Abstract
Both complement and antibody-dependent cellular cytotoxicity (ADCC) contribute to the clinical efficacy of anti-CD20 monoclonal antibody (mAb) therapy. Paradoxically, the C3b component of complement can block interaction between mAb and natural killer (NK) cells. The present study compared the effect of complement on the ability of two anti-CD20 mAbs, rituximab and GA101, to activate NK cells and mediate ADCC. Complement blocked adherence of NK cells to rituximab, but had little effect on NK binding to GA101. Target cells coated with rituximab or GA101 were able to activate NK cells in the absence of serum. Complement in serum blocked NK activation induced by rituximab, but not GA101. Complement blocked rituximab-induced NK-cell mediated ADCC, but not GA101-induced ADCC. These results demonstrate that the decreased ability of GA101 to fix complement relative to rituximab results in an enhanced ability of GA101 to bind to NK cells, activate NK cells and induce ADCC when serum is present.
Keywords: Antibody-based immunotherapy, NK cell biology, lymphoma
Introduction
The anti-CD20 monoclonal antibody (mAb) rituximab has changed the way we treat most B cell malignancies [1,2]. Despite this success, there is still much we do not know about the mechanisms responsible for this clinical efficacy. There is considerable evidence that both antibody-dependent cellular cytotoxicity (ADCC) and complement fixation contribute to the efficacy of rituximab therapy, at least in vitro and in animal models (reviewed in [3]). A better understanding of the relative roles and interactions between these two mechanisms is vital as we work to design and test the next generation of mAbs for cancer therapy.
The relationship between ADCC and complement fixation is complex. A number of investigators have found that complement mediated cytotoxicity (CMC) contributes to rituximab-induced lysis of malignant B cells [4–7]. In contrast, we recently demonstrated in vitro that the C3b component of complement can inhibit mAb-induced natural killer (NK) cell activation and ADCC by interfering with the interaction between rituximab Fc and CD16 on the NK cell [8]. We also demonstrated in a murine model that depletion of complement can enhance the efficacy of mAb therapy [9]. These studies raise the possibility that, if ADCC is a prime mechanism of action, the ability of a mAb to fix complement may actually decrease mAb efficacy.
Glennie and colleagues have demonstrated considerable variability of anti-CD20 mAb to fix complement [10]. mAbs, such as rituximab, that translocate CD20 into membrane rafts are effective at fixing complement. These anti-CD20 mAbs are designated as type I anti-CD20 mAbs. In contrast, type II anti-CD20 mAbs such as B1 [11] or GA101 [12] bind to CD20 in a different manner. They are unable to translocate CD20 into lipid rafts and do not fix complement effectively.
Other differences, such as modifications in the Fc glycosylation patterns, can also impact the ability of mAbs to fix complement. GA101 is a humanized type II anti-CD20 antibody that was derived by humanization of the parental B-Ly1 mouse antibody [13]. The Fc region of GA101 was glycoengineered to contain bisected, afucosylated carbohydrates. As a result, GA101 has increased affinity for the low- and high-affinity FcγRIIIa. GA101 mediates direct killing of some B cell lines and is poor at fixing complement [12,14–16].
Given that complement fixation can inhibit the ability of rituximab-coated target cells to activate NK cells to mediate ADCC, we assessed the effect of complement on the ability of GA101 to bind to NK cells, activate NK cells or mediate ADCC.
Materials and methods
Cell lines, antibodies and serum
Raji cells (Burkitt lymphoma B cells) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Rituximab and ofatumumab were obtained commercially. GA101 and wt GA101 (similar to GA101 without the modifications in glycosylation) were provided by Genentech (South San Francisco, CA). Normal human serum was obtained from normal donors after obtaining informed consent. Heat-inactivated serum was produced by heating normal human serum to 57°C for 30 min. Serum and NK cells from the same donor were used in each individual experiment. C5-depleted serum was obtained commercially from Complement Technology (Tyler, TX).
