Abstract
Chronic muscle pain affects 20–50% of the population, is more common in women than men, and is associated with increased pain during physical activity and exercise. Muscle fatigue is common in people with chronic muscle pain, occurs in response to exercise and is associated with release of fatigue metabolites. Fatigue metabolites can sensitize muscle nociceptors which could enhance pain with exercise. Using a mouse model we tested whether fatigue of a single muscle, induced by electrical stimulation, resulted in enhanced muscle hyperalgesia and if the enhanced hyperalgesia was more pronounced in female mice. Muscle fatigue was induced in combination with a sub-threshold muscle insult (2 injections of pH 5.0 saline) in male and female mice. We show that male and female mice, fatigued immediately prior to muscle insult in the same muscle, develop similar muscle hyperalgesia 24h later. However, female mice also develop hyperalgesia when muscle fatigue and muscle insult occur in different muscles, and when muscle insult is administered 24 hours after fatigue in the same muscle. Further, hyperalgesia lasts significantly longer in females. Finally, muscle insult with or without muscle fatigue results in minimal inflammatory changes in the muscle itself, and sex differences are not related to estradiol (ovariectomy) or changes in brainstem activity (pNR1). Thus, the current model mimics muscle fatigue-induced enhancement of pain observed in chronic muscle pain conditions in the human population. Interactions between fatigue and muscle insult may underlie the development of chronic widespread pain with an associated female predominance observed in human subjects.
1. Introduction
Chronic pain affects over 100 million Americans [31] and chronic musculoskeletal pain is the most common type of chronic pain affecting up to 47 percent of the population [16]. Fibromyalgia (FM) and myofascial pain syndrome (MPS) are syndromes of chronic muscle pain, characterized by both somatic (focal tenderness, muscle pain, fatigue) and psychological (depression, anxiety, difficulty concentrating, insomnia) symptoms [7,37]. These diseases can be devastating: 25% of FM patients are unable to work and those who can work report significant pain- and fatigue-limited loss of productivity [6,15]. Further, these conditions are more common in women than men, suggesting differences in mechanisms of widespread pain between the sexes. Aerobic exercise improves symptoms in people with FM and MPS [33,68], but can acutely exacerbate pain in this population [45,69]. This results in aversion to physical activity, sedentary lifestyle, and decreased functional capacity [74].
While these clinical observations suggest a link between fatiguing exercise and muscle pain, the pathophysiology remains elusive. Early research on FM found evidence of mitochondrial and ultrastructural changes in muscle tissue of FM patients [48], but no clear pathology or inflammation [61,62,82]. Findings of increased pain sensitivity [47], temporal summation [56,70], and decreased pain inhibition [31,46]—characteristics of central sensitization—have shifted the focus to central changes in the pain processing system [16,17]. It is, however, unclear what events initiate the sensitization.
Recently our laboratory demonstrated that a running wheel task, which fatigued the whole-body, resulted in hyperalgesia of the paw after an otherwise innocuous muscle insult [7,37,67,81], indicating generalized fatiguing exercise enhanced the nociceptive response to muscle insult. While muscle fatigue is associated with release of fatigue metabolites (H+, lactate, and ATP) that can sensitize nociceptors and produce hyperalgesia [6,9,15,29,35,38,49,57,76], the running wheel task was not associated with changes in fatigue metabolites [33,68,81]. Rather, we found blockade of central pathways during the fatigue task prevented this enhanced hyperalgesia from occurring [45,64,69]. Collectively, these data suggest that central factors are critical to the initiation of the fatigue-enhanced hyperalgesia.
The whole body nature of the running wheel task makes it difficult to localize the mechanism by which muscle fatigue alters the response to muscle insult. For example, it is unclear if fatigue of the insulted muscle is necessary for induction of hyperalgesia, suggesting fatigue metabolites bind to and sensitize peripheral terminals of nociceptors to produce the enhanced hyperalgesia. Alternatively, muscle fatigue may enhance the response to the low-dose muscle insult even when the muscle insult and fatigue are applied to different muscles, indicating that central structures are involved in producing the enhanced hyperalgesia. Whether fatigue-related signals from a single muscle are sufficient, or if fatigue across multiple muscles is required for induction of the enhanced hyperalgesia is also unanswered by the prior running wheel studies. Therefore the purpose of this study was to determine if localized muscle fatigue is sufficient to produce hyperalgesia when combined with pH 5 saline injections (muscle insult) and to test for sex differences in the initiation and nature of muscle pain.
2. Materials and Methods
The experiments outlined below induced localized muscle fatigue in male and female mice using electrically-induced isometric contractions in the gastrocnemius muscle which are expected to maximize accumulation of fatigue metabolites. We combined this localized muscle fatigue with a subthreshold muscle insult (2 pH 5.0 saline intramuscular injections) neither of which produced muscle hyperalgesia when given alone (see below for more details). For comparison we combined the muscle fatigue with neutral intramuscular saline injection (pH 7.2). Nociceptive behaviors were assessed as muscle withdrawal thresholds to pressure applied over the belly of the gastrocnemius muscle and decreases in thresholds were considered hyperalgesia (see below). All experiments were approved by the Institutional Animal Care and Use Committee and performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and IASP Ethical Guidelines for the Use of Animals in Research. Both male and female C57BL6/J mice from Jackson Laboratories (n=215), age 4–8 weeks, were used in all studies.
2.1. Electrically Stimulated Fatiguing Muscle Contractions
To induce muscle fatigue, mice were deeply anesthetized with 2–4% isoflurane. Fatiguing muscle contractions were produced by applying electrical pulses through needle electrodes implanted in the proximal portion of the gastrocnemius muscle using a modified Burke protocol [10,25,74,75] that was confirmed empirically. The electrical pulses were generated by a Grass S88 solid-state square wave stimulator (Grass Technologies, West Warwick, RI). To test total force output before and after fatiguing contraction, maximum force contractions were elicited by applying high-frequency (100 Hz), supramaximal stimulus trains: 7 volts (V) pulses with 1 ms pulse duration. Each train lasted 500 ms with 3 second (s) between trains. To produce muscle fatigue, supra-maximal, moderate frequency stimulations (40 Hz) were applied to the muscle by applying trains consisting of 90, 1 ms pulses (7 V) (train duration 3.75 s) every 8 sec (rest intervals 4.25 s between trains). Correct electrode placement in the gastrocnemius was confirmed by plantarflexion of the ankle joint without activation of toe flexors, tibialis anterior muscle, or muscles above the knee.
A trial consisted of three maximum contractions (100 Hz stimulation) to establish the baseline force, six minutes (m) of sub-maximal (40 Hz stimulation) fatiguing contractions, and three maximum contractions immediately after the fatiguing contractions. A subset of animals from the behavior experiments were analyzed for baseline force and decline in force (male n=12, female n=13). In a separate group of animals (male, n=3), force recovery was monitored at 2, 4, 6, 8, and 10 m following completion of the fatiguing task, again using 3 maximum contractions. Force was continuously measured by attaching the hindpaw to an iWorx FT-302 force transducer (iWorx, Dover, NH) and sampling the analog output at 100 Hz using LabVIEW software (National Instruments, Austin, TX). All force measurements were then computed off-line using Freemat and Python scripts. Fatigue was operationally defined as the decline in force between baseline and post-fatigue maximum force contractions.
