Abstract
Background:
The tissue-protective potential of Persea americana necessitated a look into the histopathological effects of the plant extract on the pancreas, liver, and kidneys. This study was conceived and designed based on the gaps in the research that has been performed and what is known about the plant. The hypoglycaemic and tissue-protective effects of hot aqueous Persea americana (avocado pear) seed extracts on alloxan-induced albino rats were investigated.
Methods:
Persea americana seeds were extracted using hot water, and different concentrations of the extract were prepared. The effects of different concentrations (20, 30, 40 g/L) of the hot aqueous P. americana seed extract on alloxan-induced Wistar albino rats were compared with those of a reference drug, glibenclamide. The glucose level of the rats was measured daily, and the weight of the animal was monitored on a weekly basis for 21 days. The oral glucose tolerance test (OGTT) was performed at 0, 30, 60, 90 and 120 minutes, and the histopathologies of the liver, kidneys, and pancreas were investigated. Phytochemical analysis of P. americana seed extracts indicated the presence of glycosides, tannins, saponins, carbohydrates, flavonoids, and alkaloids.
Results:
The results showed that the extract possessed a significant hypoglycaemic (P < 0.05) effect and reversed the histopathological damage that occurred in alloxan-induced diabetic rats, comparable to the effects glibenclamide. The seeds of P. americana also had anti-diabetic and protective effects on some rat tissues such as the pancreas, kidneys, and liver.
Conclusion:
In conclusion, the present study provides a pharmacological basis for the folkloric use of the hot-water extract of P. americana seeds in the management of diabetes mellitus.
Keywords: alloxan, hot aqueous, hypoglyceamic effect, persea americana, protective effect
Introduction
The International Diabetes Federation (IDF) reports that the prevalence of diabetes mellitus has reached epidemic levels globally. Recent estimates indicate that there were 366 million diabetics worldwide in 2011, and this number is expected to increase to 552 million by 2030 (1). Impaired glucose tolerance in sub-Saharan Africa is expected to rise by 75.8%, from 26.9 million in 2010 to 47.3 million in 2030, which is more than double the predicted global increase of 37% (2). Mortality that was attributable to diabetes in sub-Saharan Africa was estimated in 2010 to be 6% of the total mortality, and this value had increased from 2.2–2.5% in 2000. The absolute and relative mortality rates are highest in the 20–39 year age-group, i.e., the most economically productive population (2). In Nigeria, which has over 250 tribes and different culture and food values, the prevalence values of diabetes have not been uniform, although the values range from 1–7% of the Nigerian population (3,4).
Over 30 years, the prevalence of diabetes has steadily increased. Iloh et al. reported a prevalence of 3.9% for the Imo state (5). However, a higher prevalence rates was reported in Port Harcourt (6.8%) by Nyenwe et al. that same year (6). According to the estimates in 2004, the Diabetes Association of Nigeria (DAN) estimates the diabetic population in Nigeria to be approximately 10 million, and approximately half of that number resides in the Lagos State because of its cosmopolitan nature (7). These findings indicate that diabetes has become a major public health issue.
Plants may act on blood glucose through different mechanisms. Some plants may contain insulin-like substances (8), inhibit insulinase activity or increase beta β-cells in the pancreas by activating the regeneration of these cells (9,10), or some may serve as antioxidants by reducing the oxidative stress due to free radicals in the pancreas (11,12).
Persea americana (avocado) is a tree that belongs to the laurel family, Lauraceae, and is one of the 150 varieties of avocado pear. This plant is indigenous to Central and South America, but it is now cultivated in the United States of America, Asia, parts of Europe, and Tropical Africa and is commonly known as the avocado pear, alligator pear, or Mexican avocado. The medicinal relevance of the various parts of this tropical plant is enormous. The effects of aqueous seed extracts of Persea americana Mill. (Avocado), var. Fuerte, on the blood pressure, plasma, and tissue lipids of albino rats were investigated by Imafidon and Amaechina, and their results suggested that the use of the aqueous seed extract of this plant in the treatment of hypertension might produce a favourable lipid profile (13). Alhassan and colleagues also evaluated the hypoglycaemic activity of P. americana aqueous seed extracts on alloxan-induced diabetic rats and concluded that the anti-diabetic effects of the extract might be due to certain mineral elements and phytochemicals and that an increase in weight could be due to proper nutrient utilisation that is most likely induced by the avocado seed extract (14). However, the work by Okonta et al. suggests that P. americana can lower blood glucose levels in cases of mild hyperglycemia but not severe hyperglycemia (15). Edem et al. studied the effects of aqueous alligator pear seed extracts on normal and alloxan-induced diabetic rats, and their results suggested a restorative (protective) effect of the extract on pancreatic islet cells (16).