C1q binding enzyme-linked immunosorbent assay
Serial dilutions of anti-CD20 mAb were immobilized on a MaxiSorp 96-well plate and free binding sites were blocked with phosphate buffered saline (PBS) containing 3% bovine serum albumin (BSA). As a control, direct staining of plates with anti-human immunoglobulin G (IgG) was used to confirm equal coating of anti-CD20 mAb on the plates. For experimental samples, C1q (Sigma, St. Louis, MO) was added at a concentration of 2.2 μg/mL at room temperature for 90 min. Plates were washed, and bound C1q was detected with polyclonal rabbit anti-human C1q (Dako, Denmark) followed by detection with polyclonal goat anti-rabbit Fc-horseradish peroxidase (HRP) (Jackson ImmunoResearch, West Grove, PA). ABTS [2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)] was added as a substrate and plates read on a microplate reader (405 nm/490 nm).
CMC assay
Serum and anti-CD20 mAb at various concentrations were added to 104 51Cr-labeled Raji cells in a 96-well V-bottomed plate and incubated for 2 h at 37°C. All samples were evaluated in triplicate. After centrifugation, supernatant was collected and counts determined. Percent specific lysis was calculated as: (experimental value − spontaneous lysis)/(maximum lysis − spontaneous lysis) × 100.
NK-cell adhesion assay
The NK-cell adhesion assay has been previously reported [8]. Briefly, 96-well enzyme immunoassay/radioimmunoassay (EIA/RIA) U-bottomed microtiter plates (Corning, Corning, NY) were coated overnight at 4°C with 10 μg/mL of anti-CD20 mAb. After coating, plates were washed three times with PBS. Wells were then coated with neat heat-inactivated fetal calf serum and again washed three times with PBS. Human serum or heat-inactivated serum was added to individual wells at concentrations of 0–25% at 37°C for 1 h. Plates were washed three times with PBS. NK cells from healthy donors were obtained from leukocyte reduction filters and isolated using the magnetic activated cell sorting (MACS) NK-cell isolation kit from Milteny Biotec (Auburn, CA). NK cells (5 × 104 cells/well) were added to wells of the micro titer plates and allowed to adhere for 1 h at room temperature. Plates were spun gently at 100 g for 1 min. Absorbance at 405 nm was then read using a plate reader. When NK cells bind to the mAb Fc in the U-bottomed wells, there is a smaller pellet. The beam of the plate reader goes through the center of the well, i.e. through the pellet. Therefore, when there is limited binding of NK cells to mAb, there is a thicker pellet at the bottom of the well. This results in high absorbance. In contrast, binding of NK cells to mAb on the walls of the well results in low absorbance. Thus, this assay is a semiquantitative measure of NK cell binding to mAb on the walls of the well, with low absorbance indicating high binding of cells to mAb, and high absorbance indicating low binding of cells to mAb.
NK-cell activation
Peripheral blood mononuclear cells (PBMCs) were obtained from normal donors. Freshly isolated PBMCs were combined at a 1:1 ratio with Raji cells at a final concentration of 2.5 × 106 PBMCs and 2.5 × 106 Raji per mL. mAbs at concentrations of 0–5 μg/mL were added along with media, autologous serum or autologous heat-inactivated serum at various concentrations. Cultures were incubated for 16–20 h at 37°C before flow cytometric analysis. For analysis, cells were washed and stained with directly conjugated antibodies including anti-human CD56 AlexaFluor 647, CD54 phycoerythrin (PE) (BD Pharmingen, Franklin Lakes, NJ), CD16 fluorescein isothiocyanate (FITC) (Serotec, Raleigh, NC), CD3 PE-Cy7 (Caltag Laboratories, Burlingame, CA) and CD19 allophy-cocyanin (APC)-Cy7, per the manufacture’s protocol. Flow cytometric analysis of NK cell phenotype was performed on an LSR (BD Immunocytometric Systems, San Jose, CA). NK cells were identified as CD3−, CD56+ cells in the lymphocyte gate. Specific measurements included assessment of NK cell CD16 median fluorescence intensity and % NK cells that were CD54bright, as previously reported.
