Abstract
Neurophysiological recordings in the cerebellar cortex of awake-behaving animals are revolutionizing the way we think about the role of Purkinje cells in sensori-motor calibration. Early theorists suggested that if a movement became miscalibrated, Purkinje cell output would be changed to adjust the motor command and restore good performance. The finding that Purkinje cell activity changed in many sensori-motor calibration tasks was taken as strong support for this hypothesis. Based on more recent data, however, it has been suggested that changes in Purkinje cell activity do not contribute to the motor command directly; instead, they are used either as a teaching signal, or to predict the altered kinematics of the movement after calibration has taken place. I will argue that these roles are not mutually exclusive, and that Purkinje cells may contribute to command generation, teaching, and prediction at different times during sensori-motor calibration.
Introduction
Whether the goal is to adjust our golf swing based on the prevailing wind conditions, or to compensate for muscle weakness as we grow older, the brain must constantly monitor our movements and make adjustments when corrections are needed. Previous work indicates that the cerebellum is an essential component of the neural machinery necessary for keeping our movements finely tuned, but its specific contribution to sensori-motor calibration remains controversial. Purkinje cells, whose axons carry the entire output of the cerebellar cortex to other brain areas, are at the center of this controversy. Here, I review current hypotheses about the role of the cerebellum in light of recording studies which have examined Purkinje cell activity in a variety of sensori-motor learning tasks.
Inverse and forward models
Many ideas about the function of the cerebellum are derived from one simple observation: more often than not, Purkinje cell activity and movement kinematics are intimately linked. In many cases, including the tracking of moving objects with the hand or the eyes, the firing rate of individual Purkinje cells can be predicted with remarkable accuracy by taking into account specific parameters of the movement, like its direction, speed, acceleration and duration [1-8]. This tight relationship between neural activity and motor output implies that if the kinematics of a particular movement change during sensori-motor calibration, that is swing the golf club faster to compensate for a strong headwind, the firing rate of Purkinje cells would have to change accordingly. In support of this hypothesis, there is now overwhelming evidence clearly demonstrating parallel changes in Purkinje cell activity and motor output in many sensori-motor learning tasks [9,10,11•,12•,13••,14-18].
Although there is general agreement that Purkinje cell activity is often related to movement kinematics both before and after sensori-motor calibration, there is still debate about what this relationship really means. Much of this debate is fueled by arguments about the neural implementation of internal models in the cerebellum [19••,20••,21,22••,23,24,25••]. One view is that some component of Purkinje cell activity represents the output of an inverse model, defined computationally as an element whose output signal is fed to downstream mechanical actuators to produce a desired movement. According to this view, the firing rate of Purkinje cells contributes directly to the motor command; changes in Purkinje cell activity during sensori-motor calibration are directly responsible for modulating motoneuron signals and modifying the kinematics of the movement in a way that improves performance. An alternative view is that some component of Purkinje cell activity represents the output of a forward model, defined computationally as an element whose output signal provides a prediction about the kinematic properties of the upcoming movement (or its sensory consequences). This type of signal is thought to be essential for state estimation in motor control; when compared to the actual movement that is produced (or the actual sensory consequences), it provides an estimate of how the motor command has affected the state of our body and the world around us [21]. The feedback controllers that implement the inverse model then use information about the current state to optimize subsequent motor commands. Under the forward model scenario, changes in Purkinje cell activity after sensori-motor calibration simply reflect a prediction about the altered kinematic properties of the movement itself; these changes do not contribute directly to the motor command, and affect movement only indirectly via state estimation. In the following sections, I review recording studies looking at the activity of Purkinje cell during sensori-motor calibration, and assess whether the current neurophysiological evidence is more in line with what would be expected of an inverse or a forward model.