The work of Mahadeva et al., concentrated on the mechanism of the antidiabetic activity of P. americana. The insulin-stimulative and antioxidative effects of Persea americana were evaluated in streptozotocin (STZ)-treated rats. This group found that the activities of pathophysiological enzymes such as serum aspartate transaminase (AST), serum alanine transaminase (ALT), and serum alkaline phosphatase (ALP) were altered in the serum of rats that had been treated with glyclazide, which was used as the standard reference drug, but not control rats. These results revealed the tissueprotective nature of Persea americana fruits (17).
The aforementioned studies provided further insight into the restorative and antioxidant activities of P. americana. However, the tissueprotective potential of P. americana necessitated a look into the histopathological activity of the plant extract in the pancreas, liver and kidneys. Therefore, this study was conceived and designed based on the obvious gaps in what had been done or was known about this plant.
Materials and Methods
Sample collection, identification and preparation
The plant was identified as P. americana at the Department of Plant Science and Biotechnology, Faculty of Sciences, University of Port Harcourt, Nigeria. After identification, a ripe P. americana was washed and cleanly cut with a sharp, stainless knife, and the seed was removed and washed with clean water to remove dirt. The seed was then air dried for 3 hours, chopped into small pieces using the stainless steel knife, and the chopped seeds were again air dried for two weeks in the shade in a well-ventilated area to avoid contamination by mould. The seed matter was then pulverised with a blender to obtain 2 kg of fine powder, which was passed through a sieve (mesh size: 30 mm).
Extraction
The hot aqueous extraction method of N’guessan et al. (18) was adopted for the study because locals used hot water decoction when making extracts. Twenty grams of the dry powder was mixed in 1 L of distilled water to prepare 20 g/L of herbal extract, and the mixture was then boiled for approximately 45 minutes. The decoction was first filtered through a clean cloth and then using cotton wool, and 0.25 L of the decoction was placed in another flask to serve as filtrate 1. One litre of distilled water was added to the residual extract after boiling for 45 minutes, and 0.25 L of this decoction was collected as before (this solution served as filtrate 2). The same procedure was repeated to make filtrates 3 and 4, and the four filtrates were then pooled together to obtain a final decoction (1 L) of crude plant extract at a concentration of 20 g/L. This herbal extract was named P. americana, 20 g/L and was administered to the diabetic rats of group 3 (diabetic rat + 20 g/L P. americana).
The same procedure was used on 30 g of dry, fine powder to obtain 30 g/L of P. americana that was to be administered to the diabetic rats in group 4 (diabetic rat + 30 g/L P. americana). Following the same procedure, 40 g of dry, P. americana powder was used to prepare a 40 g/L solution, which was administered to diabetic rats of group 5 (diabetic rat + 40 g/L P. americana).
Phytochemical analysis of the sample
Phytochemical analysis of the dry, powdered seed of P. americana was carried out to identify the phytoconstituents in the extract. The analytical procedures for alkaloids, saponins, tannins, flavonoids, and resins were described by Matos (19) and in João Jaime et al. (20), and the standard method of Harbone (21) was adopted.
Animal study
Thirty adult male albino Wistar rats (weight range of 150–200 g) were obtained from the department of Biochemistry Animal House, University of Port-Harcourt, Nigeria. The rats were weight matched, grouped into six groups of five rats each and kept in well-ventilated cages at room temperature (28–30 °C) under controlled light cycles of 12 hours light/12 hours dark. The rats were allowed access to feed and water ad libitum for a period of seven days to acclimate them prior to commencement of the experiment.
Induction of the animals
After the acclimatisation period, the animals were prepared for diabetes induction by alloxan monohydrate after an 18 hours, overnight fast. The body weights and blood glucose levels of the animals were taken before and after induction. Group 1 served as the negative control and thus, was not induced. However, groups 2 to 6 were intraperitoneally injected with 150 mg/kg body weight of alloxan. Thereafter, the body weights and blood glucose levels of the rats were monitored on a daily basis for seven days to obtain a stable blood glucose level (18,22).
Sample collection and analysis
Blood samples were collected by pricking the tail of the animal tail a sharp razor, and the blood glucose level was estimated using a One Touch Glucometer (Johnson & Johnson, Zhejiang, China).
Treatment
Seven days after induction of diabetes, which was when the blood glucose levels of the rats were stable, drug treatment commenced, and was continued for 21 days as follows.
-
Group 1--- non-induced (negative control) received water
Group 2--- alloxan-induced non-treated mice (positive control) received water
Group 3--- alloxan-induced and treated with 20 g/L of the P. americana aqueous extract
Group 4---- alloxan-induced and treated with 30 g/L of the P. americana aqueous extract
Group 5---- alloxan-induced and treated with 40 g/L of the P. americana aqueous extract
Group 6--- alloxan-induced and treated with 0.5 mg/kg body weight of glibenclamide
The body weight was measured on a weekly basis, but the blood glucose level was measured on a daily basis for 21 days.
