that dill is responsible for reducing PTZ induced convulsions by blocking or reducing calcium channel activity, which decreases sodium and calcium entering the neurons; however, more studies should be undertaken to investigate this.
Dill aromatic constituents include D-carvone and D-limonene (31). D-carvone belongs to the terpenoid group and has a large chemical structure. Monoterpenes have anti-inflammatory, antinociceptive, and anticonvulsant bioactivities (32), and the +D–carvone antiepileptic role in PTZ models has been reported (33). In the newly released data, this crucial role was related to the D-carvone epoxy structure (34). Furthermore the antinociceptive activity of D-limonene in mice has been detected, and it is believed that this agent blocks the production and/or release of inflammatory mediators which cause seizure (35). Prostaglandins are known as proconvulsants, blocking inflammatory mediators and deferring and reducing seizure (36,37). It can be suggested that D-limonene is responsible for the dill anticonvulsive activity in PTZ induced seizure.
Conclusion
Based on these results, it may be postulated that dill has anticonvulsant effects. There have been contraindications; including resistance, side effects, and many adverse effects observed in anticonvulsant chemically synthesized drugs, so it is desirable to produce herbal medicines with fewer side effects for treating severe neurological disorders. Herbs are a powerful source for the pharmaceutical market, and among these various plants, dill extract has exhibited protective effects on the central nervous system which could decrease epileptic convulsions.
Acknowledgments
We are grateful to Ferdowsi University of Mashhad for plant identification. The financial support of Sabzevar University of Medical of Sciences in performing the study is gratefully acknowledged.
Footnotes
Conflict of interest
None.
Funds
None.
Authors’ contributions
Conception and design: AA, MZM, MSJ, TAM
Analysis and interpretation of the data: AA, MZM
Drafting of the article: AA, MZM, MSJ
Critical revision of the article for the important intellectual content: MZM, MSJ
Final approval of the article: AA, MSJ
Provision of study materials or patient: MZM, TAM
Statistical expertise: AA
Obtaining of funding and collection and assembly of data: AZA
Administrative, technical or logistic support: MZM, TAM, AZA
References
- 1.McNamara JO. Kindling: An animal model of complex partial epilepsy. Ann Neurol. 1984;16(1 Supp):S72–S76. doi: 10.1002/ana.410160712. doi: 10.1002/ana.410160712 . [DOI] [PubMed] [Google Scholar]
- 2.Rossi J. Sensitization induced by kindling and kindling- related phenomena as a model for multiple chemical sensitivity. Toxicology. 1996;111(1–3):87–100. doi: 10.1016/0300-483x(96)03394-x. doi: 10.1016/0300-483X(96)03394-X . [DOI] [PubMed] [Google Scholar]
- 3.McNamara JO. Drugs effective in the therapy of the epilepsies. Goodman and Gilman's The Pharmacological Basis of Therapeutics. In: Hardman JG, Limbird LE, Gilman AG, editors. 10th ed. New York (NY): McGraw-Hill; 2001. p. 532. [Google Scholar]
- 4.Samren EB, van Duijn CM, Koch S, Hiilesmaa VK, Klepel H, Bardy AH, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38(9):981–990. doi: 10.1111/j.1528-1157.1997.tb01480.x. doi: 10.1111/j.1528-1157.1997.tb01480.x . [DOI] [PubMed] [Google Scholar]
- 5.Abdullah JM. Interesting asian plants: their compounds and effects on electrophysiology and behaviour. Malays J Med Sci. 2011;18(4):1–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Zohary D, Hopf M. New York (NY): Oxford University Press; 1988. Domestication of plants in the old world. [Google Scholar]
- 7.Shyu YS, Lin JT, Chang YT, Chiang CJ, Yang DJ. Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower. Food Chemistry. 2009;115(2):515–521. doi: 10.1016/j.foodchem.2008.12.039 . [Google Scholar]