Purkinje cell activity in tasks with sensory disturbances
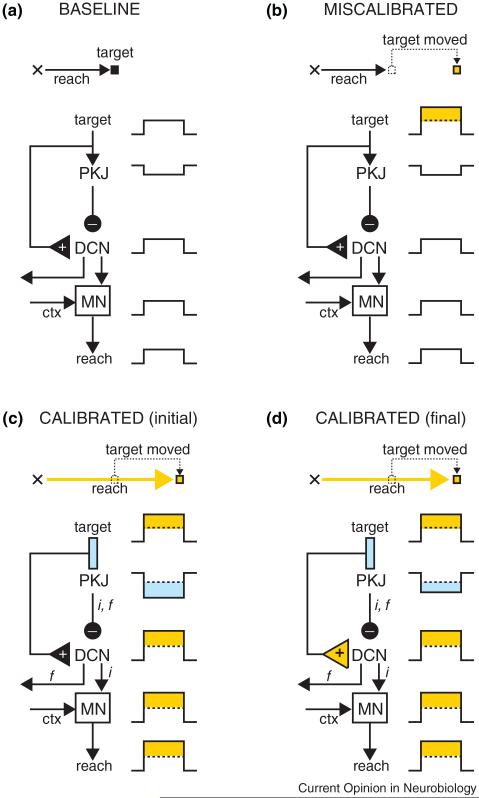
We first consider Purkinje cell activity in sensori-motor calibration tasks that feature a predictable sensory disturbance of the external environment. In Figure 1, for example, the task is to reach for the target, something that is accomplished with accuracy during the ‘baseline’ phase because the reach that is produced in response to the target stimulus is well-calibrated. In the ‘miscalibrated’ phase, the target is suddenly displaced to a new position just as the hand is about to reach it. If the target is always moved to the same new position, the reach will be recalibrated over the course of many trials. Eventually the hand will go straight to the place where the target is expected to move, ignoring the initial target location (Figure 1; ‘calibrated’ phase).
Figure 1.
A hypothesis about sensori-motor calibration after a sensory disturbance. In the baseline phase (a), the hand starts at the position indicated by the cross and the reach takes it to the target location. The activity of Purkinje cells (PKJ), deep cerebellar nucleus (DCN), and motor neurons (MN) is indicated schematically as either increases or decreases in firing rate throughout the entire reach. In (b), the target is shifted to the right (indicated in orange) and as a result the reach falls short. During the early stages of calibration (c), the kinematics of the reach have been altered (indicated in orange) and the hand is moved to the new target location. Note that Purkinje cell activity has changed (cyan) and that it is collaborating with signals from motor cortex (ctx) to improve the motor command generated by the MN (inverse model: ‘i’). Consistent with a forward model (‘f’), the change in Purkinje cell activity is also a prediction of sensory input (i.e. the target will move), and future movement (i.e. the reach will go further). The output of the forward model could be sent out by the DCN to cortex for further processing and state estimation. After extensive training in this task (d), there is a transfer of plasticity from cerebellar cortex to DCN (orange triangle). However in this task, the amount of transfer is small, and Purkinje cell output remains largely unaffected relative to (c).
The reaching task described in Figure 1 is analogous to many other sensori-motor calibration tasks commonly used to examine cerebellar function. I will refer to these as ‘sensory disturbance’ (SD) tasks. All SD tasks have two key features in common: first, the need to calibrate the motor command arises because a predictable change in the sensory stimulus alters the movement that is necessary to achieve the particular goal of the task. Second, this type of sensori-motor calibration entails making adjustments to the motor command in a way that ultimately changes the kinematics of the movement relative to the baseline period.
Purkinje cell activity has been recorded in a variety of SD tasks, including classic examples in sensori-motor calibration known to be cerebellar-dependent. Many of these are based on target shifts, for example the reaching example in Figure 1 [26], saccade adaptation to double-steps [27,28], and learning in smooth pursuit eye movements [29], and the vestibular-ocular reflex using predictable displacement of the target relative to the head. Eyeblink conditioning [30], another staple in the field of cerebellar-dependent learning, can also be construed as an SD task [31]; here, the sensory disturbance is an air-puff to the cornea, and sensori-motor calibration can be thought of as a process that gradually adjusts the motor command in a way that ultimately leads to the protective closing of the eyelid in response to a conditioned stimulus. In every single one of these cerebellar-dependent SD tasks, the modification of movement kinematics is accompanied by obvious alterations in the activity of Purkinje cells in the relevant parts of cerebellar cortex [10,11•,12•,13••,14-16].