ADCC assay
Raji cells served as target cells and were radiolabeled with 51Cr. NK cells were isolated using the MACS NK isolation kit per the manufacturer’s protocol as outlined above. NK cells were added to Raji cells at a concentration of 1 × 104 cells/well at effector:target ratios of 50:1, 25:1, 12.5:1, 1:1 and 0:1. Rituximab or GA101 was then added at a concentration of 5 μg/mL. In select samples, C5-depleted serum or heat-inactivated C5-depleted serum was added at a final concentration of 25%. Samples were incubated for 4 h at 37°C, centrifuged at 400 g for 5 min and supernatant removed. Percent specific lysis was calculated as: (experimental value − spontaneous lysis)/(maximum lysis − spontaneous lysis) × 100.
Results
C1q binding to rituximab is greater than to GA101
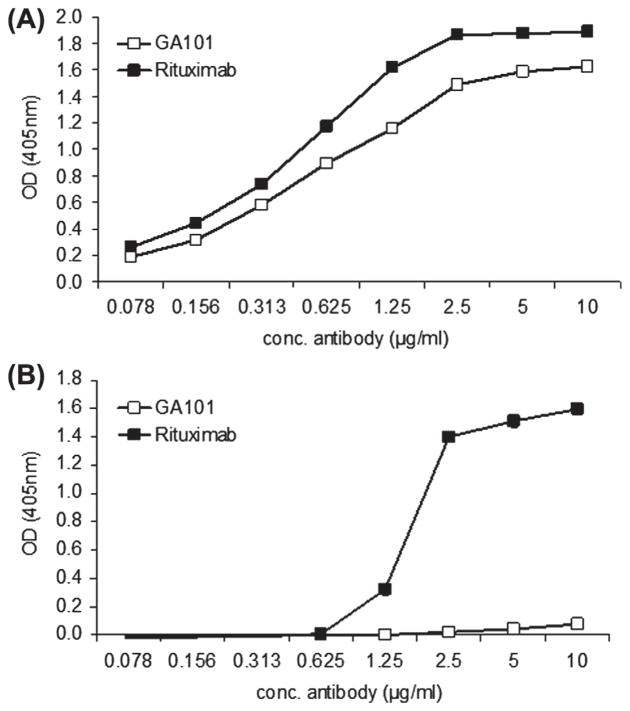
Plates were coated with serial dilutions of anti-CD20 antibody. As illustrated in Figure 1(A), staining with anti-human IgG confirmed that similar amounts of rituximab and GA101 were present on the plate. On separate plates, C1q was added, and the presence of bound C1q detected with anti-C1q antibody. C1q bound to rituximab beginning at rituximab concentrations of 1.25 μg/mL, but did not bind to GA101 even when wells were coated with 10 μg/mL [Figure 1(B)].
Figure 1.
C1q binds to rituximab but not GA101. Serial dilutions of GA101 and rituximab were added to 96-well plates. (A) Direct staining of plates with anti-human IgG confirming similar coating of GA101 and rituximab on the plates. (B) Human C1q was added in equal concentrations to all wells, and bound C1q detected with polyclonal rabbit anti-human C1q followed by polyclonal goat anti-rabbit Fc-HRP. Data shown are representative of three independent experiments.
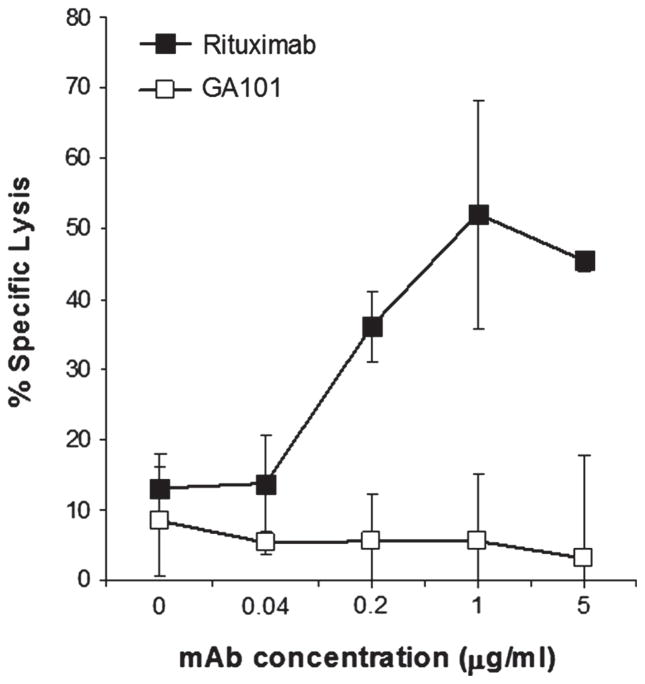
Rituximab mediates CMC of Raji cells while GA101 does not mediate CMC
To confirm that rituximab is more effective than GA101 at mediating CMC, we assessed the ability of these mAbs to induce lysis of opsonized Raji cells in the presence of complement. As illustrated in Figure 2, rituximab was effective at mediating CMC while GA101 was ineffective at concentrations of mAb up to 5 μg/mL.