2.2. Low-Intensity Muscle Insult
The low-intensity muscle insult consisted of two intramuscular (i.m.) injections of 20 μl normal saline into the gastrocnemius muscle 5 days apart. The pH of the normal saline was adjusted with HCl to pH 5.0 ± 0.1. Control injections consisted of two injections of normal saline (pH 7.2 ± 0.1) 5 days apart. The unbuffered pH 5 saline injections reduce muscle pH to approximately 6.9, which is comparable to decreases seen after intense exercise [2–4,65]. Neither 2 injections of pH 5.0 nor 2 injections of pH 7.2 produce hyperalgesia [61,62,81,82].
2.3. Pain Behavior
Muscle withdrawal thresholds (MWT) were measured by applying force sensitive tweezers to the belly of the gastrocnemius as previously described, where lower thresholds indicate greater sensitivity [47,63]. Mice were acclimated to this behavioral paradigm in two 5 minute sessions over a two day period prior to the first injection. Briefly, mice were placed in a gardener’s glove, the hindlimb was held in extension, and the muscle was squeezed with force sensitive tweezers until the animal withdrew its hinblimb. An average of 3 trials per animal was taken at each time period. A decrease in withdrawal thresholds was interpreted as muscle hyperalgesia.
2.4 Ovariectomy
To test the role of estrogen on sex-dependent effects observed in the current study, female C57BL6/J mice were ovariectomized. Briefly, each animal was deeply anesthetized with 2–4% isoflurane and the ovaries were removed by an abdominal approach as we previously published [67]. Animals were given 100 μl of 0.3mg/ml buprenorphine every 12h for 3 days and monitored daily for 5 days. One week after ovariectomy, behavioral experiments were done.
2.5. Experimental Protocol: Induction of Muscle Hyperalgesia
2.5.1. Standard Protocol
We performed a set of 3 experiments to address our aims, with slight variations in each. However, the common protocol across all experiments was as follows. On day 0, baseline muscle withdrawal thresholds were assessed and the first saline injection was administered. Fatiguing muscle contractions were induced prior to the second saline injection on day 5. On day 6, the muscle withdrawal thresholds were reassessed. See below for experiment-specific details.
2.5.2. Experiment 1: Necessity of Acidic Saline and Fatigue Parameters in Initiating Muscle Hyperalgesia
This experiment tested if localized (i.e., single muscle) muscle fatigue combined with a low-intensity muscle insult (2 injections of pH 5.0) produced muscle hyperalgesia compared to four control conditions. On day 1, mice (males n=6, females n=6 for each condition, total of 60 mice) were tested for baseline pain behaviors and given the first saline injection. On day 5, the second saline injection was administered immediately after completing the set of fatiguing muscle contractions to the homonymous gastrocnemius muscle. Each cohort of 12 animals was exposed to one of the following conditions: (1) two pH 5 injections with fatiguing muscle contractions (experimental),;(2) two pH 5 saline injections without fatiguing muscle contractions (control); (3) two pH 5 saline injections with two sets of maximum contractions 6 minutes apart (without the fatiguing muscle contractions)(control); (4) fatiguing muscle contractions with two pH 7.2 saline injections (control); or (5) a single pH 5 saline injection immediately after the fatiguing muscle contractions (control). Muscle withdrawal thresholds were measured bilaterally 24h after the second (or single for condition ‘5’) saline injection.
2.5.3. Experiment 2: Course of Muscle Hyperalgesia
This experiment tested the duration of hyperalgesia induced by combining muscle fatigue with pH 5.0 injections (males n=6, females n=6). Muscle withdrawal thresholds were assessed 24h, 3d, 5d, 7d, and weekly afterwards until the pain behavior returned to baseline (6 weeks).
2.5.4. Experiment 3: Final Acidic Saline Injection after Muscle Fatigue
To test for a time-dependent effect between pH 5 saline injections and muscle fatigue, we varied the time between the second acid injection and fatiguing muscle contractions (for each condition males n=6, females n=6, total of 36). For each condition, mice received the first pH 5 saline injection on day 0 and on day 5 received the second pH 5 saline injection. We applied the fatiguing muscle contractions to the homonymous muscle either (1) immediately, (2) 2h, or (3) 24h (i.e., day 4) before the second pH 5 saline injection. Muscle withdrawal thresholds were measured at baseline and 24h after the second pH 5 saline injection. Muscle withdrawal thresholds were measured bilaterally 24h after the second pH 5 saline injection.
2.5.5. Experiment 4: Separation of Acidic Saline Injections and Muscle Fatigue
To test the spatial characteristics of the fatigue-enhanced hyperalgesia response, the pH 5.0 injections and muscle fatigue were applied to homonymous and heteronymous muscles (for each condition males n=6, females n=6, total of 24 animals) and mechanical hyperalgesia tested bilaterally. Two pH 5 saline injections spaced 5 days apart were combined with the fatiguing muscle contractions immediately prior to the second injection. However, the two conditions varied by whether the pair of saline injections were given to the fatigued (ipsilateral) gastrocnemius muscle or the unfatigued (contralateral) gastrocnemius muscle. Muscle withdrawal thresholds were measured bilaterally 24h after the second pH 5 saline injection.
2.6. Experiment 5: Assessment of Inflammatory Changes in Treated Muscle
Inflammatory changes were assessed by histopathologic evaluation of tissues as well as by measuring tissue levels of myeloperoxidase. Mice were given two pH 5.0 saline injections five days apart with muscle fatigue immediately before the second injection. Controls consisted of animals that received (1) no treatment, i.e. naïve, (2) two pH 5 saline injections without muscle fatigue, (3) two pH 7.2 saline injections with muscle fatigue, (4) or a single injection of 3% carrageenan (positive control). 24 h after the treatment, mice were euthanized by exposure to 100% CO2 for 5 minutes followed by thoracotomy. For histological analysis, the gastrocnemius muscles (for each condition, males n=2, total of 10 mice) were fixed in 10% neutral buffered formalin for 72 hours. These specimens were then embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E) for histological examination. A board-certified veterinary pathologist, who was blinded to treatment group, assessed the slides.
