Abstract
The midbrain periaqueductal gray (PAG) is an integrative neural site in regulating several physiological functions including cardiovascular activities driven by sympathetic nervous system. Specifically, activation of the dorsolateral PAG (dl-PAG) leads to increases in sympathetic nervous activity and arterial blood pressure. Our recent studies demonstrated that angiotensin-(1-7) [Ang-(1-7)] plays an inhibitory role in neuronal activity of the dl-PAG via a Mas-R [Ang-(1-7) receptor] and neuronal NO dependent signaling pathway (Mas-R-nNOS). Because sympathetic nervous activity is augmented in chronic heart failure (HF), the present study was to determine 1) the levels of Ang-(1-7) and Mas-R-nNOS expression within the dl-PAG of control rats and rats with HF and 2) the role for Ang-(1-7) in modulating activity of dl-PAG neurons in both groups. Results showed that chronic HF decreased the levels of Ang-(1-7) and attenuated Mas-R-nNOS pathways. Also, we demonstrated that the discharge rates of dl-PAG neurons of HF rats (5.52±0.52 Hz, n=21, P < 0.05 vs. control) were augmented as compared with control rats (4.03±0.39 Hz, n=28) and an inhibitory role played by Ang-(1-7) in neuronal activity of the dl-PAG was significantly decreased in HF (51±6%, P < 0.05 vs. control) as compared with controls (72±8%). Our findings suggest that the inhibitory effects of Ang-(1-7) on dl-PAG neurons are impaired in HF, likely due to attenuated Mas-R-nNOS signaling pathways.
INTRODUCTION
The midbrain periaqueductal gray (PAG) is an important neural site for numerous physiological functions including cardiovascular regulation [1, 3, 18]. Among regions of the PAG, the dorsolateral (dl) region receives abundant afferent inputs from the spinal cord [10, 16] and sends descending neuronal projections to the medulla in regulating cardiovascular activities [19, 26]. Activation of the dl-PAG contributes to increases in sympathetic nerve activity (SNA) and blood pressure (BP) [1, 3]. Moreover, the dl-PAG plays a functional role in regulating pain, adaptive behavior, emotion, and anxiety etc. as a key relay area that receives numerous neuronal projections from other brain regions [18]. Also, during those physiological and behavioral activities SNA and BP responses are notably observed.
The brain renin angiotensin system (RAS) plays an essential role in control of SNA, BP and balance of hydromineral and fluid volume [5, 20]. Also, the RAS contributes to the development of hypertension and cardiac hypertrophy [6, 25]. In the RAS, angiotensin II (Ang II) has been widely studied and findings suggest that brain Ang II represents the most important effective hormone of this system. Ang II injected into the PAG of rats increases BP via AT1 receptors [11], suggesting that the RAS is engaged in regulation of BP in the PAG. Additionally, the role played by Ang II of the PAG in modulating nociceptive and behavioral responses has been previously reported [22, 24].
The heptapeptide angiotensin-(1-7) [Ang-(1-7)] was traditionally considered as an inactive metabolic breakdown product of Ang II. Because angiotensin converting enzyme 2 (ACE2) is identified to cleave directly Ang II to Ang-(1-7) and the G-protein coupled receptor Mas (Mas-R) is recognized as the first binding site for Ang-(1-7) [13, 23, 30], many studies demonstrated that this peptide is involved in cardiovascular actions. Opposed to Ang II, the effects of Ang-(1-7) are primarily beneficial via counter-regulating Ang II actions [15, 23]. In the brain, Ang-(1-7) and Mas-R are expressed in cardiovascular related-regions [2]. The role for Ang-(1-7) in central regulation of cardiovascular activities and in the pathogenesis of neurogenic hypertension has been reported [7, 14]. Our recent study [28] has demonstrated that Mas-R appears within dl-PAG and Ang-(1-7) decreases the discharge rate of dl-PAG neurons via Mas-R and neuronal NO dependent signaling pathway (Mas-R-nNOS).