Oral glucose tolerance test (OGTT)
The oral glucose tolerance test was performed on overnight-fasted, normal diabetic, and treated mice on the 21st day of treatment. Glucose (2 g/kg body weight) was given orally, and the blood glucose level was measured at 0, 30, 60, 90, and 120 minutes after the administration of glucose.
The animals were sacrificed at the end of the study by anaesthetising with chloroform vapour. The liver, pancreas and kidneys of each rat were then dissected for histology.
Histopathology analysis
Dissected pancreas, livers, and kidneys from control, diabetic and treated mice were fixed in 10% formaldehyde, and processed and used for histopathological analysis. Tissue processing was carried out using an autotechnicon and the prepared, 5 μm thick sections were mounted on slides and stained with haematoxylin and eosin. The stained sections were morphologically evaluated by two independent histopathologists.
Statistical analysis
Blood glucose level data were evaluated using the Mann Whitney test, and groups were considered to be significantly different if P ≤ 0.05.
Results
The phytochemical tests of the P. americana seeds revealed the presence of carbohydrates, alkaloids, glycosides, saponins, tannins, and flavonoids.
The effects of P. americana and glibenclamide treatment on the blood glucose levels of alloxaninduced diabetic rats are shown in Table 1. The administration of alloxan increased the serum glucose levels of the rats to approximately 72.2% (P < 0.05) of the control rats in a time-dependent manner. Three weeks of daily, oral P. americana aqueous extract treatment (20, 30, or 40 mg/L) or glibenclamide (5 mg/kg) significantly reduced the blood glucose levels of diabetic rats (P < 0.05). The reductions that were observed when treating with plant extracts compared to those of the positive control ranged from 45.8% (200 mg/kg on day 7) to 58.9% (400 mg/kg on day 21). The reference drug glibenclamide produced its maximum response on day 14 (58.9%), which was similar to the response that was observed for the 40 g/L P. americana extract on day 21.
Table 1.
Blood glucose level of treated and non-treated alloxan-induced diabetic rats
| Treatment /day | Day 0 (mg/dL) | Day 7 (mg/dL) | Day 14 (mg/dL) | Day 21 (mg/dL) |
|---|---|---|---|---|
| N.C. | 117.0 (7.7) | 117.8 (11.3) | 116.4 (11.6) | 117.6 (9.8) |
| P. C. (DI alone) | 421.4 (55.1)a | 414.6 (45.7)a | 404.0 (82.0)a | 413.0 (88.1)a |
| DI + 20 g/L P.a. | 441.4 (61.4) | 239.2 (46.4)b (↓ 45.8%) | 221.4 (45.3)b (↓ 49.8%) | 216.6 (42.4)b (↓ 50.9%) |
| DI + 30 g/L P.a. | 436.8 (50.8) | 215.6 (25.3)b (↓ 50.6%) | 195.2 (12.3)b (↓ 55.3%) | 192.0 (19.0)b (↓ 56.0%) |
| DI + 40 g/L P.a. | 460.2 (83.8) | 210.6 (15.8)b (↓ 54.2%) | 192.8 (15.5)b (↓ 58.1%) | 189.0 (11.4)b (↓ 58.9%) |
| DI + GBM | 448.2 (54.9) | 186.2 (8.9)b (↓ 58.5%) | 184.2 (14.9)b (↓ 58.9%) | 187.2 (7.0)b (↓ 58.2%) |
*All values are expressed as the mean (SD) (n = 5); a P < 0.05 vs negative control group; b P < 0.05 vs. diabetic control group (NC = Negative Control; PC = Positive Control, DI = diabetic-induced, and GBM = glibenclamide. Figures in parentheses indicate a percentage increase or decrease in BGL (blood glucose level) when compared to the NC.
Based on the OGTT, glucose-induced hyperglycemia reached a maximum after 60 minutes and was reversed to its normal levels after 120 minutes in the negative-control rats. The tested extracts at different dose levels and glibenclamide reduced the blood-glucose-level peak 120 minutes after glucose loading to approximately the level at 0 min (Table 2). These changes in glucose levels were significant (P < 0.05) when compared to those of the normal control rats.
Table 2.