As a marker for neutrophilic infiltration, myeloperoxidase concentration was quantified by spectrophotometry. The gastrocnemius muscle (for each condition, males n=6, total of 30 mice) was harvested and weighed. The tissue was minced with scissors before being homogenized in 1 mL 0.5% hexadecyltrimethyl ammonium bromide (HTAB) on ice. The homogenate was then treated with 3 freeze-thaw cycles by immersion in 95% ethanol chilled by crushed dry ice followed by immersion in hot water. Lysates were centrifuged at 1,000 RPM for 15 minutes and 10 μL was plated in triplicate on 96 well flat bottom cell culture plates. 190 μl of freshly prepared assay reagent (10 mg o-dianisidine dihydrochloride with 1 μl 30% hydrogen peroxide dissolved in 50 mL 1M phosphate buffer) was then added to the lysate. Each plate also included a serial dilution of stock myeloperoxidase as a standard curve. Absorbance of light at 450 nm was measured using a microplate reader (SpectraMax Plus384) running SoftMax.
2.7 Experiment 6: Quantification of pNR1 Positive Cells in the RVM
To test if sex differences were due to altered modulation of central facilitatory pathways, previously found to show sexual dimorphism in response to noxious stimuli [50,74], we tested if there was increased phosphorylation of the NR1 (pNR1) subunit of the NMDA receptor (pNR1). We examined pNR1 in the rostral ventomedual medulla (RVM) since previous studies in our laboratory using animal models of muscle pain show 1) increased pNR1 immunoreactivity, 2) blockade of NMDA receptors reduces hyperalgesia, 3) downregulation of NR1 reduces muscle hyperalgesia, and 4) over-expression of NR1 induces muscle hyperalgesia [20,21,64,72]. Male and female mice were given two pH 5.0 saline injections five days apart with muscle fatigue immediately before the second injection. Comparison groups consisted of naïve mice for each sex. 24 h after the second injection, mice were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 4% paraformaldehyde. Brains were removed, and the RVM was blocked, embedded in OCT, and frozen at −20 C until analysis. Sections were cut on a cryostat at 20 μm through the medulla and placed on slides. Serial sections containing the RVM were immunohistochemically stained as previously described [21,64]. Sections from all animals were stained simultaneously using a primary rabbit polyclonal antibody against phosphorylated serine 897 of the NMDA R1 subunit (pNR1) (Millipore, 1:500 dilution). Sections were incubated overnight in the primary antibody followed the next day by 1h incubation with biotinylated goat anti-rabbit (Life Technologies, 1:200) and then 1h incubation in streptavidin-Alexa 568 conjugate (Life Technologies, 1:200). Images of the RVM were taken at 20× magnification on an Olympus BX-51 light microscope using the same settings for each section and between animals. Quantification was performed of line using Image J and counting the number of stained cells in the RVM in five sections per animal as previously described [21,64]. As a control for staining, the facial nucleus, contained in the same sections as the RVM, was similarly quantified.
2.8 Statistical Analysis
Data are reported as the mean ± S.E.M. The effects of the fatiguing muscle contractions on force output were compared to baseline using student’s t tests. Repeated measures ANOVA were used for assessing fatigue recovery and behavioral measurements, with Duncan’s post-hoc tests for between group follow-up assessments. Student’s t-tests with rank ordered Bonferroni corrections were used to test for differences between sexes across time. One-way ANOVA with Duncan’s post-hoc test was used to analyze the myeloperoxidase assay, and immunohistochemistry data. For all experiments, p<0.05 was considered statistically significant.
3. Results
3.1. Electrical stimulation of muscle produces short-lasting muscle fatigue
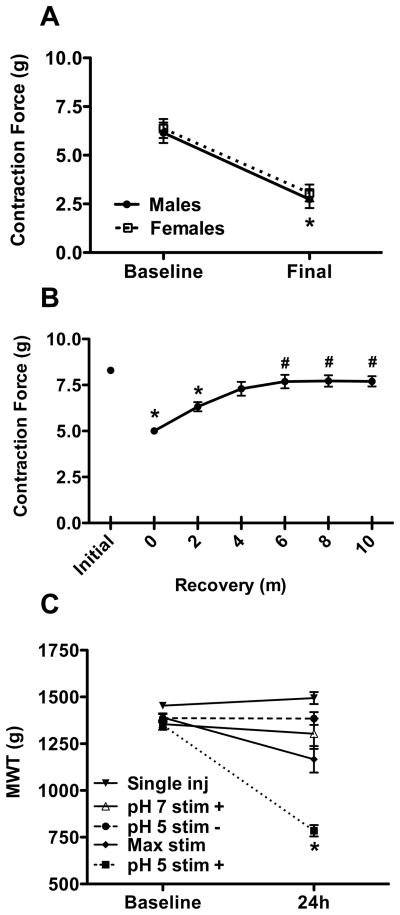
To induce local muscle fatigue we electrically stimulated the gastrocnemius muscle for 6 minutes using a modified Burke protocol [11,25,56,70,75] to produce isometric contractions that resulted in an approximately 50% decrease in force. Maximum intensity, high frequency electrical stimulation produced 6.2 (+/− 0.5 SEM) and 6.4 (+/− 0.5 SEM) grams (g) of force at baseline in male and female mice, respectively (fig 1A), and there was a similar decrease in force between the sexes. This decrease in force recovered rapidly, returning to 93% of baseline by 10 minutes (fig 1B). There were no significant differences in force (pH 5 = 6.8 ± 0.4g; pH 7.2 = 5.5 ± 0.6g) or fatigue magnitude (pH 5 = 54.1 ± 4.5%; pH 7.2 = 60.9 ± 3.8%) between animals treated with pH 5.0 or pH 7.2 saline.
Fig. 1.
Electrically stimulated muscle contractions result in muscle fatigue and enhance the response to pH 5 saline injections. (A) Force elicited by 100 Hz electrical stimuli before and after 6 m fatiguing contractions (males n=12, females n=13) * p < 0.05, difference from baseline. (B) Recovery of force after 6 m fatiguing contractions (n=3) * p < 0.05, difference from initial, # p < 0.05, difference from start of recovery. (C) Behavioral measure of sensitivity to mechanical stimuli at baseline and after treatment with a single pH 5 saline injection with electrically stimulated muscle contractions (males, n=6), two pH 5 acidic saline injections alone (pooled, males & females n=6 each), two pH 7.2 saline injections with electrically stimulated muscle contractions (pooled, males & females n=6 each), two pH 5 saline injections with test contractions but not fatigue (pooled, males & females n = 6 each), or two pH 5 saline injections with electrically stimulated muscle contractions (pooled, males & females n=6 each) * p < 0.05, difference from baseline and control groups at 24h.
3.2 Muscle fatigue combined with sub-threshold muscle insult produces hyperalgesia in a sex-dependent manner
3.2.1. Combination of pH 5.0 Saline and Muscle Fatigue is Necessary to Produce Muscle Hyperalgesia
To determine if pH 5.0 saline must be combined with local muscle fatigue to produce hyperalgesia, we measured MWT in mice exposed to either pH 5.0 saline or local muscle fatigue, or both in combination. There was a significant decrease in muscle withdrawal thresholds in mice that received two pH 5.0 saline injections in combination with muscle fatigue (Fig 1c) when compared to baseline or controls. Mice that received two pH 5.0 saline injections without muscle fatigue, a single pH 5.0 saline injection with muscle fatigue, two pH 7.2 saline injections with muscle fatigue, or pH 5.0 with only the maximum contractions but no fatigue showed no change in their muscle withdrawal thresholds (F4,51 = 19.957, p < 0.001) (fig 1C).