Figure 2.
Rituximab, but not GA101, mediates CMC of Raji cells. 51Cr-labeled Raji cells were coated with various concentrations of rituximab or GA101, and 25% normal human serum added for 2 h. Percent lysis was determined. Error bars indicate SD of the mean. All samples were run in triplicate (n = 3).
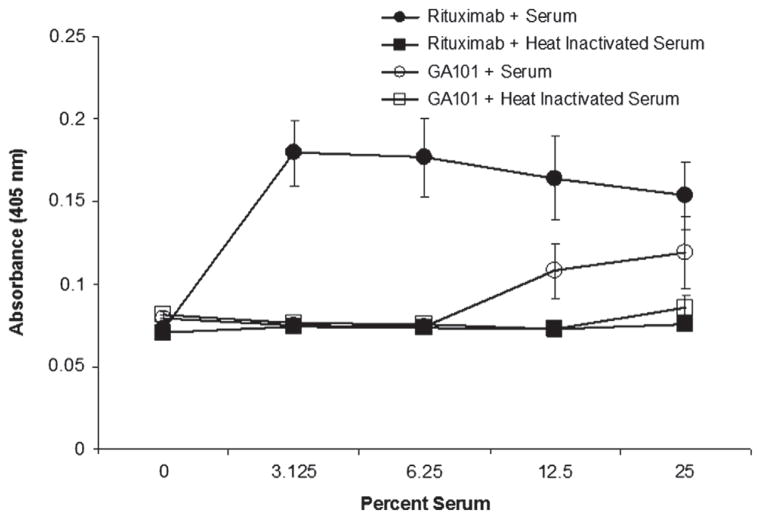
Serum inhibits binding of NK cells to rituximab but not to GA101
Our prior studies demonstrated that serum complement blocks NK cell binding to rituximab [8]. More specifically, C3b binds to rituximab Fc and blocks binding of the Fc to CD16. Given that GA101 fixes complement poorly, we assessed whether complement in serum is able to block binding of NK cells to GA101. We used an adherence assay to assess interactions between NK cells and plastic-bound rituximab or GA101. As reported previously, NK cells bound to rituximab in the absence of serum. This interaction was blocked by even low concentrations of serum (3.125%). NK cells also bound to GA101. However, in contrast to rituximab, serum complement had little effect on NK binding to GA101 (Figure 3). The mAbs in this assay are bound directly to plastic, and thus this assay reflects differences that are independent of the epitope specificity of the mAb and whether it is a type I or a type II anti-CD20 mAb. The differences in the ability of NK cells to adhere to GA101 and rituximab is therefore due, at least in part, to the Fc structure of the mAbs. Most importantly, these results demonstrate that complement interferes with the interaction between rituximab and NK cells, but not GA101 and NK cells.
Figure 3.
Complement blocks adherence of NK cells to rituximab but not GA101. U-bottomed 96-well microtiter plates were coated with 5 μg/mL rituximab or GA101. Wells were then incubated with various concentrations of normal human serum or heat-inactivated serum. High absorbance indicates less binding of NK cells to mAb and resulted from NK-cell pelleting. In contrast, low absorbance indicates binding of NK cells to walls of well and reflects greater binding of NK cells to mAb. Error bars indicate SD of the mean. All samples were run in triplicate.