Effect of Persea americana and glibenclamide on serum glucose levels on oral glucose tolerance test (OGTT) rats
| Treatment | Basal (mg/dL) | 30 min (mg/dL) | 60 min (mg/dL) | 120 min (mg/dL) |
|---|---|---|---|---|
| NC | 117.6 9.8 (9.8) | 113.6 (8.7) | 140.6 (5.3) (↑ 19.2%) | 117.0 (8.0) |
| PC (non treated DI) | 413.0 (88.1) | 427.0 (89.8) | 110.6 (24.3) | 0 |
| DI + 20 g/L P.a. | 216.6 (42.4) | 220.2 (44.0) | 275.6 (37.2) (↑ 20.1%) | 223.6 (40.3) |
| DI + 30 g/L P.a. | 192.0 (19.0) | 199.0 (19.0) | 262.2 (37.2) (↑ 24.1%) | 187.6 (11.1) |
| DI + 40 g/L P.a. | 189.0 (11.4) | 192.4 ± 11.8 | 246.6 ± 6.2 (↑ 22.0%) | 190.4 (9.0) |
| DI + GBM | 187.2 (7.0) | 189.0 (7.2) | 256.8 (32.9) (↑ 26.4%) | 187.2 (3.2) |
*All values are expressed as the mean (SD) (n = 5); diabetic control group (NC = Negative Control; PC = Positive Control, DI = diabetic induced, GBM = glibenclamide). Figures in parentheses indicate a percentage increase in the BGL of OGTT rats at different time intervals when compared to the basal blood glucose leve
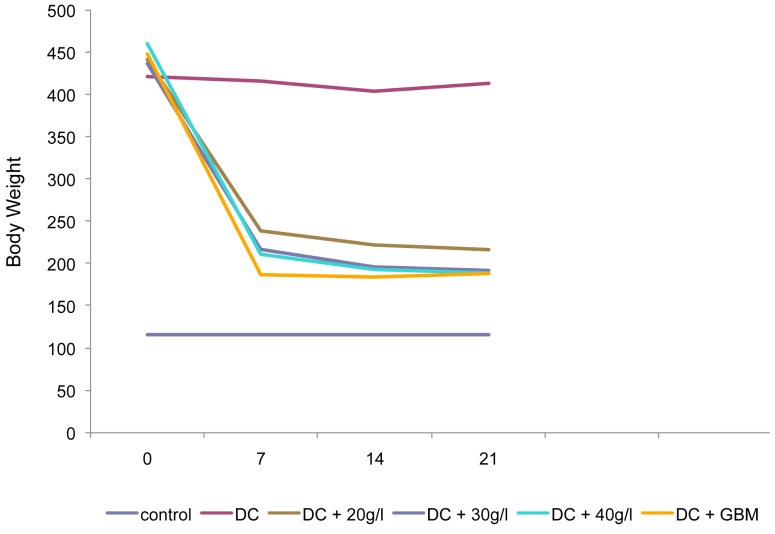
Figure 1 shows that the administration of P. americana increased the body weight of alloxan-induced diabetic rats.
Figure 1:
Effect of the aqueous extract of P. americana and glibenclamide on the body weight (g) of alloxaninduced diabetic rats.
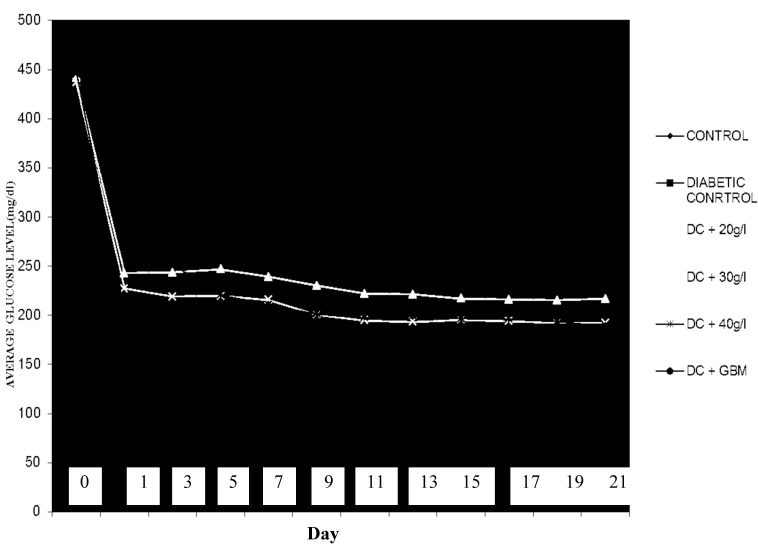
The effect of different concentrations of the P. americana extract on the blood glucose levels of the rats compared with that of glibenclamide indicated that the extract is as effective and potent as glibenclamide (Figure 2).
Figure 2:
Glycaemia variation curve for normal, control rats and rats treated with glibenclamide or P. americana extracts.