- 8.Rafii F, Shahverdi AR. Comparison of essential oils from three plants for enhancement of antimicrobial activity of nitrofurantoin against enterobacteria. Chemotherapy. 2007;53(1):21–25. doi: 10.1159/000098246. doi: 10.1159/000098246 . [DOI] [PubMed] [Google Scholar]
- 9.Yazdanparast R, Alavi M. Antihyperlipidaemic and antihypercholesterolaemic effects of Anethum graveolens leaves after the removal of furocoumarins. Cytobios. 2001;105(410):185–191. [PubMed] [Google Scholar]
- 10.Tian J, Ban X, Zeng H, Huang B, He J, Wang Y. In vitro and in vivo activity of essential oil from dill (Anethum graveolens L.) against fungal spoilage of cherry tomatoes. Food Control. 2011;22(12):1992–1999. doi: 10.1016/j.foodcont.2011.05.018 . [Google Scholar]
- 11.Zargari A. Iran (IR): Tehran University Publications; 1996. Medicinal plants. [Google Scholar]
- 12.Kaur G, Arora D. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement Altern Med. 2009;9:30. doi: 10.1186/1472-6882-9-30. doi: 10.1186/1472-6882-9-30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moehle B, Heller W, Wellmann E. UV-induced biosynthesis of quercetin 3-o-beta-d-glucuronide in dill Anethum graveolens cell cultures. Phytochemistry. 1985;24(3):465–468. [Google Scholar]
- 14.Wolfman C, Viola H, Paladini A, Dajas F, Medina JH. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. Pharmacol Biochem Behav. 1994;47(1):1–4. doi: 10.1016/0091-3057(94)90103-1. doi: 10.1016/0091-3057(94)90103-1 . [DOI] [PubMed] [Google Scholar]
- 15.Medina JH, Paladini AC, Wolfman C, Levi de Stein M, Calvo D, Diaz LE, et al. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem Pharmacol. 1990;40(10):2227–2231. doi: 10.1016/0006-2952(90)90716-x. doi: 10.1016/0006-2952(90)90716-X . [DOI] [PubMed] [Google Scholar]
- 16.Olsen RW, Bureau M, Houser CR, Delgado-Escueta AV, Richards JG, Möhler H. GABA/benzodiazepine receptors in human focal epilepsy. Epilepsy Res. 1992;(8 Suppl):383–391. doi: 10.1016/b978-0-444-89710-7.50053-7. [DOI] [PubMed] [Google Scholar]
- 17.Olfert ED, Cross BM, McWilliam AA. Ottawa (CD): Canadian Council on Animal Care; 1993. Guide to the care and use of experimental animals. [Google Scholar]
- 18.Yehuda S, Mostofsky DI. Circadian effects of betaendorphin, melatonin, DSIP, and amphetamine on pentylenetetrazol-induced seizures. Peptides. 1993;14(2):203–205. doi: 10.1016/0196-9781(93)90030-k. doi: 10.1016/0196-9781(93)90030-K . [DOI] [PubMed] [Google Scholar]
- 19.Golmohammadi R, Pejhan A, Azhdari-Zarmehri H, Mohammad-Zadeh M. The role of ethanol on the anticonvulsant effect of valproic acid and cortical microvascular changes after epileptogenesis in mice. Neurol Sci. 2013;34(7):1125–1131. doi: 10.1007/s10072-012-1190-y. [DOI] [PubMed] [Google Scholar]
- 20.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–94. doi: 10.1016/0013-4694(72)90177-0. doi: 10.1016/0013-4694(72)90177-0 . [DOI] [PubMed] [Google Scholar]
- 21.Hosseinzadeh H, Karimi G, Ameri M. Effects of Anethum graveolens L. seed extracts on experimental gastric irritation models in mice. BMC pharmacology. 2002;2:21. doi: 10.1186/1471-2210-2-21. doi: 10.1186/1471-2210-2-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janahmadi M, Niazi F, Danyali S. Kamalinejad M. Effects of the fruit essential oil of Cuminum cyminum Linn. (Apiaceae) on pentylenetetrazol-induced epileptiform activity in F1 neurones of Helix aspersa. J Ethnopharmacol. 2006;104(1–2):278–282. doi: 10.1016/j.jep.2005.09.019. doi: 10.1016/j.jep.2005.09.019 . [DOI] [PubMed] [Google Scholar]
- 23.Hosseinzadeh H, Karimi GR, Rakhshanizadeh M. Anticonvulsant effect of Hypericum perforatum: role of nitric oxide. J Ethnopharmacol. 2005;98(1–2):207–208. doi: 10.1016/j.jep.2004.12.007. doi: 10.1016/j.jep.2004.12.007 . [DOI] [PubMed] [Google Scholar]