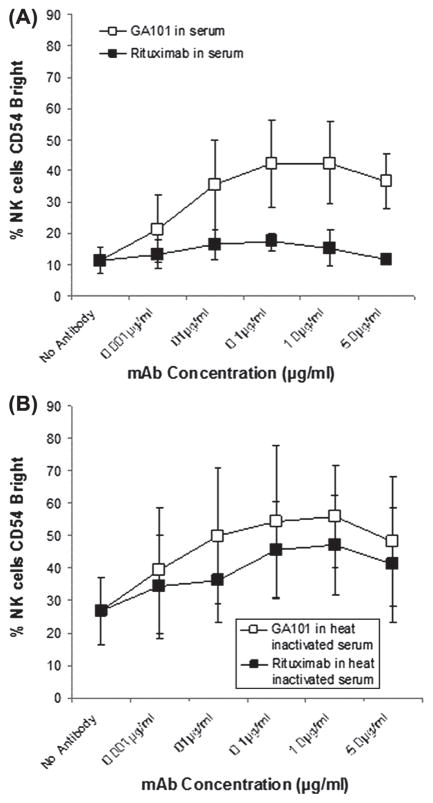
GA101 is superior to rituximab in activating NK cells in the presence of complement
Previously, we demonstrated that the addition of rituximab to a mixture of target B-cells and PBMCs induces activation of NK cells, as measured by a number of phenotypic changes including up-regulation of CD54. Furthermore, we found that serum complement, more specifically C3b, blocks NK activation induced by rituximab-coated target cells. In the present study we compared the ability of rituximab and GA101 to induce NK activation and assessed the effect of complement on this activation. In the absence of serum, Raji cells coated with either rituximab or GA101 were able to induce up-regulation of CD54 (data not shown). Similar results were seen when heat-inactivated autologous serum was added. In the presence of unmodified serum, rituximab-coated Raji cells were unable to induce CD54 up-regulation. In contrast, GA101-coated Raji cells induced up-regulation of NK cell CD54 even in the presence of serum (Figure 4). This suggests that the ability of rituximab to fix complement limits its ability to activate NK cells in the presence of complement, while the inability of GA101 to fix complement allows GA101 to activate NK cells even in the presence of complement. We also analyzed the effect of serum on NK activation using ofatumumab, a type I anti-CD20 mAb with an enhanced ability to bind complement and wt GA101, a type II anti-CD20 antibody that lacks the glycomodifications found in GA101. As with GA101, complement had little effect on the ability of wt GA101 to activate NK cells. The impact of complement on the ability of ofatumumab to activate NK cells was similar to that seen with rituximab (data not shown). Thus, the effect of complement on NK activation is dependent on the ability of the mAb to fix complement, which is dependent in large part on the type of anti-CD20 antibody, not on glycomodification.
Figure 4.
Complement blocks ability of rituximab-coated target cells to activate NK cells, but not GA101-coated target cells to activate NK cells. Raji cells were coated with various concentrations of rituximab or GA101 in the presence or absence of 20% normal human serum or heat-inactivated serum from the same donor. NK cell activation, as determined by % cells CD54bright, was determined. (A) NK activation in the presence of normal human serum. (B) NK activation in the presence of heat-inactivated normal human serum. All samples were run in triplicate. Error bars indicate SD of the mean.
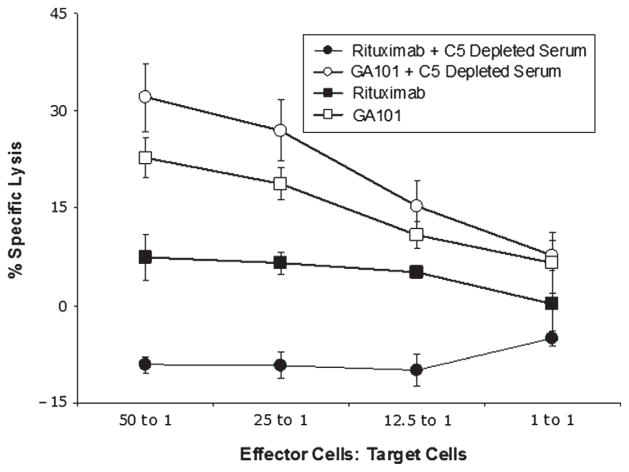
C5-depleted serum blocks ADCC mediated by rituximab but not ADCC mediated by GA101
We next evaluated whether there is a difference between rituximab and GA101 in their ability to mediate ADCC in the presence of the early components of complement. These experiments were done using C5-depleted serum, because such serum allows for activation of the earlier steps in the complement cascade, including C3b deposition, without development of the membrane attack complex. As expected, C5-depleted serum was unable to induce CMC of rituximab or GA101-coated Raji cells (data not shown). In the absence of complement, purified NK cells induced ADCC of both rituximab-coated Raji cells and GA101-coated Raji cells. ADCC mediated by rituximab was inhibited by C5-depleted serum. In fact, C5a-depleted serum plus rituximab enhanced the survival of lymphoma cells, as indicated by lysis lower than in the serum-free controls. This was likely because there was limited CMC, ADCC was blocked, and other serum factors such as growth factors in the C5a-depleted serum helped maintain lymphoma cell viability. In contrast, C5-depleted serum had no effect on ADCC mediated by GA101 (Figure 5). These studies demonstrate that complement fixation upstream of C5 activation impairs the ADCC of rituximab-coated target cells by NK cells, but has no effect on ADCC mediated by GA101.