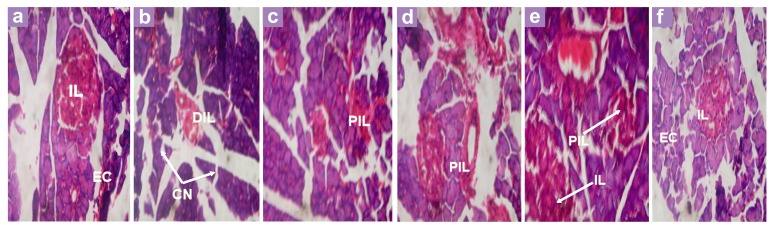
The histology of the pancreas (Figure 3) reveals that the normal control rats had intact pancreatic islets and exocrine cells. However, alloxan-induced diabetic rats (diabetic control rats) showed depleted islet cells (DIL) and areas of cell necrosis (CN). Diabetic rats that had been treated with the 20 g/L extract had small, preserved islet cells (PIL), which is an improvement from what occurred in the untreated alloxan-induced diabetic rats. Further improvements were observed in rats that had been treated with 30 g/L and 40 g/L of extract, such as more prominent islet cells and exocrine cells, which indicated an improvement in the architecture of the pancreas as the concentration of the extract increased. The standard drug glibenclamide also led to recovery of the pancreatic tissue, as evident from the intact pancreatic islet that is observed in Figure 3.
Figure 3:
Histopathological Effect of Extracts and Glibenclamide on the Pancreas of alloxan-induced diabetic rats. (a) Negative Control - Pancreas showing exocrine acini, endocrine islets (IL) and areas of cell necrosis (CN); (b) Diabetic control - Shows depleted islets (DIL); (c) Diabetic rats treated with 20 g/L of extracts - Shows exocrine acini and small preserved islets (PIL); (d) Diabetic rats treated with 30 g/L of extracts -Shows preserved islets (PIL); (e) Diabetic rats treated with 40 g/L of extracts - Shows full regeneration of islets and exocrine (EC); (f) Diabetic rats treated with glibenclamide - Pancreas showing islets (IL) and exocrine acini (EC). All panels were stained with hematoxylin and eosin, 400× magnification.
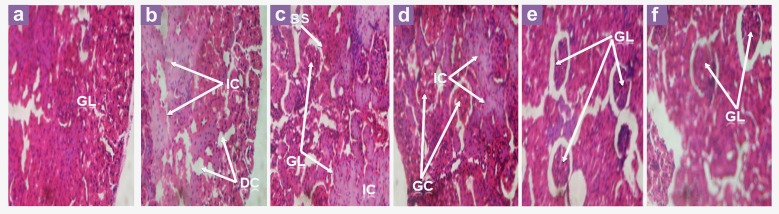
The histology of the kidneys is shown in Figure 4. The kidney of a normal rat has glomeruli (GL) and a compact-tissue appearance (Figure 4a), the tissue of untreated, alloxanised rats (positivecontrol rats) showed the presence of dissolved cells (DC) due to cell necrosis and widespread infiltrations by inflammatory cells (IC) (Figure 4b). However, as the concentration of the extract increased from 20 g/L to 40 g/L, noticeable improvements in the tissue architecture were evident, and more visible glomeruli and fewer inflammatory cells were observed (Figure 4c–4e). Glibenclamide also caused a similar improvement in the kidney tissue (Figure 4f).
Figure 4:
Histopathological effect of extracts and glibenclamide on the kidneys of alloxan-induced diabetic rats. (a) Control mice - Normal rat kidney showing the architecture of glomeruli (GL) and compact tissue; (b) Diabetic control rats - Kidney showing profuse infiltration by inflammatory cells (IC), areas with dissolved cells (DC) and an absence of glomerulus; (c). Diabetic rats treated with 20 g/L of extract - Kidney showing Bowman’s space (BS), glomeruli and inflammatory cells (IC); (d) Diabetic rats treated with 30 g/L of extract - Kidney showing glomeruli (GL) and some inflammatory cells (IC); (e) Diabetic rats treated with 40 g/L of extract - Kidney showing glomeruli (GL) and a near compact kidney architecture; (f) Glibenclamide-treated rats - Kidney showing glomeruli (GL) and a near compact kidney architecture. (hematoxylin and eosin 400× magnification).
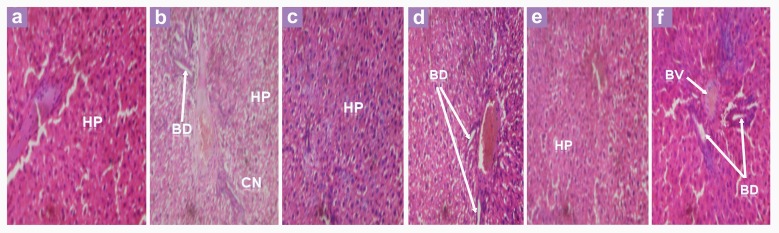
The histology of the liver is shown in Figure 5. Compared to the histology of normal, control rats, cell necrosis was evident in the untreated, alloxanised rats (positive-control rats) (Figure 5a,5b). However, this was not the case for those treated with the extracts or glibenclamide (Figure 5c–5f). In these instances, the liver tissues were compact and healthy.