What is the significance of the changes in Purkinje cell firing rate observed during sensori-motor calibration in SD tasks? One interpretation is that Purkinje cell activity is the output of an inverse model (‘i’ in Figure 1), responsible for correcting the kinematics of the movement by contributing directly to the motor command. In favor of this hypothesis, Purkinje cells are connected to motor neurons by just a few synapses in many cerebellar-dependent SD tasks (via the deep cerebellar or vestibular nuclei), and could therefore exert a strong influence in the generation of movement [32-34]. Further support for the inverse model hypothesis comes from previous work in saccade adaptation [11•], and in learning of smooth pursuit [35•], and vestibular-ocular reflex [18]. In these studies, it is possible to demonstrate quantitatively that the specific changes observed in Purkinje cells during sensori-motor calibration are well-suited to provide a motor command to downstream areas responsible for driving movement.
An alternative to the inverse model interpretation is that Purkinje cell activity is the output of a forward model (‘f’ in Figure 1). Under this scenario, the firing rate of Purkinje cells provides an estimate of the upcoming movement (i.e. motor forward model), or an estimate of the upcoming sensory stimulus (i.e. sensory forward model). Thus, in the ‘initial calibration’ phase of Figure 1, the change in Purkinje cell activity (cyan rectangle next to ‘PKJ’) would indicate that the upcoming reach has different kinematics than during baseline (orange rectangle next to ‘reach’), or that the target is expected to move from its initial position (orange rectangle next to ‘target’). Because in SD tasks, both the motor command and the movement differ in the calibration phase relative to baseline, it is impossible to determine whether Purkinje cell activity is indicative of an inverse model, a forward model, or both [19••]. Or could its function be something else altogether?
Purkinje cell activity as a teaching signal
In addition to its potential role as the output of a forward and/or inverse model, recent work suggests that Purkinje cell activity during sensori-motor calibration could also serve an ancillary function as a ‘teacher’. A series of in vitro studies have revealed that signals typically present in Purkinje cells during sensori-motor calibration [36••,37,38] can be used to induce synaptic plasticity in target cells of the deep cerebellar and the vestibular nucleus [39••,40••,41,42•,43,44. These findings lend support to the trigger-and-storage hypothesis of sensori-motor calibration [45], whose roots can be traced back to the original Miles and Lisberger model of vestibularocular adaptation [46]. According to this proposal, cerebellar-dependent learning is a two-step process in which the change in Purkinje cell activity during the early stages of sensori-motor calibration is relatively labile, and is used as a teaching signal to drive more stable and permanent plasticity in downstream neurons (Figure 1; final calibration).
Pharmacological inactivation studies in prototypal cerebellar-dependent learning tasks have shed some light on the trigger-and-storage hypothesis [45,47-49,50•,51]. In eyeblink conditioning, the data suggest that Purkinje cell activity serves as a teaching signal to transfer plasticity from cerebellar cortex to downstream areas in the deep nuclei. However, the transfer is only partial; even after long-term conditioning (Figure 1; final calibration), both sites of plasticity, one in the cortex and one in the nuclei, are necessary and contribute to different aspects of the conditioned eyelid movement (i.e. Purkinje cells controlling the timing, and deep cerebellar neurons controlling the amplitude of the blink) [45,47]. In contrast, there seems to be an almost complete transfer of plasticity in adaptation of the vestibular-ocular reflex using miniaturizing spectacles in cats [48,49] and monkeys [50•], and the optokinetic response in mice [51]. As discussed below, the extent to which plasticity is transferred from cortex to nucleus has important implications for interpreting the role of Purkinje cell signals during sensori-motor calibration.