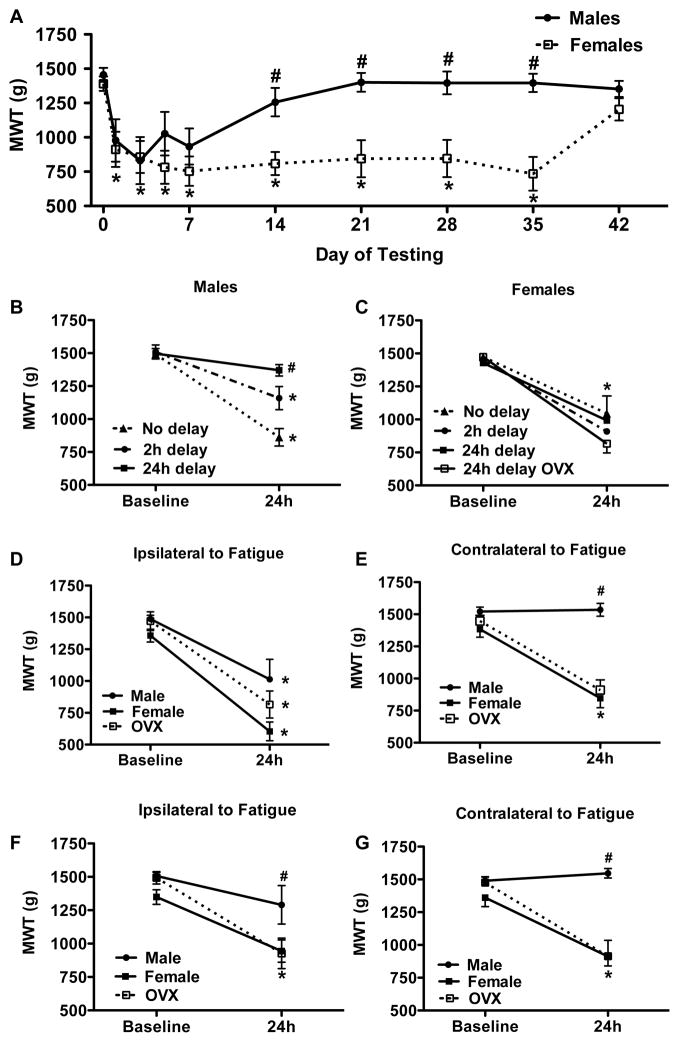
3.2.2. Muscle Hyperalgesia Lasts Significantly Longer in Female Mice
To examine the duration of the decreased muscle withdrawal thresholds we followed mice for up to 6 weeks after the second acidic saline injection. There was a significant sex difference in the duration of the decreased muscle withdrawal thresholds after combining muscle fatigue with muscle insult (F1,10 = 16.765, p=0.002). Muscle withdrawal thresholds returned to baseline by day 14 in males, but not until day 42 in females (fig 2G). Post-hoc tests confirmed significant differences in withdrawal thresholds between males and female mice after the 14 day period.
Fig. 2.
Sex differences in mechanical hypersensitivity after repeated pH 5 saline injection and electrically stimulated muscle contractions. (A) Duration of mechanical hypersensitivity (n=6) * p < 0.05, difference from baseline, # p < 0.05, difference from females. (B & C) The final pH 5 saline injection was delayed after electrically stimulated muscle contractions for 0, 2, or 24 h (n=6 per group) * p < 0.05, difference from baseline, # p < 0.05 difference from intact and ovariectomized females. (D, E, F, G) A spatial relationship between pH 5 saline injection and electrically stimulated muscle contractions was tested by varying the location of acidic saline injection (n=6) * p < 0.05, difference from baseline, # p < 0.05, difference from intact and ovariectomized females. (D & E) pH 5 saline injections and electrically stimulated muscle contractions were given into the same muscle and mechanical hypersensitivity was tested in the gastrocnemius muscles (D) ipsilateral and (E) contralateral to the site of treatment. (F & G) pH 5 saline injections were given to the gastrocnemius muscle contralateral to the site of electrically stimulated muscle contractions. Mechanical hypersensitivity was tested in the muscle (F) ipsilateral and (G) contralateral to the site of fatigue.
3.2.3. Delaying Final Acidic Saline Injection after Muscle Fatigue Results in Time-Dependent Decrease in Hyperalgesia for Male, but not Female Mice
To determine if the muscle fatigue had to be done in close time proximity to the muscle insult, we performed the muscle fatigue task immediately, 2h, or 24h prior the muscle insult. Significant sex differences were observed between the time for induction of muscle fatigue and the muscle insult (F6,37 = 3.208, p=0.012). For males the greatest decrease in withdrawal thresholds occurred when muscle fatigue was induced immediately before the second injection, an intermediate effect was seen for 2h delay, and no decrease in withdrawal thresholds occurred when the fatigue task was given 24h before the second injection (fig 2A). Surprisingly, in females withdrawal thresholds did not vary significantly between time intervals – muscle fatigue induced immediately, 2h, or 24h before the second injection resulted in comparable decreases in withdrawal thresholds (fig 2B). Post-hoc tests revealed a significant difference between males and females for the group in which muscle fatigue was induced 24h before the second injection.
3.2.4. Spatial Separation of Acidic Saline Injections and Muscle Fatigue Fails to Produce Hyperalgesia in Male, but not Female Mice
To test the spatial characteristics of the fatigue-enhanced response to muscle insult, muscle fatigue and muscle insult were applied to different muscles and hyperalgesia was tested bilaterally. Again, significant sex differences were observed (F11,64 = 10.786, p < 0.001). In males when muscle fatigue and muscle insult occurred in the same muscle there was a significant decrease in withdrawal thresholds ipsilaterally, but not contralaterally. When the muscle fatigue and muscle insult were given to heteronymous gastrocnemius muscles, male mice showed no change in withdrawal thresholds (figs 2D–G). In contrast, female mice showed decreased muscle withdrawal thresholds bilaterally regardless of whether the localized muscle fatigue and muscle insult occurred in the same or contralateral muscles (figs 2D & 2E).