Figure 5:
Histopathological effect of extracts and glibenclamide on the liver of alloxan-induced diabetic rats. (a) Control rats - Liver of control rat shows normal arrangement of hepatocytes (HP) with no visible lesion; (b) Diabetic control rats - Liver of alloxan-induced rat shows bile duct (BD) and degeneration of the hepatocytes (HP) with areas of cell necrosis (CN); (c–f). Liver of treated, alloxan-induced rats showing hepatocytes (HP), bile duct (BD) and blood vessels (BV) with normal architecture. 400× magnification.
Discussion
The present study has shown a significant decrease (P < 0.05) in blood glucose levels with the use of different concentrations of aqueous seed extracts of P. americana. This finding may indicate the presence of hypoglycaemic agents in the seeds of P. americana that were concentrated in the aqueous extracts. The results suggest that P. americana seed extracts exhibit hypoglycaemic and antihyperglycaemic effects, which is consistent with the results of various works (14,18,23). The medicinal effects of these phytochemicals have been documented. Flavonoids have been found to be an active principle in many herbal medicines (24), and are a known to be powerful antioxidants that may help protect organs against toxicity or oxidative stress due to agents such as alloxan (25). Saponins have been reported to possess hypoglycaemic activity, which may be due to the inhibition of liver glycogenesis or glycolysis (26), and may have contributed to the observed hypoglycaemic activity of the plant extract. Furthermore, flavonoids, tannins, and saponins were reported by Tiwari and Rao (27), to possess hypoglycaemic properties via an inhibitory action on the sodium-glucose transporter 1 (S-GLUT1).
Histopathological studies of diabetic control rats showed degeneration of pancreatic islet cells, which was similar to earlier observations (18,22). This phenotype most likely gave rise to insulin deficiency. Insulin deficiency (or diabetes mellitus) causes excessive elevation and poor utilisation of blood glucose and leads to hyperglycemia. This histopathological study of diabetic-treated groups indicated an increased volume density of islets and increased percentage of beta cells in diabetic rats that received the extracts, which may be a sign of regeneration. Signs of regeneration of β-cells, the potentiation of insulin secretion from surviving β-cells of the islets of Langerhans and a decrease in blood glucose levels have been reported to occur after consuming some plant extracts (9,10). The P. americana seed may have some chemical components that exert regenerative effects on β-cells, stimulate these cells to produce more insulin (pancreatotrophic action) or have some insulin-like substances because higher concentrations of the extract had a greater restorative effect on the islet cells of diabetic rats than the lower-dose extracts.
The tissue-protective effect of P. americana can be observed by its ability to restore and reverse the already damaged tissues of alloxaninduced rats, and the observed effect is in agreement with what other researchers have reported. A study by Adewole et al. (11) reported that, the anti-diabetic property that was observed for Catharanthus roseus Linn (Apocyaceae) was due to its antioxidant effect on the pancrease, which prevented damage by oxygen-free radicals. John et al. (28), reported that Terminalia arjuna stem bark reversed pathological lesions that were evoked in cells of alloxan-induced diabetic rats. Additionally, the ethanolic extracts of Parinari polyandra and Spondias mombin seeds were useful in reducing hyperglycaemia and managing hepatic complications in alloxan-induced diabetic rats.
Item et al. (29) investigated the antidiabetic mechanism of combined Vernonia amydalina and Azadirrachta indica extracts by evaluating their effects on the histology of the pancreas and livers of normal and diabetic rats. This group reported a recovery/reversal of liver and pancreatic damage in streptozotocin-induced diabetic rats. Another report by Teoh et al. (30), showed the protective effect of Momordica charantia, which is a bitter gourd that is known for its antidiabetic properties, on the kidneys of streptozotocininduced diabetic rats. Studies by Edem et al. (16) suggested the restorative effect of P. americana extracts on the pancreatic islet cells of alloxaninduced diabetic rats, and the work of Mahadeva et al. (17) demonstrated the insulin-stimulative and antioxidative properties of P. americana fruits on streptozotocin-induced diabetic rats, thus revealing their tissue-protective nature.