Purkinje cell activity in tasks with motor disturbances
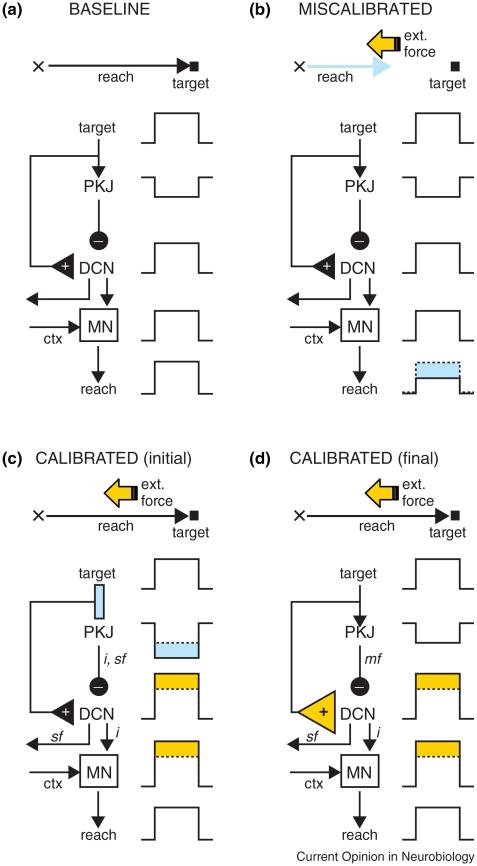
Figure 2 shows an example of sensori-motor calibration in a ‘motor disturbance’ (MD) task. In the ‘miscalibrated’ phase, the reach falls short of the target because we have turned on an external force that opposes the movement of the hand. As long the force stays the same and can be predicted, the movement will be adjusted gradually until eventually the hand reaches the target once again (Figure 2; ‘calibrated’ phase).
Figure 2.
A hypothesis about sensori-motor calibration after a motor disturbance. In the baseline phase (a), the hand starts at the position indicated by the cross and the reach takes it to the target location. In (b), an opposing force is applied during the movement, and as a result the reach falls short (cyan). During the early stages of calibration (c), the motor command generated by the MN has been altered (orange), and the hand is able to reach the target once again. Note that Purkinje cell activity has changed (cyan) and is contributing to the improved motor command (inverse model: ‘i’). The change in Purkinje cell activity is also a good prediction of sensory input (i.e. there will be an opposing force, sensory forward model: ‘sf’), but not of future movement (i.e. the reach kinematics are the same as in the baseline phase). After extensive training in this task (d), there is a full transfer of plasticity from cerebellar cortex to DCN (orange triangle), and Purkinje cell modulation returns to its baseline state. At this point, Purkinje cells are not contributing directly to the improvements made to the motor command; instead their output can be used to predict the kinematics of the reaching movement, which are essentially the same as during the baseline period (motor forward model: ‘mf’).
MD tasks, many of which have been used extensively to study cerebellar-dependent learning and adaptation, share the same two basic features: first, calibration is needed to compensate for a change in the relationship between the motor command and the movement (or the perception of movement). Second, this type of sensori-motor calibration entails making adjustments to the motor command in a way that ultimately produces a movement (or the perception of a movement) with kinematics that are similar to those in the baseline period (Figure 2). Examples of cerebellar-dependent MD tasks include sensori-motor calibration of arm movements within externally applied force fields [17,52], blink adaptation under lid restraint [53], and saccade and vestibular-ocular adaptation after surgically weakening of the extraocular muscles [54]. Other tasks in which the perception of movement (rather than movement itself) is distorted by altering visual feedback could be included in this category as well [55,56].
Electrophysiological recordings during sensori-motor calibration of arm movements in MD tasks have been particularly useful for disentangling hypotheses about the link between Purkinje cells and internal models. A number of studies have demonstrated that the firing rate of many Purkinje cells changes in a manner that is consistent with the adjustments being made to the motor command (measured as changes in EMG). Although these findings have been interpreted as strong evidence for an inverse model [9,57•], the kinematics of the movements were not controlled, and may have differed significantly between the baseline and the recalibrated stages [19••]. Thus, a forward model in which Purkinje cells change their firing during the calibration process to provide an accurate estimate of the altered kinematics could not be ruled out entirely.
An ingenious study of arm movements has provided the most rigorous test to date of whether Purkinje cell activity in MD tasks may provide a signal to modify the motor command directly (i.e. inverse model), or to estimate movement kinematics (i.e. forward model) [58]. The task required tracking a small moving target with the hand under two very different conditions: in some trials, a viscous force was applied to the hand; in others the force was elastic. Although the motor commands required to track the target differed greatly depending on the type of force applied, after extensive training the movement kinematics were essentially the same in both conditions. Purkinje cell activity after extensive training was linked to different kinematic aspects of the movement, like the position or velocity of the hand, but it did not differ between the two force field conditions. This finding indicates that at least for this particular task, and after extensive training, Purkinje cells in the areas recorded (lobules IV–VI) do not contribute directly to the adjustments made to the motor command; instead, Purkinje cell activity appears to represent the output of a forward model that codes for the kinematics of the upcoming movement.