Congestive heart failure (HF) is a chronic condition that is characterized by impaired cardiac function that leads to a decrease in blood supply to metabolizing tissues. It is well known that sympathoexcitation plays a prominent role in disease progression [4]. Moreover, sympathoexcitation is inversely related to disease prognosis [8]. Since activation of dl-PAG increases SNA and BP, in this report, we examined neuronal activity of dl-PAG in rats with chronic HF. Also, we examined the effects of HF on the protein levels of Ang-(1-7) and Mas-R-nNOS expression within dl-PAG. Furthermore, we examined the role played by Ang-(1-7) in modulating neuronal activity in dl-PAG of control rats and HF rats. We hypothesized that Ang-(1-7) and Mas-R-nNOS pathways are attenuated after HF, which leads to a decrease in the inhibitory effects of Ang-(1-7) on dl-PAG neurons.
METHODS
All procedures were approved by the Institutional Animal Care and Use Committee of Penn State College of Medicine and complied with the NIH guidelines.
Coronary Artery Ligation
Male Sprague-Dawley rats (160 to 200 g) were anesthetized, intubated, and artificially ventilated. A left thoracotomy between the fourth and fifth ribs was performed, exposing the left ventricular wall. The left coronary artery was ligated [27]. Age and body weight-matched rats that underwent the same procedure as described except that a suture was placed below the coronary artery but was not tied served as controls. The PAG tissues were removed to perform the experiments six weeks after the surgery.
Transthoracic echocardiography was performed before the experiments [27]. The rats were anesthetized by inhalation of an isoflurane-oxygen mixture. The transducer was positioned on the left anterior chest, and left ventricular dimensions were measured. In addition, a Millar catheter was inserted into the right carotid artery and was threaded into the left ventricle for measurement of left ventricular end-diastolic pressure (LVEDP) to further examine the rats’ cardiac function before the neuronal tissues were taken.
Measure Levels of Ang-(1-7)
Eight control rats and ten HF rats were anesthetized and sacrificed. The brain was removed quickly and the dl-PAG tissue was dissected under a Zeiss anatomical microscope. The levels of Ang-(1-7) in dl-PAG were measured using a two-site ELISA. Briefly, polystyrene 96-well microtitel immunoplates were coated with affinity-purified goat anti-Ang-(1-7) antibody. After overnight incubation, plates were washed and the diluted samples and Ang-(1-7) standard solutions (0–1000 pg/ml) were distributed in each plate and left at room temperature overnight. The plates were washed and incubated with anti-Ang-(1-7) galactosidase. The optical density was measured at 575 nm using a microplate spectrophotometer.
Expression of Mas-R-nNOS Pathways
Six control rats and eight HF rats were anesthetized and decapitated. The brain was removed and dl-PAG was dissected. The tissues were processed using a standard procedure to determine Mas-R and nNOS as reported previously [28]. Briefly, total protein was extracted by homogenizing dl-PAG sample in ice-cold radioimmunoprecipitation assay buffer and protein concentrations were measured. After being denatured, the samples containing 20 μg of protein were loaded onto gels and electrically transferred to a polyvinylidene fluoride membrane and incubated overnight with primary antibodies. Next, the membranes were washed and incubated with secondary antibodies, respectively. The immunoreactive proteins were detected by enhanced chemiluminescence. The bands recognized by the primary antibody were visualized by exposure of the membrane onto an x-ray film. Then, the film was scanned and the optical density of Mas-R, nNOS and β-actin bands was analyzed using the NIH Scion Image software.
Neuronal Activity of the dl-PAG
Fourteen control rats and fourteen HF rats were anesthetized and decapitated. Briefly, the brain was quickly removed and placed in ice-cold artificial cerebral spinal fluid (aCSF) solution as described previously [28, 29]. A tissue block containing the PAG was cut from the brain coronal slice (300 μm).
A whole cell current-clamp technique was used to record the spontaneous firing activity of dl-PAG neurons in a recording chamber with the controlled conditions as reported previously [28, 29]. Whole cell recordings from dl-PAG neurons were performed visually using differential interference contrast (DIC) optics on an upright microscope. A tight giga-ohm seal was subsequently obtained in dl-PAG neuron. Signals were recorded and saved in a computer using a MultiClamp 700B amplifier and pClamp 10.1 software. A liquid junction potential of −15.0 mV was corrected during off-line analysis [28, 29]. An equilibration period of 5–10 min was allowed after the recording reached a steady state.