- 24.Sayyah M, Mandgary A, Kamalinejad M. Evaluation of the anticonvulsant activity of the seed acetone extract of Ferula gummosa Boiss. against seizures induced by pentylenetetrazole and electroconvulsive shock in mice. J Ethnopharmacol. 2002;82(2–3):105–109. doi: 10.1016/s0378-8741(02)00166-6. doi: 10.1016/S0378-8741(02)00166-6 . [DOI] [PubMed] [Google Scholar]
- 25.Mohammadzadeh M, Pajhhan A, Mirnajafizadeh SJ, Rakhshani MH. Effects of the aqueous and alcoholic extracts of anethum graveolens L (dill) on pentylenetetrazol-induced seizures in male mice. J Rafsanjan University medical Sci. 2012;11(1):45–54. [Google Scholar]
- 26.Kojuri J, Vosoughi AR, Akrami M. Effects of anethum graveolens and garlic on lipid profile in hyperlipidemic patients. Lipids Health Dis. 2007;6:1476–1512. doi: 10.1186/1476-511X-6-5. doi: 10.1186/1476-511X-6-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahramikia S, Yazdanparast R. Efficacy of different fractions of Anethum graveolens leaves on serum lipoproteins and serum and liver oxidative status in experimentally induced hypercholesterolaemic rat models. Am J Chin Med. 2009;37(4):685–99. doi: 10.1142/S0192415X09007168. doi: 10.1142/S0192415X09007168 . [DOI] [PubMed] [Google Scholar]
- 28.Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88(2):308–316. doi: 10.1046/j.1365-2672.2000.00969.x. doi: 10.1046/j.1365-2672.2000.00969.x . [DOI] [PubMed] [Google Scholar]
- 29.Stavri M, Gibbons S. The antimycobacterial constituents of dill (Anethum graveolens) Phytother Res. 2005;19(11):938–941. doi: 10.1002/ptr.1758. doi: 10.1002/ptr.1758 . [DOI] [PubMed] [Google Scholar]
- 30.Naseri MKG, Heidari A. Antispasmodic effect of Anethum graveolens fruit extract on rat ileum. Inter J Pharmacol. 2007;3(3):260–264. [Google Scholar]
- 31.Jirovetz L, Buchbauer G, Stoyanova AS, Georgiev EV, Damianova ST. Composition, quality control, and antimicrobial activity of the essential oil of longtime stored dill (Anethum graveolens L.) seeds from Bulgaria. J Agric Food Chem. 2003;51(13):3854–3857. doi: 10.1021/jf030004y. doi: 10.1021/jf020004y . [DOI] [PubMed] [Google Scholar]
- 32.Gonçalves JC, Oliveira Fde S, Benedito RB, de Sousa DP, de Almeida RN, de Araújo DA. Antinociceptive activity of (-)-carvone: evidence of association with decreased peripheral nerve excitability. Biol Pharm Bull. 2008;31(5):1017–1020. doi: 10.1248/bpb.31.1017. doi: 10.1248/bpb.31.1017 . [DOI] [PubMed] [Google Scholar]
- 33.de Sousa DP, de Farias Nobrega FF, de Almeida RN. Influence of the chirality of (R)-(-)- and (S)-(+)- carvone in the central nervous system: a comparative study. Chirality. 2007;19(4):264–268. doi: 10.1002/chir.20379. doi: 10.1002/chir.20379 . [DOI] [PubMed] [Google Scholar]
- 34.de Almeida RN, de Sousa DP, Nobrega FF, Claudino Fde S, Araujo DA, Leite JR, et al. Anticonvulsant effect of a natural compound alpha, beta-epoxycarvone and its action on the nerve excitability. Neurosci Lett. 2008;443(1):51–55. doi: 10.1016/j.neulet.2008.07.037. doi: 10.1016/j.neulet.2008.07.037 . [DOI] [PubMed] [Google Scholar]
- 35.do Amaral JF, Silva MI, Neto MR, Neto PF, Moura BA, de Melo CT, et al. Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol Pharm Bull. 2007;30(7):1217–1220. doi: 10.1248/bpb.30.1217. doi: 10.1248/bpb.30.1217 . [DOI] [PubMed] [Google Scholar]
- 36.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46(11):1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. doi: 10.1111/j.1528-1167.2005.00298.x . [DOI] [PubMed] [Google Scholar]
- 37.Oliveira MS, Furian AF, Royes LF, Fighera MR, Fiorenza NG, Castelli M, et al. Cyclooxygenase-2/PGE2 pathway facilitates pentylenetetrazol-induced seizures. Epilepsy Res. 2008;79(1):14–21. doi: 10.1016/j.eplepsyres.2007.12.008. doi: 10.1016/j.eplepsyres.2007.12.00 . [DOI] [PubMed] [Google Scholar]