3.3. Muscle insult and fatigue is not associated with acute muscle inflammation
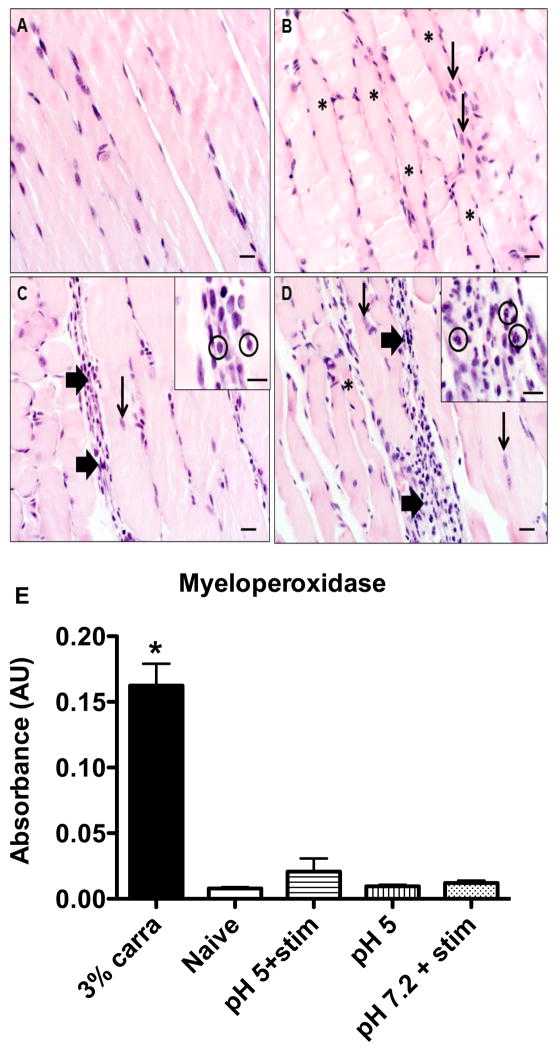
To determine if there was muscle damage and inflammation the muscles were examined histologically. All groups, naïve controls, pH 7.2 controls, pH 4.0 injection, and fatigued cohorts demonstrated mild, multifocal myofiber degeneration, as characterized by myocyte hypereosinophilia and loss of cross-striations. Similarly, all groups also exhibited signs of mild, multifocal regeneration as characterized by centralized rowing of nuclei. Compared to naïve controls, animals treated with acidic saline, with or without fatigue, had a mild, multifocal lymphoplasmacytic infiltrate, but notably lacked neutrophils (fig. 3A–C). In contrast, 3% carrageenan was used as a positive control and showed primarily a moderate to sometimes marked multifocal to coalescing neutrophilic infiltrate (fig. 3D).
Fig. 3.
Inflammation in muscle treated with repeated pH 5 saline injections and electrically stimulated muscle contractions. Representative hemotoxylin and eosin stained gastrocnemius muscle sections taken from (A) naive mice and 24h after treatment with (B) repeated pH 5 saline injections alone, (C) electrically stimulated muscle contractions with pH 5 saline injections, and (D) 3% carrageenan. Thin arrows indicate centralized rowing of myocyte nuceli, indicative of regeneration, thick arrows show sites of multifocal inflammatory cell infiltrates which are magnified within the insets where specific cell types are circled (in (C) it is lymphocytes, in (D) it is neutrophils) and asterisks indicate degenerative myocytes, scale bars = 20 μm (inset scale bars = 10 μm). (E) Colorimetric assay to quantify myeloperoxidase, a neutrophil marker, in whole, homogenized gastrocnemius muscle tissue in naive mice (n=7) and after treatment with 3% carrageenan (n=6), repeated pH 5 saline injections alone (n=7), repeated pH 5 injections with electrically stimulated muscle contractions (n=7), and repeated pH 7.2 saline injections with electrically stimulated muscle contractions (n=7). * p < 0.05.
The absence of neutrophils in response to muscle fatigue and muscle insult was confirmed by assaying for myeloperoxidase from whole muscle tissue. Mice treated with muscle fatigue and pH 7.2 saline, pH 5 saline alone, or muscle fatigue with pH 5.0 saline showed quantities of myeloperoxidase comparable to the naïve control while those treated with 3% carrageenan had significantly greater concentrations (F6,47 = 61.167, p < 0.001) (fig. 3E).
3.4 Ovariectomy has no effect on sex differences in the induction of muscle hyperalgesia
To determine if circulating estrogen levels were responsible for the sex differences observed in the behavioral experiments, we compared ovariectomized females to intact males and females in the acidic saline fatigue model. We found that ovariectomized mice behave no differently than gonadally intact females—they develop similar bilateral hyperalgesia whether acidic saline is given to the heteronymous or homonymous muscle (fig. 2D–G) and when acidic saline administration is delayed by 24 h (fig. 2B).
3.5 No changes in pNR1 in the RVM after Treatment with Acidic Saline and Muscle Fatigue
To examine if the sex differences were related to changes in central excitability, we examined if there was enhanced pNR1 in the RVM, previously shown to facilitate pain through NMDA receptors and the NR1 subunit [21,64,67]. Sections of RVM stained for pNR1 were examined for the number of positively-staining cells. No significant differences in the number of pNR1 positive cells in the RVM were found between naïve and treated animals, or between sexes (see Table 1). The facial nucleus similarly showed no significant differences between sexes or treatment.
Table 1.
pNR1 staining in the RVM of mice treated with repeated pH 5 injections and electrically stimulated muscle contractions. Total number of cells stained for pNR1 across 5 sections containing the RVM or facial nucleus. There were no significant differences by sex, treatments or location.
| Group | RVM | Facial Nucleus | |
|---|---|---|---|
| Males- Fatigue & Acid | 24 +/− 1.5 | 29.3 +/− 2.0 | N=3 |
| Males – No Treatment | 26.75 +/− 2.1 | 26.5 +/− 3.6 | N=4 |
| Females – Fatigue & Acid | 27 +/− 5.1 | 24.7 +/− 5.2 | N=3 |
| Females – No Treatment | 28.5+/− 2.9 | 24.75 +/− 3.9 | N=4 |
4.0 Discussion
The current study shows that combining localized muscle fatigue with a low-intensity muscle insult results in long-lasting muscle hyperalgesia that parallels previous studies using whole-body fatiguing exercise [67]. Accordingly, priming of innocuous muscle insult to produce hyperalgesia does not require widespread input from multiple fatiguing muscles. Initiation of this hyperalgesia is sex-dependent with female mice showing a wider window of time and a greater distance between muscle fatigue and muscle insult to produce hyperalgesia, and longer duration of hyperalgesia. Finally, this model does not produce acute inflammation or tissue damage. These data suggest that combining two low intensity muscle insults with localized muscle fatigue produces sexually dimorphic patterns of muscle hyperalgesia.
Chronic musculoskeletal pain is more prevalent among women [7,8,37,42,73]. Consistent with this, we found significant differences between sexes in both the requirements for initiation of hyperalgesia and the nature of hyperalgesia induced by combining muscle fatigue with a subthreshold muscle insult. Our preliminary experiment showed ovariectomy does not alter the hyperalgesia in female mice, suggesting release of estradiol from the ovaries at the time of induction is not necessary for the development of hyperalgesia and differs from our prior study showing ovariectomy reduces sex-differences in whole-body fatigue induced pain [67]. The mechanisms for the sex differences in the current study are unclear. . Masculinization of the brain by testosterone or sex chromosome-linked genes remain potential mechanisms for these sex differences and future studies are needed [5].