Ang-(1-7), A-779 (Mas-R antagonist) and S-methyl-L-thiocitrulline (SMTC, nNOS inhibitor) were dissolved in the aCSF solution immediately before being used. The drugs were delivered into the recording chamber at final concentrations using syringe pumps during the experiment [28, 29]. Based on prior studies [9, 28, 31], 100 nM of Ang-(1-7), 10 μM of A-779 and 1 μM of SMTC were chosen in this experiment. The responses of action potentials (AP) of dl-PAG neurons to drugs were recorded after baseline control data were collected. The discharge rates of PAG neurons were analyzed off-line with a peak detection program (MiniAnalysis).
Statistical Analysis
One-way ANOVA was used to determine differences between groups. Data represent means ± SEM. For all analyses, differences were considered significant at P<0.05.
RESULTS
General and Echocardiographic Measurements
The left ventricular fractional shortening (LVFS) was determined by echocardiographic measurements. Rats with the left ventricular FS <30% showed increases in heart weight, LVEDP, and left ventricular diastolic dimension. These rats were used as a HF group. In addition, FS is >40% in all control rats. The key measurements of the cardiac function are shown in Figure 1.
Figure 1.
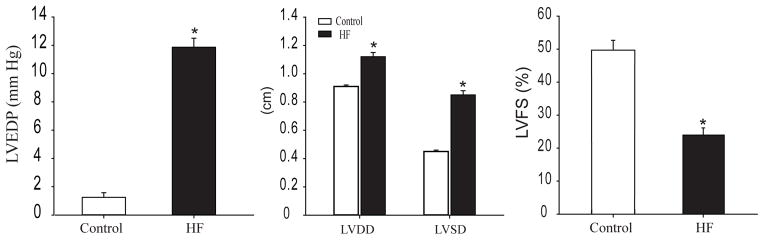
Left ventricular end-diastolic pressure (LVEDP) and echocardiographic measurements. *P <0.05, HF (n=32) vs. control (n = 28). LVDD: left ventricular diastolic dimension; LVSD: left ventricular systolic dimension; and LVFS: left ventricular fractional shortening.
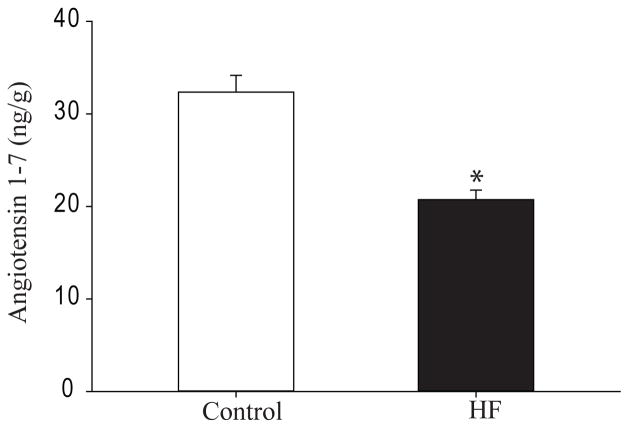
Levels of Ang-(1-7)
The levels of Ang-(1-7) in dl-DRG of control rats (n=8) and HF rats (n=10) induced were examined (Figure 2). As compared with control rats, the levels of Ang-(1-7) in dl-PAG of HF rats were significantly decreased.
Figure 2.
The levels of Ang-(1-7) (ng/g protein) in dl-PAG tissues of control rats (n=8) and HF rats (n=10). *P <0.05 vs. control group.
Expression of Mas-R and nNOS
The protein levels of Mas-R and nNOS in dl-PAG of control rats (n=6) and HF rats (n=8) were analysed (Figure 3). Mas-R and nNOS were localized within dl-PAG. Figure 3 illustrates that expression of Mas-R and nNOS was significantly lower within dl-PAG of HF rats as compared with their expression in control rats.
Figure 3.

(Top panels in A&B): Dual representative bands illustrate Mas-R and nNOS expression in control rats and HF rats. (Bottom panels in A&B): Average data show that Mas-R and nNOS are significantly attenuated in HF (n=8) compared with control (n= 6). Note that the same amount of protein was sampled to examine their individual expression. Beta-actin was used as control for equal loading of protein. *P <0.05 vs. control.
Effects of Ang-(1-7) on Neuronal Activities
Data were collected from a total of 98 dl-PAG neurons responsive to applied agents that were obtained from 14 control rats and 14 HF rats. The amplitude of AP in both groups was >60 mV. The input resistance was 655.9±26.3 MΩ in control group and 642.5±34.2 MΩ in HF group (P >0.05 vs. control).