Based on the results of the double-force field experiment described above [58], it is tempting to rule out the notion that Purkinje cell activity represents the output of an inverse model, at least for this particular MD task. Certainly, that conclusion seems appropriate for Purkinje cells within the recorded areas, primarily lateral and intermediate zones in lobules IV–VI. However, it remains possible that Purkinje cells in other areas of cerebellar cortex might have more direct connections to motoneurons and directly influence the motor command. Furthermore, the recordings in the double-force field experiment were done in highly trained monkeys after they had mastered the task, raising the possibility that the same Purkinje cells whose activity was consistent with the output of a forward model after extensive training may have contributed to the output of an inverse model earlier on in training. As discussed in the next section, the idea that the role of Purkinje cells in sensori-motor calibration may vary over time is indeed consistent with the trigger-and-storage model presented above (see ‘Purkinje cell activity as a teaching signal’).
Hypothesis — the role of Purkinje cells varies during training
Figure 2 describes the contribution of Purkinje cell activity to the output of forward and inverse models in a task for which there is full transfer of plasticity from cerebellar cortex to deep cerebellar nuclei. According to the trigger-and-storage model, during the early stages of sensori-motor calibration in MD tasks, Purkinje cell signals contain an ‘sf’ component used to estimate the strength of the external force, and also an ‘i’ component used for directly modifying the motor command (Figure 2; early calibration). In the final phase of the calibration process, however, plasticity has been transferred completely from the cerebellar cortex to the deep nuclei. At this stage, Purkinje cell activity has returned to the same level it was during baseline, and thus provides a good estimate of the movement kinematics, which are also the same as in the baseline phase. This is consistent with the results of the double-force field experiment, which suggest that after extensive training in this particular MD task, Purkinje cell activity in certain areas of cerebellar cortex represents the output of a motor forward model (‘mf’ in Figure 2), but not a sensory forward model (‘sf’), or an inverse model (‘i’). A less than perfect transfer of plasticity would result in Purkinje cell activity that is also related to the motor command and muscle EMG, as reported recently in similar MD tasks [57•,59].
An imaging study investigating the long-term adaptation of arm movements to force fields provides some support for the hypothesis presented in Figure 2 regarding the role of Purkinje cells at different stages of training. After good performance was achieved, activity in the cerebellar cortex started decreasing gradually over many days if training was extended, and was followed by an increase of activity in the deep nuclei [60]. This work, and related studies supporting the transfer of plasticity from cortex to nuclei in other cerebellar-dependent tasks [45,47-49,50•,51], suggest the tantalizing hypothesis that depending on the stage and the type of learning, Purkinje cells may accomplish their function by wearing different hats: forecaster, teacher and commander.
Conclusions and perspectives
Careful analysis of Purkinje cell activity in awake-behaving animals provides hints about the role of the cerebellar cortex in sensori-motor calibration. It is clear that many Purkinje cells change their activity in tasks that require making motor adjustments to maintain good performance. However, the functional significance of these neural changes is much less clear. I advance a new proposal in which Purkinje cell activity is used in three different ways in the early stages of sensori-motor calibration: first as the output of a forward model that generates sensory and motor predictions for state estimation, second as a teaching signal that ‘transfers’ plasticity from the cerebellar cortex to neurons of the vestibular and deep cerebellar nuclei, and third as the output of an inverse model that contributes directly to the motor command. The role of Purkinje cells switches later on, depending on the amount of ‘transfer’ that takes place. In sensori-motor tasks driven by a disturbance of the sensory stimulus, transfer is often partial and as a result, Purkinje cell activity continues to act as the output of both forward and inverse models. In sensori-motor tasks driven by a motor disturbance, transfer is much more complete. In this case, Purkinje cells stop making adjustments to the motor command; instead their activity is used as the output of a forward model that predicts the kinematic properties of future movement. Testing these predictions will require sampling the activity of Purkinje cells at different stages of the sensori-motor calibration process, and developing new tasks in which changes to the motor command (inverse model) can be dissociated from the kinematics of the movement (forward model).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Fu QG, Flament D, Coltz JD, Ebner TJ. Relationship of cerebellar Purkinje cell simple spike discharge to movement kinematics in the monkey. J Neurophysiol. 1997;78:478–491. doi: 10.1152/jn.1997.78.1.478. [DOI] [PubMed] [Google Scholar]