Alternatively, differential processing of nociceptive input centrally could also contribute to sex differences. Previous studies show that contralateral hyperalgesia in uninjured tissue is mediated by central mechanisms, since removal of afferent fiber input from the site of insult has no effect on the contralateral hyperalgesia [18,19,23,31,44,79,80]. Bilateral hyperalgesia develops in both sexes after unilateral treatment with pH 4.0 saline or pH 5.0 saline (low-intensity insult) in combination with whole-body fatigue [16,65,81], suggesting central sensitization occurs in both sexes when the stimuli are high intensity (pH 4.0) or widespread (whole-body fatigue). However, the present study shows sex differences emerge when animals are treated with low intensity, focal stimuli. In human subjects, females have greater central excitability in a number of measures: temporal summation [7,27,37,59,60], secondary hyperalgesia [6,15,39], referred pain [30,33,68], and decreased conditioned pain modulation [1,32,45,69]. The basis for greater central excitability is unknown, but sex differences occur in the connectivity and activity of the periaqueductal gray (PAG) and rostral ventromedial medulla (RVM), regions implicated in pain modulation [50,74]. While increased levels of pNR1 staining in the RVM has been associated with fatigue and pain [64], the current study found no differences between treated and naïve mice. Higher intensity stimuli may be necessary for elevations in pNR1. Whether this means widespread muscle hyperalgesia is mediated by different molecular mechanisms in the RVM, a different structure in the CNS, or some other process requires further study.
The current study also shows that females develop hyperalgesia even when there is a substantial delay between muscle fatigue and the muscle insult. This suggests that females fail to attenuate an ongoing central response to muscle fatigue. This has significant consequences for the initiation of muscle pain, as seemingly innocuous stimuli—pH 5.0 saline and isolated muscle fatigue—given far apart in time and space, are able to converge on central structures and produce widespread hyperalgesia in females. The prolonged nature of the hyperalgesia observed in females compared to males also points to greater central sensitivity as the hyperalgesia presumably exceeds the duration of muscle insult. These data indicate that females have less stringent requirements for the onset and significantly greater duration of muscle hyperalgesia, which could account for the greater probability of developing muscle pain observed in female patients.
Chronic muscle pain syndromes, such FM and MPS, are characterized by constant pain at rest, enhanced pain in response to pressure applied to the muscle, and enhanced pain with acute exercise [7,37,40,61,62,69,82]. We chose isometric contractions at a force sufficient to occlude muscle perfusion in order to maximize accumulation of fatigue metabolites while minimizing tissue damage [47,58,59]. Fatigue metabolites, such as protons, lactate and ATP, contribute to loss of muscle force [43,56,70] and activate receptors located on nociceptors, acid sensing ion channels (ASICs) and purinergic receptors, respectively [34,35,46,52,54,57]. ASICs and purinergic receptors are well-established to play a role in nociceptive processing including that from the muscle [17,22,36,41,66,77]. Further, acid, lactate and ATP can interact to produce a potentiated response. ASICs demonstrate enhanced sensitivity to pH changes when bound by lactate or in the presence of ATP [9,38,67,81]. Similarly, dorsal root ganglia treated with acid, lactate and ATP show enhanced intracellular calcium compared to those receiving each treatment alone [9,29,35,38,49,57,76]. Primary afferent fibers show robust responses to both ATP and acid [35,57,81]. Notably, these metabolites return to normal within minutes to hours after a fatiguing task with a concomitant recovery of muscle force [43,64].
A range of pH solutions and exercise durations have previously been examined in the acidic saline model of muscle pain. pH 4 saline injections produce bilateral hyperalgesia regardless of exposure to whole-body exercise and pH 5 saline injections produce hyperalgesia only in animals that perform fatiguing exercise. Higher pH solutions, 6 or 7.2, do not produce hyperalgesia even in exercising animals [10,25,75,81]. Further, mice treated with 30 min or 2h of running wheel activity alone do not develop hyperalgesia [64,81]. While this data does not indicate if the interaction between fatigue and acidic saline is additive or synergistic, it does suggest that the threshold for muscle hyperalgesia is significantly lower when acidic saline injections are paired with even brief bouts of fatiguing exercise.
Release of metabolites from fatiguing stimuli may sensitize and prime the nociceptors, resulting in enhanced response to a subsequent low-intensity muscle insult [9,38,63]. Thus, fatigue metabolites likely contribute to the development of muscle pain by priming muscle nociceptors for a greater response to the subsequent muscle insult. Interestingly, delaying the final muscle insult after muscle fatigue resulted in a time-dependent decrease in hyperalgesia in male mice, but not female mice. Presumably, this delay reduced concentrations of fatigue metabolites and allowed partial recovery from priming effects. When considered with the localized nature and shorter duration of hyperalgesia in male mice, these findings suggest that, in the absence of central sensitization, hyperalgesia in response to low-intensity muscle insult in male mice derives from these peripheral mechanisms.
Acute exercise has profound effects on both the innate and acquired immune system, leading to catecholamine- and cortisol-related transient elevations of circulating leukocytes [11,25,75,78]. In the present study, no neutrophils or macrophages were observed in the tissue 24h after treatment, likely due to the fact that circulating levels of these cells return to normal before 24h [67,78]. Neutrophils are also recruited to sites of tissue damage and some types of acute exercise, such as eccentric contractions, result in damage and inflammation of muscle tissue with subsequent delayed onset muscle soreness (DOMS) [7,8,26,37,42,71,73]. In the current study, the absence of neutrophils in the muscle suggests that acute inflammation is not contributing to the hyperalgesia observed after isometric muscle contractions, distinguishing fatigue-enhanced hyperalgesia from DOMS. While we find no evidence of recruitment of these cells in the affected muscle, it is possible that release of cytokines from local or circulating inflammatory cells could contribute to muscle pain.
Consistent with earlier work, we found mild lymphoplasmacytic infiltration in mice that received pH 5.0 injections, but not those receiving pH 7.2 with electrically stimulated muscle contractions[65]. Lymphocytes are a functionally diverse population and the exact nature of the lymphoplasmacytic infiltrate after injection of pH 5.0 is unknown. Lymphocytes have the potential to enhance pain states by releasing pro-algesic factors, e.g. TNF-α, IL-1, and prostaglandins [53,67] and are present in chronically inflamed tissue [12,13,24,55]. However cultured lymphocytes (cytotoxic T-cells) exposed to decreases in pH show pH-dependent decreases in inflammatory cytokine release evoked by specific antigens [14,28,51]. Further, the current study shows comparable lymphocyte infiltration between the group that developed hyperalgesia (pH 5.0 saline with muscle fatigue) and a control that failed to develop hyperalgesia (pH 5.0 saline alone), suggesting lymphocytes could contribute to but are not by themselves sufficient for the observed hyperalgesia.