Basal discharges of dl-PAG neurons were first examined (Figure 4). The frequency of AP was higher in dl-PAG neurons of HF rats (5.52±0.52 Hz, n=21, P < 0.05 vs. control) than that in control rats (4.03±0.39 Hz, n=28). Then, the effects of Ang-(1-7) on the discharges of dl-PAG neurons were examined by using the same dosage of Ang-(1-7) (100 nM) in both groups. Figure 4 showed that Ang-(1-7) significantly decreased the discharge rate of dl-PAG neurons in both groups. After Ang-(1-7), the rate was decreased from 4.03±0.39 Hz to 1.1±0.28 Hz (P<0.05 vs. baseline) in 28 dl-PAG neurons of control rats; and from 5.52±0.52 Hz to 2.43±0.41 Hz (P<0.05 vs. baseline). Nevertheless, an inhibitory role played by Ang-(1-7) in neuronal activity of dl-PAG was significantly decreased in HF rats (51±6%, P < 0.05 vs. control) as compared with control rats (72±8%). Note that the responses of AP to Ang-(1-7) developed at a latency of 15 to 60 s. Then, the responses recovered during washout of the perfusion solution and completely returned to the levels of control without Ang-(1-7) at 5–10 min.
Figure 4.
(A): Original tracings from dl-PAG neurons from a control rat and a HF rat show baseline spontaneous discharges and discharges during 100 nM of Ang-(1-7) perfusion. (B): Average data (n=28 in control and n=21 in HF) for the effects of Ang-(1-7) on the firing activity of dl-PAG neurons. *P < 0.05 vs. baseline; # P < 0.05 vs. control. (C): Average data showing the percentage of inhibitory effects of Ang-(1-7) on the firing activity of dl-PAG neurons. *P < 0.05 vs. control.
In additional experiments, the firing activities of dl-PAG neurons of both control (n=30) and HF rats (n=19) were first observed following application of 100 nM of Ang-(1-7), and a recovery was allowed. Then, the firing activities were examined during Ang-(1-7) in the presence of A-779, and SMTC, respectively. Subsequent application of Ang-(1-7) failed to decrease the spontaneous neuronal activities in the presence of A-779 and SMTC in both groups. i.e. The discharge rates in dl-PAG neurons of control rats (n=16) were 4.06±0.50 Hz after A-779 vs. 4.12±0.42 Hz during infusion of Ang-(1-7) as Mas-R was blocked (P > 0.05 vs. baseline and A-779 application). The discharge rates in dl-PAG neurons of HF rats (n=10) were 5.65±0.55 Hz after A-779 vs. 5.52±0.45 Hz during infusion of Ang-(1-7) as Mas-R was blocked (P > 0.05 vs. baseline and A-779 application). Similarly, the discharge rates in dl-PAG neurons of control rats (n=14) were 4.10±0.36 Hz after SMTC and 4.07±0.37 Hz after Ang-(1-7) with pretreatment of SMTC (P > 0.05 vs. baseline and SMTC perfusion); and the discharge rates in dl-PAG neurons of HF rats (n=9) were 5.63±0.43 Hz after SMTC and 5.67±0.56 Hz after Ang-(1-7) with pretreatment of SMTC (P > 0.05 vs. baseline and SMTC perfusion).
DISCUSSION
As a part of the RAS, Ang II has been widely studied and findings suggest that brain Ang II represents the important effective hormone of this system [5, 20, 32, 33]. Ang II acts as a neurotransmitter in the CNS and plays an important role in the control of balance of fluid volume, as well as sympathetic and cardiovascular activities [5, 20]. Ang II injected into the PAG of rats increases BP via AT1 receptors [11], suggesting that the RAS is engaged in regulation of BP in the PAG. Also, the RAS contributes to the development of hypertension and cardiac hypertrophy [33]. As cardiac output is reduced in HF, SNA is enhanced and the RAS including angiotensinogen, ACE, and the Ang II and its receptors is altered [33]. Ang II receptor subtypes, termed AT1 and AT2, are engaged in action of Ang II [12]. The prior studies revealed the presence of both AT1 and AT2 throughout the rat brain [17, 21]. Studies further suggest that Ang II mediated AT1 upregulation in the CNS plays an important role in augmented SNA in HF [33].