- 2.Roitman AV, Pasalar S, Johnson MT, Ebner TJ. Position, direction of movement, and speed tuning of cerebellar Purkinje cells during circular manual tracking in monkey. J Neurosci. 2005;25:9244–9257. doi: 10.1523/JNEUROSCI.1886-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greger B, Norris SA, Thach WT. Spike firing in the lateral cerebellar cortex correlated with movement and motor parameters irrespective of the effector limb. J Neurophysiol. 2004;91:576–582. doi: 10.1152/jn.00535.2003. [DOI] [PubMed] [Google Scholar]
- 4.Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol. 1990;63:1241–1261. doi: 10.1152/jn.1990.63.5.1241. [DOI] [PubMed] [Google Scholar]
- 5.Medina JF, Lisberger SG. Variation, signal, and noise in cerebellar sensory-motor processing for smooth-pursuit eye movements. J Neurosci. 2007;27:6832–6842. doi: 10.1523/JNEUROSCI.1323-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung HC, Suh M, Kettner RE. Cerebellar flocculus and paraflocculus Purkinje cell activity during circular pursuit in monkey. J Neurophysiol. 2000;83:13–30. doi: 10.1152/jn.2000.83.1.13. [DOI] [PubMed] [Google Scholar]
- 7.Shidara M, Kawano K, Gomi H, Kawato M. Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature. 1993;365:50–52. doi: 10.1038/365050a0. [DOI] [PubMed] [Google Scholar]
- 8.Thier P, Dicke PW, Haas R, Barash S. Encoding of movement time by populations of cerebellar Purkinje cells. Nature. 2000;405:72–76. doi: 10.1038/35011062. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert PF, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- 10.Norris SA, Greger B, Hathaway EN, Thach WT. Purkinje cell spike firing in the posterolateral cerebellum: correlation with visual stimulus, oculomotor response, and error feedback. J Neurophysiol. 2004;92:1867–1879. doi: 10.1152/jn.01251.2003. [DOI] [PubMed] [Google Scholar]
- 11•.Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci. 2010;30:3715–3727. doi: 10.1523/JNEUROSCI.4953-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Based on the known connections in the pathway between cerebellar cortex and downstream motoneurons, the authors demonstrate that some of the changes observed in Purkinje cells during saccade adaptation are well suited to modify the motor command directly in a way that improves performance.
- 12•.Catz N, Dicke PW, Thier P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proc Natl Acad Sci USA. 2008;105:7309–7314. doi: 10.1073/pnas.0706032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changes in the population burst, but not in the burst of individual Purkinje cells, can account for the altered kinematics and duration of adapted saccades.
- 13••.Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–1192. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Activation of the climbing fiber input can be used to predict how the activity of each individual Purkinje cell changes trial-by-trial during sensori-motor calibration of smooth pursuit eye movements.
- 14.Kahlon M, Lisberger SG. Changes in the responses of Purkinje cells in the floccular complex of monkeys after motor learning in smooth pursuit eye movements. J Neurophysiol. 2000;84:2945–2960. doi: 10.1152/jn.2000.84.6.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27:2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green JT, Steinmetz JE. Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning. Learn Mem. 2005;12:260–269. doi: 10.1101/lm.89505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojakangas CL, Ebner TJ. Purkinje cell complex and simple spike changes during a voluntary arm movement learning task in the monkey. J Neurophysiol. 1992;68:2222–2236. doi: 10.1152/jn.1992.68.6.2222. [DOI] [PubMed] [Google Scholar]
- 18.Lisberger SG, Pavelko TA, Bronte-Stewart HM, Stone LS. Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J Neurophysiol. 1994;72:954–973. doi: 10.1152/jn.1994.72.2.954. [DOI] [PubMed] [Google Scholar]
- 19••.Ebner TJ, Hewitt AL, Popa LS. What features of limb movements are encoded in the discharge of cerebellar neurons? Cerebellum. 2011 doi: 10.1007/s12311-010-0243-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkinje cell discharge is more consistent with the output of a forward model predicting future movement kinematics, than with the output of an inverse model influencing the motor command directly.