Figure 5.
Complement in C5-depleted serum blocks NK-cell mediated ADCC of rituximab-coated target cells but not GA101-coated target cells. 51Cr-labeled Raji target cells were coated with 5 μg/mL rituximab or GA101, and purified NK cells added as effector cells in various E:T ratios along with 25% C5-depleted serum or medium alone. Percentage of specific lysis was determined. All samples were run in triplicate (n = 3). Error bars represent SD of the mean.
Discussion
The anti-CD20 mAb rituximab has changed our approach to the treatment of B cell malignancies. Nevertheless, rituximab alone does not cure patients, and many patients relapse following combination therapy with rituximab and chemotherapy. There is still much room for improvement. Understanding mechanisms of action and mechanisms of resistance to anti-CD20 mAb therapy is key to the rational design and evaluation of the next generation of such treatments [17].
The role of complement in mediating the anti-tumor activity of anti-CD20 mAb has received considerable attention, and is proving to be quite complex. There is solid evidence that complement can induce CMC of rituximab-coated target cells in vitro [4,18] and can contribute to the anti-tumor activity of mAb in animal models [7]. Early clinical studies indicate that a next-generation anti-CD20 mAb with the greatest ability to fix complement (ofatumumab) is effective, particularly in chronic lymphocytic leukemia (CLL) [19], where malignant cells are exposed to mAb in the circulation.
On the other hand, as outlined above, we have found that complement in serum can limit the ability of rituximab-coated targets to activate NK cells. Taylor and colleagues have demonstrated that monocytes and macrophages may “shave” rituximab–complement complexes off the surface of CLL cells [20,21], resulting in viable malignant cells that no longer have surface antigen on their surface. While these results are provocative, it remains to be seen whether the ability of complement to inhibit NK activation, or the “shaving” of immune complex from the surface of CLL cells, impacts on the efficacy of therapy.
Complement components are almost always studied in the serum, but much of the anti-tumor activity of anti-CD20 mAbs takes place in the extravascular compartment. We previously found that complement in the extravascular compartment had limited ability to mediate lysis in a traditional CH50 assay but was present in sufficient concentration to inhibit activation of NK cells by rituximab-coated target cells [9]. Thus, the fixation of complement may be a “friend” for anti-CD20 mAb-coated target cells in the circulation, where complement levels are high and CMC is important, but a “foe” in the tissues, where complement levels are lower and ADCC may play a more significant role.
Indeed, data from both laboratory models and correlative clinical studies suggest that ADCC plays a significant role in the anti-tumor effects of mAbs in general, and rituximab in particular. The therapeutic effect of mAbs is lost in Fcγ-receptor knock-out mice [22]. In the clinic, three independent studies have demonstrated that single-agent rituximab is more effective in patients with Fcγ receptor III (CD16) polymorphisms associated with higher affinities for human IgG, suggesting that Fc receptors on effector cells play a key role in the therapeutic effect of rituximab [23–25]. This evidence pointing toward ADCC and CD16 as being vital to the clinical activity of rituximab has led to the generation and evaluation of a number of next-generation anti-CD20 mAbs with an enhanced ability to bind to CD16 [26]. These mAbs look promising in vitro, but whether they are more effective clinically remains to be determined.