In summary, we show that localized fatiguing isometric contractions in a single muscle treated with acidic saline is sufficient for the development of long-lasting muscle hyperalgesia. Taken together, these data suggest that seemingly mild muscle insults, when combined, are capable of producing long-lasting and widespread muscle pain out of proportion to the injury in a sex-dependent manner. Multiple low-intensity insults could be an underlying factor in the transition from acute to chronic pain and could underlie the female predominance of muscle pain observed clinically. Understanding the mechanisms of exercise-induced pain could lead to treatments targeting the deleterious consequences of acute exercise resulting in improved compliance and a more active lifestyle in individuals with musculoskeletal pain. Therefore, this model could be valuable for exploring the mechanisms underlying fatigue-enhanced muscle pain, the greater prevalence of chronic muscle pain among females, and the factors that influence the transition from acute to chronic pain.
Summary.
Characterization of a pre-clinical model of chronic muscle pain in which muscle fatigue combined with a subthreshold muscle insult produces long lasting, sex-dependent hypersensitivity with minimal tissue damage in mice—findings consistent with clinical observations of chronic muscle pain patients.
Acknowledgments
We thank S. A. Gregory for developing software for interpreting muscle fatigue data.
Funding: National Institutes of health AR053509, AR052316 and AR061371.
Footnotes
Author contributions: NSG, LFL, and KAS planned the muscle fatigue experiments. NSG and KAS planned the behavioral experiments. NSG performed the muscle fatigue and behavioral experiments. NSG, LFL, and KAS contributed to the analysis of muscle fatigue and behavioral experiments. NSG, KGC, and KAS planned and contributed to the analysis of the histology experiments. NSG and KGC performed the histology. NSG and KAS planned and analyzed the myeloperoxidase experiment. NSG performed the myeloperoxidase experiment. All authors read, commented on, and contributed to the writing of the manuscript.
Competing Interests: The authors have no competing interests to disclose.
References
- 1.Arendt-Nielsen L, Sluka KA, Nie HL. Experimental muscle pain impairs descending inhibition. PAIN. 2008;140:465–471. doi: 10.1016/j.pain.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangsbo J, Johansen L, Graham T, Saltin B. Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J Physiol (Lond) 1993;462:115–133. doi: 10.1113/jphysiol.1993.sp019546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangsbo J, Juel C, Hellsten Y, Saltin B. Dissociation between lactate and proton exchange in muscle during intense exercise in man. J Physiol (Lond) 1997;504 ( Pt 2):489–499. doi: 10.1111/j.1469-7793.1997.489be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol (Lond) 1996;495 ( Pt 2):587–596. doi: 10.1113/jphysiol.1996.sp021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 6.Bennett RM. Fibromyalgia and the disability dilemma. A new era in understanding a complex, multidimensional pain syndrome. Arthritis Rheum. 1996;39:1627–1634. doi: 10.1002/art.1780391004. [DOI] [PubMed] [Google Scholar]
- 7.Bennett RM. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2007;21:427–445. doi: 10.1016/j.berh.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–80. doi: 10.1017/s0140525x97221485. discussion 435–513. [DOI] [PubMed] [Google Scholar]
- 9.Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke RE, Levine DN, Tsairis P, Zajac FE. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol (Lond) 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke RE, Levine DN, Tsairis P, Zajac FE. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol (Lond) 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2001;86:782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- 13.Castrillon EE, Cairns BE, Wang K, Arendt-Nielsen L, Svensson P. Comparison of glutamate-evoked pain between the temporalis and masseter muscles in men and women. PAIN. 2012;153:823–829. doi: 10.1016/j.pain.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Chanda ML, Mogil JS. Sex differences in the effects of amiloride on formalin test nociception in mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R335–42. doi: 10.1152/ajpregu.00902.2005. [DOI] [PubMed] [Google Scholar]
- 15.Chandran A, Schaefer C, Ryan K, Baik R, McNett M, Zlateva G. The comparative economic burden of mild, moderate, and severe fibromyalgia: results from a retrospective chart review and cross-sectional survey of working-age u.s. Adults J Manag Care Pharm. 2012;18:415–426. doi: 10.18553/jmcp.2012.18.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25:173–183. doi: 10.1016/j.berh.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Clauw DJ, Crofford LJ. Chronic widespread pain and fibromyalgia: what we know, and what we need to know. Best Pract Res Clin Rheumatol. 2003;17:685–701. doi: 10.1016/s1521-6942(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 18.Coderre TJ, Melzack R. Cutaneous hyperalgesia: contributions of the peripheral and central nervous systems to the increase in pain sensitivity after injury. Brain Res. 1987;404:95–106. doi: 10.1016/0006-8993(87)91359-x. [DOI] [PubMed] [Google Scholar]
- 19.Coderre TJ, Melzack R. Increased pain sensitivity following heat injury involves a central mechanism. Behavioural brain research. 1985;15:259–262. doi: 10.1016/0166-4328(85)90181-0. [DOI] [PubMed] [Google Scholar]
- 20.Da Silva LF, DeSantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. J Pain. 2010;11:378–387. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Da Silva LFS, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. PAIN. 2010;151:155–161. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dessem D, Ambalavanar R, Evancho M, Moutanni A, Yallampalli C, Bai G. Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X(3) expression in a functionally relevant manner. PAIN. 2010;149:284–295. doi: 10.1016/j.pain.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donaldson LF. Unilateral arthritis: contralateral effects. Trends Neurosci. 1999;22:495–496. doi: 10.1016/s0166-2236(99)01481-2. [DOI] [PubMed] [Google Scholar]
- 24.Dong X-D, Mann MK, Kumar U, Svensson P, Arendt-Nielsen L, Hu JW, Sessle BJ, Cairns BE. Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. NSC. 2007;146:822–832. doi: 10.1016/j.neuroscience.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enoka R, Rankin L, Joyner M. Fatigue-related changes in neuromuscular excitability of rat hindlimb muscles. Muscle Nerve. 1988 doi: 10.1002/mus.880111104. [DOI] [PubMed] [Google Scholar]
- 26.Faulkner JA, Brooks SV, Opiteck JA. Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention. Phys Ther. 1993;73:911–921. doi: 10.1093/ptj/73.12.911. [DOI] [PubMed] [Google Scholar]
- 27.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. PAIN. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 28.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 29.Forrester T, Lind AR. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol (Lond) 1969;204:347–364. doi: 10.1113/jphysiol.1969.sp008917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. PAIN. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Ge H-Y, Madeleine P, Arendt-Nielsen L. Sex differences in temporal characteristics of descending inhibitory control: an evaluation using repeated bilateral experimental induction of muscle pain. PAIN. 2004;110:72–78. doi: 10.1016/j.pain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Graff-Radford SB. Myofascial pain: Diagnosis and management. Curr Pain Headache Rep. 2004;8:463–467. doi: 10.1007/s11916-004-0068-y. [DOI] [PubMed] [Google Scholar]