- 20••.Lisberger SG. Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience. 2009;162:763–776. doi: 10.1016/j.neuroscience.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkinje cell discharge during smooth pursuit eye movements provides three types of signals: first eye velocity feedback, second a motor command, and third an internal model of the physics of the orbit.
- 21.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Green AM, Angelaki DE. Internal models and neural computation in the vestibular system. Exp Brain Res. 2010;200:197–222. doi: 10.1007/s00221-009-2054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Analysis of 3D-ocular motion suggests that Purkinje cell output provides a signal related to the kinematics of movement (forward model), but not to the motor command (inverse model)
- 23.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 24.Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16:645–649. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 25••.Imamizu H, Kawato M. Brain mechanisms for predictive control by switching internal models: implications for higher-order cognitive functions. Psychol Res. 2009;73:527–544. doi: 10.1007/s00426-009-0235-1. [DOI] [PubMed] [Google Scholar]
- Reviews functional imaging and neurophysiological evidence in support of a modular organization of multiple forward and inverse models in the cerebellum.
- 26.Diedrichsen J, et al. Neural correlates of reach errors. J Neurosci. 2005;25:9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkinson N, Miall RC. Disruption of saccadic adaptation with repetitive transcranial magnetic stimulation of the posterior cerebellum in humans. Cerebellum. 2010;9:548–555. doi: 10.1007/s12311-010-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci. 2008;27:132–144. doi: 10.1111/j.1460-9568.2007.05996.x. [DOI] [PubMed] [Google Scholar]
- 29.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol. 2000;83:2047–2062. doi: 10.1152/jn.2000.83.4.2047. [DOI] [PubMed] [Google Scholar]
- 30.Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. J Neurosci. 1999;19:10940–10947. doi: 10.1523/JNEUROSCI.19-24-10940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- 32.Highstein SM. Synaptic linkage in the vestibulo-ocular and cerebello-vestibular pathways to the VIth nucleus in the rabbit. Exp Brain Res. 1973;17:301–314. doi: 10.1007/BF00234668. [DOI] [PubMed] [Google Scholar]
- 33.Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990;302:330–348. doi: 10.1002/cne.903020211. [DOI] [PubMed] [Google Scholar]
- 34.Morcuende S, Delgado-Garcia JM, Ugolini G. Neuronal premotor networks involved in eyelid responses: retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat. J Neurosci. 2002;22:8808–8818. doi: 10.1523/JNEUROSCI.22-20-08808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Medina JF, Lisberger SG. Encoding and decoding of learned smooth-pursuit eye movements in the floccular complex of the monkey cerebellum. J Neurophysiol. 2009;102:2039–2054. doi: 10.1152/jn.00075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkinje cell output changes during adaptation of smooth pursuit, but the relationship between neural firing and eye-movement kinematics is not the same before and after adaptation.
- 36••.Ke MC, Guo CC, Raymond JL. Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci. 2009;12:1171–1179. doi: 10.1038/nn.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adaptation of the vestibular-ocular reflex is possible in the absence of teaching signals from climbing fiber inputs, suggesting that inherent modulation of Purkinje cell output during the process of sensori-motor calibration may act as a teaching signal itself.
- 37.Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. J Neurosci. 1998;18:9112–9129. doi: 10.1523/JNEUROSCI.18-21-09112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medina JF, Mauk MD. Simulations of cerebellar motor learning: computational analysis of plasticity at the mossy fiber to deep nucleus synapse. J Neurosci. 1999;19:7140–7151. doi: 10.1523/JNEUROSCI.19-16-07140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.McElvain LE, Bagnall MW, Sakatos A, du Lac S. Bidirectional plasticity gated by hyperpolarization controls the gain of postsynaptic firing responses at central vestibular nerve synapses. Neuron. 2010;68:763–775. doi: 10.1016/j.neuron.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First to show that the patterns of Purkinje cell activity present during adaptation of the vestibular-ocular reflex can be used as a teaching signal to induce plasticity in the vestibular nuclei.