The ability of alloxan to induce weight loss in untreated rats mimics what is commonly observed in clinical diabetes (WHO, 2003) (31). During the 21 days experimental period, the body weight of diabetic rats was reduced, whereas a significant (P < 0.05) gain in body weight occurred in treated rats. The administration of P. americana extracts corrected the loss in body weight and significantly restored these levels (P < 0.05) towards normal. The ability of the P. americana extracts to restore body weight seems to be a result of its ability to reduce hyperglycemia by increased glucose metabolism, and this may be due to the protective effect of the extract in controlling muscle wasting, i.e., reversal of gluconeogenesis.
Conclusion
In summary, the plant extract exerted a dose-dependent protective effect on the pancreas, kidneys and liver, like the reference drug glibenclamide did. Taken together, the results of present study provide a pharmacological basis for the folkloric use of the hot-water extract of P. americana seeds in the management of diabetes mellitus.
Acknowledgments
None.
Footnotes
Conflict of interest
None.
Funds
None.
Authors’ contributions
Conception and design, analysis and interpretation of the data, drafting of the article and final approval of the article: NAE, OEO
Critical revision of the article for the important intellectual content: OEO
Provision of study materials or patient and obtaining of funding: NAE, AO
Statistical expertise, administrative, technical or logistic support and collection and assembly of data: AO
References
- 1.International Diabetes Federation . 5th ed. Diabetes Atlas 2011. [Internet] Available from: http://www.idf.org/diabetesatlas/diabetesandimpairedglucosetolerance . [Google Scholar]
- 2.International Diabetes Federation . 4th ed. Brussels (BE): International Diabetes Federation; 2009. The IDF Diabetes Atlas. [Google Scholar]
- 3.Fabiyi AK, Kolawole BA, Adeshinto O, Ikem RT. The impact of knowledge, attitude, practice and belief of type two Nigerian diabetes patients on drug compliance. Diabetes Int. 2002;12(1):15–17. [Google Scholar]
- 4.Wokoma FS. Diabetes and hypertension in Africa- An overview. Diabetes Int. 2002;12(12):36–40. [Google Scholar]
- 5.Iloh G, Amadi AN, Nwankwo BO, Ugwu VC. Obesity in adult Nigerians: A study of its pattern and common primary co-morbidities in a rural Mission General Hospital in Imo state, south-eastern Nigeria. Niger J Clin Pract. 2011;14(2):212–218. doi: 10.4103/1119-3077.84019. [DOI] [PubMed] [Google Scholar]
- 6.Nyenwe AE, Osaretin JO, Anele EI, Aaron O, Seye B. Type II diabetes in adult Nigerians: A study of its prevalence and risk factors. Diabet Res Clin Pract. 2003;62(3):177–185. doi: 10.1016/j.diabres.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Ogbera AO, Adedokun A, Fasanmade OA, Ohwovoriole AE, Ajani M. The foot at risk in Nigerians with diabetes mellitus: The Nigerian scenario. International J Endocr Metab. 2005;4:165–173. [Google Scholar]
- 8.Gray AM, Flatt PR. Insulin–releasing and insulin–like activity of the traditional anti-diabetic plant Coriander sativum (coriander) Br J Nutr. 1999;81(3):208–209. doi: 10.1017/s0007114599000392. [DOI] [PubMed] [Google Scholar]
- 9.Abdel MA, El-Feki M, Salah E. Effect of Nigella sativa, fish oil and gliclazide on alloxan diabetic rats. Biochemical and Histopathological studies. J Egy Ger Soci Zool. 1997;23:237–265. [Google Scholar]
- 10.Shanmugasundaram ER, Gopith KI, Radha SK, Rajendram VM. Possible regeneration of the islets of Langerhans in streptozocin diabetic rats given Gymnemasylvester leaf extracts. J Ethnopharmcol. 1990;30:265–269. doi: 10.1016/0378-8741(90)90106-4. [DOI] [PubMed] [Google Scholar]
- 11.Adewole SO, Ojewole JAO. Insulin-induced immunohistochemical and morphological changes in pancreatic β-cells of streptozotocin-treated diabetic rats. Methods Find in Exp Clin Pharmacol. 2007;29(7):447–455. doi: 10.1358/mf.2007.29.7.1119168. [DOI] [PubMed] [Google Scholar]