GA101 is unique among these anti-CD20 mAbs. It is a type II anti-CD20 mAb, in contrast to most of the other anti-CD20 mAbs that are type I anti-CD20 mAbs [12]. This is thought to be a potential advantage, in that cross-linking by type II anti-CD20 mAbs in vitro induces a greater degree of cell death of target B-cells when compared to type I anti-CD20 mAbs [27]. Type II anti-CD20 mAbs are less effective at fixing complement and mediating CMC than type I anti-CD20 mAbs. Our findings are consistent with those of Pievani et al. who also demonstrated that human serum inhibited NK-cell activation induced by rituximab, but not GA101 [28].
The differences between rituximab and GA101 are not due solely to the binding properties of the different types of anti-CD20 mAb to antigen. Both the C1q enzyme-linked immunosorbent assay (ELISA) and in vitro adherence assay measuring NK cell binding to mAbs demonstrated differences when rituximab and GA101 were placed directly on plastic. Thus, these assays did not involve target cells or target antigen. The epitope specificity of the mAb, or movement of the mAb – antigen complex in the target cell membrane, were not factors, as there was no movement of plastic-bound mAbs. C1q bound to rituximab to a greater extent than to GA101. NK cells bound to either rituximab or GA101 on the plastic in the absence of complement. Nevertheless, these two mAbs behaved very differently in the presence of complement. Complement blocked the ability of NK cells to bind to rituximab, but had little effect on the ability of NK cells to bind to GA101. Further studies are needed to understand the structural factors that determine whether an anti-CD20 mAb is a type I or type II.
It is important to emphasize that our studies focused on the relationship between anti-CD20 mAbs and NK cells. Other immune effector cells express different Fc receptors, and the effect of complement on those interactions is very different from that seen with NK cells, as we have previously reported [8]. Indeed, a recent report by Rafiq et al. highlights the many differences between ofatumumab and GA101 in how they interact with various Fc receptors and activate distinct effector cell types [29].
In summary, the ability of anti-CD20 mAbs to fix complement has been thought for many years to be a desirable feature. Our prior work suggests that complement in serum or in extravascular fluid can paradoxically limit the ability of NK cells to bind to rituximab, limit NK cell activation induced by rituximab-coated target cells and limit rituximab-mediated ADCC. The ability to fix complement may therefore be a foe, not a friend, to successful mAb therapy, at least under circumstances where ADCC is a primary mechanism of action. Here we compared GA101, an anti-CD20 mAb that does not fix complement well, with rituximab. Even in the presence of complement, NK cells adhered well to GA101, GA101-coated target cells activated NK cells and GA101 mediated ADCC. Clinical evaluation of GA101 is ongoing. The results of such clinical trials, and ongoing performance of translational studies evaluating the relationship between complement fixation and anti-CD20 mAb efficacy, will be crucial as we work to understand how we can take advantage of the complex interactions between complement and ADCC with the goal of optimizing the efficacy of this valuable therapeutic modality.
Supplementary Material
Acknowledgments
The authors thank Tina Otz for technical assistance, and Suresh Veeramani, Christopher Dahle and Sue Blackwell for helpful advice.