- 34.Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol. 2004;96:1166–1169. doi: 10.1152/japplphysiol.01020.2003. [DOI] [PubMed] [Google Scholar]
- 35.Hoheisel U, Reinöhl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. PAIN. 2004;110:149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 36.Hori K, Ozaki N, Suzuki S, Sugiura Y. Upregulations of P2X(3) and ASIC3 involve in hyperalgesia induced by cisplatin administration in rats. PAIN. 2010;149:393–405. doi: 10.1016/j.pain.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Hsu ES. Acute and Chronic Pain Management in Fibromyalgia: Updates on Pharmacotherapy. Am J Ther. 2010 doi: 10.1097/MJT.0b013e3181d6b6d4. [DOI] [PubMed] [Google Scholar]
- 38.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 39.Jensen MT, Petersen KL. Gender differences in pain and secondary hyperalgesia after heat/capsaicin sensitization in healthy volunteers. The Journal of Pain. 2006;7:211–217. doi: 10.1016/j.jpain.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain. 2007;11:39–47. doi: 10.1016/j.ejpain.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, Cook SP, Kane S, Urban MO. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol. 2010;161:950–960. doi: 10.1111/j.1476-5381.2010.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karibe H, Goddard G, Gear RW. Sex differences in masticatory muscle pain after chewing. J Dent Res. 2003;82:112–116. doi: 10.1177/154405910308200207. [DOI] [PubMed] [Google Scholar]
- 43.Kent-Braun JA, Fitts RH, Christie A. In: Comprehensive Physiology. Terjung R, editor. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2011. [Google Scholar]
- 44.Koltzenburg M, Torebjörk HE, Wahren LK. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117 ( Pt 3):579–591. doi: 10.1093/brain/117.3.579. [DOI] [PubMed] [Google Scholar]
- 45.Kosek E, Ekholm J, Hansson P. Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. PAIN. 1996;64:415–423. doi: 10.1016/0304-3959(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 46.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Lautenbacher S, Rollman GB, McCain GA. Multi-method assessment of experimental and clinical pain in patients with fibromyalgia. PAIN. 1994;59:45–53. doi: 10.1016/0304-3959(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 48.Le Goff P. Is fibromyalgia a muscle disorder? Joint, bone, spine : revue du rhumatisme. 2006;73:239–242. doi: 10.1016/j.jbspin.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J Cancer. 2012;131:633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- 52.Mense S. Algesic agents exciting muscle nociceptors. Exp Brain Res. 2009;196:89–100. doi: 10.1007/s00221-008-1674-4. [DOI] [PubMed] [Google Scholar]
- 53.Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. NSC. 2004;129:767–777. doi: 10.1016/j.neuroscience.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 54.Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Molecular pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pène J, Chevalier S, Preisser L, Vénéreau E, Guilleux M-H, Ghannam S, Molès J-P, Danger Y, Ravon E, Lesaux S, Yssel H, Gascan H. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 56.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. PAIN. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 57.Reinöhl J, Hoheisel U, Unger T, Mense S. Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neurosci Lett. 2003;338:25–28. doi: 10.1016/s0304-3940(02)01360-5. [DOI] [PubMed] [Google Scholar]
- 58.Rohde T, MacLean DA, Richter EA, Kiens B, Pedersen BK. Prolonged submaximal eccentric exercise is associated with increased levels of plasma IL-6. Am J Physiol. 1997;273:E85–91. doi: 10.1152/ajpendo.1997.273.1.E85. [DOI] [PubMed] [Google Scholar]
- 59.Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. PAIN. 2004;109:115–123. doi: 10.1016/j.pain.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 60.Sarlani E, Greenspan JD. Evidence for generalized hyperalgesia in temporomandibular disorders patients. PAIN. 2003;102:221–226. doi: 10.1016/S0304-3959(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 61.Simms RW. Fibromyalgia is not a muscle disorder. Am J Med Sci. 1998;315:346–350. doi: 10.1097/00000441-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Simms RW. Is there muscle pathology in fibromyalgia syndrome? Rheum Dis Clin North Am. 1996;22:245–266. doi: 10.1016/s0889-857x(05)70271-4. [DOI] [PubMed] [Google Scholar]
- 63.Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. The Journal of Pain. 2005;6:41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Sluka KA, Danielson J, Rasmussen L, DaSilva LF. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med Sci Sports Exerc. 2012;44:420–427. doi: 10.1249/MSS.0b013e31822f490e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 66.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. PAIN. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. PAIN. 2010;148:188–197. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Staud R. Treatment of fibromyalgia and its symptoms. Expert Opin Pharmacother. 2007;8:1629–1642. doi: 10.1517/14656566.8.11.1629. [DOI] [PubMed] [Google Scholar]
- 69.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. PAIN. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 70.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. PAIN. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 71.Taguchi T, Sato J, Mizumura K. Augmented mechanical response of muscle thin-fiber sensory receptors recorded from rat muscle-nerve preparations in vitro after eccentric contraction. J Neurophysiol. 2005;94:2822–2831. doi: 10.1152/jn.00470.2005. [DOI] [PubMed] [Google Scholar]
- 72.Tillu D, Gebhart G, Sluka K. Descending facilitatory pathways from the RVM initiate and maintain bilateral. PAIN. 2007 doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Troeltzsch M, Troeltzsch M, Cronin RJ, Brodine AH, Frankenberger R, Messlinger K. Prevalence and association of headaches, temporomandibular joint disorders, and occlusal interferences. J Prosthet Dent. 2011;105:410–417. doi: 10.1016/S0022-3913(11)60084-X. [DOI] [PubMed] [Google Scholar]
- 74.Valkeinen H, Häkkinen A, Alen M, Hannonen P, Kukkonen-Harjula K, Häkkinen K. Physical fitness in postmenopausal women with fibromyalgia. Int J Sports Med. 2008;29:408–413. doi: 10.1055/s-2007-965818. [DOI] [PubMed] [Google Scholar]
- 75.Vassilakos G, James RS, Cox VM. Effect of stimulation frequency on force, net power output, and fatigue in mouse soleus muscle in vitro. Can J Physiol Pharmacol. 2009;87:203–210. doi: 10.1139/y09-002. [DOI] [PubMed] [Google Scholar]
- 76.Voss AA. Extracellular ATP inhibits chloride channels in mature mammalian skeletal muscle by activating P2Y1 receptors. J Physiol (Lond) 2009;587:5739–5752. doi: 10.1113/jphysiol.2009.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA. Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. PAIN. 2011;152:2348–2356. doi: 10.1016/j.pain.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 79.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 80.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. PAIN. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 81.Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle fatigue increases the probability of developing hyperalgesia in mice. The Journal of Pain. 2007;8:692–699. doi: 10.1016/j.jpain.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yunus MB, Kalyan-Raman UP. Muscle biopsy findings in primary fibromyalgia and other forms of nonarticular rheumatism. Rheum Dis Clin North Am. 1989;15:115–134. [PubMed] [Google Scholar]