- 12.Singh SN, Vats P, Suri S, Shyam R, Kumria MM, Ranganathan S, et al. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmcol. 2001;73(3):269–277. doi: 10.1016/s0378-8741(01)00254-9. [DOI] [PubMed] [Google Scholar]
- 13.Imafidon KE, Amaechina FC. Effects of Aqueous Seed Extract of Persea Americana Mill. (Avocado) on Blood Pressure and Lipid Profile in Hypertensive Rats. Advan Biol Res. 2010;4(2):116–112. [Google Scholar]
- 14.Alhassan AJ, Sule MS, Atiku MK, Wudil AM, Abubakar H, Mohammed SA. Effects of aqueous avocado pear (Persea americana) seed extract on alloxan induced diabetes rats. Greener J Med Sci. 2012;2(1):5–11. [Google Scholar]
- 15.Okonta M, Okonta L, Cletus NA. Blood Glucose Lowering Activities of Seed of Persea americana On Alloxan Induced Diabetic Rats. Nig J Nat Prod and Med. 2007;11:26–28. [Google Scholar]
- 16.Edem DO, Ekanem IS, Ebong PE. Effect of aqueous extracts of alligator pear seed (Persea americana Mill) on blood glucose and histopathology of pancreas in alloxan-induced diabetic rats. Pak J Pharm Sci. 2009;22(3):272–276. [PubMed] [Google Scholar]
- 17.Mahadeva RUS, Mainul H, Atif AB. Insulin Stimulative and Anti-Oxidative Effects of Persea americana Fruit Extract on Streptozotocin Induced Hyperglycemic Rats. J Med Biol Sci. 2011;4(1):1–10. [Google Scholar]
- 18.N'guessan K, Amoikon KE, Soro D. Effect of Aqueous Extract of Persea Americana Seeds on the Glycemia of Diabetic Rabbits. Eur j Sci Res. 2009;26(3):376–385. [Google Scholar]
- 19.Matos FJA. 2nd ed. Brasil (BR): Fortaleza; 2000. Introdução a Fitoquímica Experimental. [Google Scholar]
- 20.João JJGL, Érika HSB, Rossana AC, Raimunda SNB, José JCS, Luciana MB, et al. Chemical composition, toxicity and larvicidal and antifungal activities of Persea americana (avocado) seed extracts. Revista da Sociedade Brasileira de Medicina Tropical. 2009;42(2):110–113. doi: 10.1590/s0037-86822009000200003. [DOI] [PubMed] [Google Scholar]
- 21.Harborne JB. London (UK): Chapman and Hall Ltd; 1973. Phytochemical Methods: A guide to modern techniques of plant analysis; –49.pp. 188 [Google Scholar]
- 22.Antia BS, Okokon JE, Okon PA. Hypoglycemic activity of aqueous leaf extract of Persea americana Mill. Indian J Pharmacol. 2005;37(5):325–326. [Google Scholar]
- 23.Edem DO. Hypoglycemic Effects of Ethanolic Extracts of Alligator Pear Seed (Persea Americana Mill) in Rats. Euro J Sci Res. 2009;33(4):669–678. [Google Scholar]
- 24.Bonilla JV, Gilbertsville KY. Methods and Composition for regulation of blood cholesterol. Afri J Traditional Complement Altern Med. 2009;6(4):573–578. [Google Scholar]
- 25.Lukačínová AJ, Mojžiš R, Beňačka J, Keller T, Maguth P, Kurila L, et al. Ništiar: Preventive Effects of Flavonoids on Alloxan-Induced Diabetes Mellitus in Rats. Acta Vet Brno. 2008;77(2):175–182. [Google Scholar]
- 26.Nakashima N, Kimura I, Kimura M, Matsura H. Isolation of pseudoprototimosaponin AIII from rhizomes of anemarrhenaasphodeloides and its hypoglycemic activity in steptozotocin-induced diabetic mice. J Nat Prod. 1993;56(30):345–350. doi: 10.1021/np50093a006. [DOI] [PubMed] [Google Scholar]
- 27.Tiwari AK, Rao JM. Diabetic mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Curr Sci. 2002;83(1):30–37. [Google Scholar]
- 28.Sievenpiper JL, Arnason JT, Leiter LA, Vuksan V. Null and Opposing Effects of Asian Ginseng (Panax ginseng C A Meyer) on Acute Glycaemia: Result of Two Acute Dose Escalation Studies. J Am Coll Nutr. 2003;22(6):524–532. doi: 10.1080/07315724.2003.10719331. [DOI] [PubMed] [Google Scholar]
- 29.Item JA, Patrick EE, Godwin EE, Mfon IA, Edem EA. Histological Effect of Combined Extracts of Vernoniaamygdalina and AzadirachtaIndica on Normal and Diabetic Rats: the Pancreas and Liver. Res J Agr Biol Sci. 2010;6(4):514–521. [Google Scholar]
- 30.Teoh SL, Azian AL, Das S. Histological changes in the kidneys of experimental diabetic rats fed with Momordica charantia (bitter gourd) extract. Romanian J Morphol Embryo. 2010;51(1):91–95. [PubMed] [Google Scholar]
- 31.World Health Organization (WHO/FOA) Geneva (SE): WHO/FOA; 2003. Diet, nutrition, and the prevention of chronic diseases. [PubMed] [Google Scholar]