This work was supported by the Leukemia and Lymphoma Society, grant # 6100-07 and grants R01 CA137198 and P50 CA97274.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Maloney DG, Grillo-Lopez AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma [see comments] J Clin Oncol. 1997;15:3266–3274. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 3.Wang SY, Weiner G. Complement and cellular cytotoxicity in antibody therapy of cancer. Expert Opin Biol Ther. 2008;8:759–768. doi: 10.1517/14712598.8.6.759. [DOI] [PubMed] [Google Scholar]
- 4.Bellosillo B, Villamor N, Lopez-Guillermo A, et al. Complement-mediated cell death induced by rituximab in B-cell lymphoproliferative disorders is mediated in vitro by a caspase-independent mechanism involving the generation of reactive oxygen species. Blood. 2001;98:2771–2777. doi: 10.1182/blood.v98.9.2771. [DOI] [PubMed] [Google Scholar]
- 5.Weng WK, Levy R. Expression of complement inhibitors CD46, CD55, and CD59 on tumor cells does not predict clinical outcome after rituximab treatment in follicular non-Hodgkin lymphoma. Blood. 2001;98:1352–1357. doi: 10.1182/blood.v98.5.1352. [DOI] [PubMed] [Google Scholar]
- 6.Di Gaetano N, Cittera E, Nota R, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–1587. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 7.Golay J, Cittera E, Di Gaetano N, et al. The role of complement in the therapeutic activity of rituximab in a murine B lymphoma model homing in lymph nodes. Haematologica. 2006;9:176–183. [PubMed] [Google Scholar]
- 8.Wang SY, Racila E, Taylor RP, et al. NK-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood. 2008;111:1456–1463. doi: 10.1182/blood-2007-02-074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang SY, Veeramani S, Racila E, et al. Depletion of the C3 component of complement enhances the ability of rituximab-coated target cells to activate human NK cells and improves the efficacy of monoclonal antibody therapy in an in vivo model. Blood. 2009;114:5322–5330. doi: 10.1182/blood-2009-01-200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cragg MS, Morgan SM, Chan HT, et al. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood. 2003;101:1045–1052. doi: 10.1182/blood-2002-06-1761. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov A, Beers SA, Walshe CA, et al. Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J Clin Invest. 2009;119:2143–2159. doi: 10.1172/JCI37884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct- and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robak T. GA-101, a third-generation, humanized and glycoengineered anti-CD20 mAb for the treatment of B-cell lymphoid malignancies. Curr Opin Investig Drugs. 2009;10:588–596. [PubMed] [Google Scholar]
- 14.Bologna L, Gotti E, Manganini M, et al. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol. 2011;186:3762–3769. doi: 10.4049/jimmunol.1000303. [DOI] [PubMed] [Google Scholar]
- 15.Patz M, Isaeva P, Forcob N, et al. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol. 2011;152:295–306. doi: 10.1111/j.1365-2141.2010.08428.x. [DOI] [PubMed] [Google Scholar]
- 16.Dalle S, Reslan L, Besseyre de Horts T, et al. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther. 2011;10:178–185. doi: 10.1158/1535-7163.MCT-10-0385. [DOI] [PubMed] [Google Scholar]
- 17.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golay J, Lazzari M, Facchinetti V, et al. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood. 2001;98:3383–3389. doi: 10.1182/blood.v98.12.3383. [DOI] [PubMed] [Google Scholar]
- 19.Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood. 2008;111:1094–1100. doi: 10.1182/blood-2007-09-111781. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy AD, Beum PV, Solga MD, et al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004;172:3280–3288. doi: 10.4049/jimmunol.172.5.3280. [DOI] [PubMed] [Google Scholar]
- 21.Beum PV, Lindorfer MA, Taylor RP. Within peripheral blood mononuclear cells, antibody-dependent cellular cytotoxicity of rituximab-opsonized Daudi cells is promoted by NK cells and inhibited by monocytes due to shaving. J Immunol. 2008;181:2916–2924. doi: 10.4049/jimmunol.181.4.2916. [DOI] [PubMed] [Google Scholar]
- 22.Clynes R, Takechi Y, Moroi Y, et al. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci USA. 1998;95:652–656. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 24.Treon SP, Hansen M, Branagan AR, et al. Polymorphisms in FcgammaRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom’s macroglobulinemia. J Clin Oncol. 2005;23:474–481. doi: 10.1200/JCO.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 25.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Bowles JA, Wang SY, Link BK, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108:2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beers SA, Chan CH, James S, et al. Type II (tositumomab) anti-CD20 monoclonal antibody outperforms type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112:4170–4177. doi: 10.1182/blood-2008-08-172999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pievani A, Belussi C, Klein C, et al. Enhanced killing of human B-cell lymphoma targets by combined use of cytokine-induced killer cell (CIK) cultures and anti-CD20 antibodies. Blood. 2011;117:510–518. doi: 10.1182/blood-2010-06-290858. [DOI] [PubMed] [Google Scholar]
- 29.Rafiq S, Butchar JP, Cheney C, et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol. 2013;190:2702–2711. doi: 10.4049/jimmunol.1202588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.