- 40••.Zheng N, Raman IM. Synaptic inhibition, excitation, and plasticity in neurons of the cerebellar nuclei. Cerebellum. 2010;9:56–66. doi: 10.1007/s12311-009-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reviews evidence suggesting that the patterns of Purkinje cell activity present in many types of sensori-motor calibration can be used as a teaching signal to induce plasticity in the deep cerebellar nuclei.
- 41.Person AL, Raman IM. Deactivation of L-type Ca current by inhibition controls LTP at excitatory synapses in the cerebellar nuclei. Neuron. 2010;66:550–559. doi: 10.1016/j.neuron.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Menzies JR, Porrill J, Dutia M, Dean P. Synaptic plasticity in medial vestibular nucleus neurons: comparison with computational requirements of VOR adaptation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long-term depression of synapses onto vestibular neurons can be induced by hyperpolarizing patterns of Purkinje cell activity present during VOR adaptation and classical conditioning.
- 43.Medina JF. A recipe for bidirectional motor learning: using inhibition to cook plasticity in the vestibular nuclei. Neuron. 2010;68:607–609. doi: 10.1016/j.neuron.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51:113–123. doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Medina JF, Nores WL, Ohyama T, Mauk MD. Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr Opin Neurobiol. 2000;10:717–724. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 46.Miles FA, Lisberger SG. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–299. doi: 10.1146/annurev.ne.04.030181.001421. [DOI] [PubMed] [Google Scholar]
- 47.Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. J Neurosci. 2001;21:4081–4089. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broussard DM, Kassardjian CD. Learning in a simple motor system. Learn Mem. 2004;11:127–136. doi: 10.1101/lm.65804. [DOI] [PubMed] [Google Scholar]
- 49.Kassardjian CD, Tan YF, Chung JY, Heskin R, Peterson MJ, Broussard DM. The site of a motor memory shifts with consolidation. J Neurosci. 2005;25:7979–7985. doi: 10.1523/JNEUROSCI.2215-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Anzai M, Kitazawa H, Nagao S. Effects of reversible pharmacological shutdown of cerebellar flocculus on the memory of long-term horizontal vestibulo-ocular reflex adaptation in monkeys. Neurosci Res. 2010;68:191–198. doi: 10.1016/j.neures.2010.07.2038. [DOI] [PubMed] [Google Scholar]
- Pharmacological inactivation of Purkinje cells in the flocculus of monkeys after short-term adaptation in the VOR impaired memory and returned the gain to normal, whereas the same inactivation after long-term adaptation had no effect.
- 51.Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao S. Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience. 2006;139:767–777. doi: 10.1016/j.neuroscience.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 52.Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- 53.Pellegrini JJ, Evinger C. Role of cerebellum in adaptive modification of reflex blinks. Learn Mem. 1997;4:77–87. doi: 10.1101/lm.4.1.77. [DOI] [PubMed] [Google Scholar]
- 54.Optican LM, Robinson DA. Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol. 1980;44:1058–1076. doi: 10.1152/jn.1980.44.6.1058. [DOI] [PubMed] [Google Scholar]
- 55.Baizer JS, Kralj-Hans I, Glickstein M. Cerebellar lesions and prism adaptation in macaque monkeys. J Neurophysiol. 1999;81:1960–1965. doi: 10.1152/jn.1999.81.4.1960. [DOI] [PubMed] [Google Scholar]
- 56.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119(Pt 4):1183–1198. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- 57•.Holdefer RN, Miller LE. Dynamic correspondence between Purkinje cell discharge and forelimb muscle activity during reaching. Brain Res. 2009;1295:67–75. doi: 10.1016/j.brainres.2009.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursts in Purkinje cells were correlated with muscle EMG activity during a sequential button-pressing task.
- 58.Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci. 2006;9:1404–1411. doi: 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto K, Kawato M, Kotosaka S, Kitazawa S. Encoding of movement dynamics by Purkinje cell simple spike activity during fast arm movements under resistive and assistive force fields. J Neurophysiol. 2007;97:1588–1599. doi: 10.1152/jn.00206.2006. [DOI] [PubMed] [Google Scholar]
- 60.Nezafat R, Shadmehr R, Holcomb HH. Long-term adaptation to dynamics of reaching movements: a PET study. Exp Brain Res. 2001;140:66–76. doi: 10.1007/s002210100787. [DOI] [PubMed] [Google Scholar]