In contrast, Ang-(1-7) is traditionally considered as an inactive metabolic breakdown product of Ang II. Because ACE2 is identified to cleave directly Ang II to Ang-(1-7) and the G-protein coupled receptor Mas-R is recognized as the first binding site for Ang-(1-7) [13, 23, 30], some studies demonstrated that this peptide is involved in cardiovascular actions. Opposed to Ang II, the effects of Ang-(1-7) are primarily beneficial via counter-regulating Ang II actions [13, 23, 30]. In the brain, Ang-(1-7) and Mas-R are expressed in cardiovascular related-regions [2]. The role for Ang-(1-7) in central regulation of cardiovascular activities and in the pathogenesis of neurogenic hypertension has been reported [7, 14].
Our recent study has demonstrated that Mas-R is present within the dl-PAG and Ang-(1-7) has an inhibitory effect on neuronal activities of the dl-PAG via Mas-R [28]. Also, among three isoforms of NOS nNOS largely appears within the dl-PAG and a blockade of nNOS significantly attenuates the effects of Ang-(1-7) on discharges of dl-PAG neurons [28]. Those data suggest that stimulation of Mas-R inhibits neuronal activities of the dl-PAG via a NO dependent signaling pathway. This provides a base for the current study to examine the role played by Ang-(1-7) in modulating activities of dl-PAG neurons in rats with HF given that chronic HF exaggerates SNA and activation of the dl-PAG increases SNA and arterial BP.
Results of the present study showed that the levels of Ang-(1-7) decreases in the dl-PAG region of rats with chronic HF, thereby likely leading to attenuated expression of Mas-R-nNOS pathways within the dl-PAG. Furthermore, regulatory effects of Ang-(1-7) on activities of dl-PAG neurons were determined in control rats and HF rats. The results demonstrated that Ang-(1-7) significantly attenuated the discharge rates of AP in the dl-PAG of both groups. These effects were abolished in the presence of Mas-R antagonist and nNOS inhibitor. This is consistent with our previous report [28], suggesting that Ang-(1-7) plays a role via a NO signaling pathway. Importantly, an inhibitory role played by Ang-(1-7) in neuronal activity of the dl-PAG is significantly decreased in HF rats as compared with control rats when the same dosage of Ang-(1-7) is applied. This result is compatible with the data demonstrating that Mas-R-nNOS is downregulated after development of chronic HF. Nevertheless, our present study provide the first evidence demonstrating that Ang-(1-7) and Mas-R-nNOS pathways are attenuated in the dl-PAG region of HF rats, which leads to a decrease in the inhibitory effects of Ang-(1-7) on dl-PAG neurons, the effects which exaggerate SNA.
A few issues should be addressed. First, a specific group of neurons related to cardiovascular regulation needs to be studied since not all the neurons in the dl-PAG are functionally related to the SNA and BP regulation. Second, we have determined the level of Ang-(1-7) protein within the dl-PAG in this study, however; the changes of Ang-(1-7) in HF may not be restricted solely in this region since SNA is also regulated by other brain nuclei e.g. brain stem and hypothalamus. Third, regarding Ang-(1-7) the similar studies in human HF patients are still lacking and such studies should increase a value of the translational work.
In summary, our findings suggest that the inhibitory effects of Ang-(1-7) on dl-PAG neurons are impaired in chronic HF, likely due to attenuated Mas-R-nNOS signaling pathways. This result offers the basis to study the potentially pharmacological role of Ang-(1-7) and its pathways within the brain in improving enhanced sympathetic nervous activity in HF.
Highlights.
Ang-(1-7) plays an inhibitory role in regulating the dorsolateral PAG neurons.
We examined the role of Ang-(1-7) in the PAG of control and heart failure rats.
Ang-(1-7) is decreased in the dorsolateral PAG of rats with heart failure.
Mas-R-nNOS pathways are attenuated in the dorsolateral PAG of heart failure.
The inhibitory role played by Ang-(1-7) is impaired in heart failure.
Acknowledgments
This study was supported by NIH R01 HL090720 & AHA Established Investigator Award 0840130N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- 1.Bandler R, Carrive P, Zhang SH. Integration of somatic and autonomic reactions within the midbrain periaqueductal gray: Viscerotopic, somatotopic and functional organization. Prog In Brain Res. 1991;87:269–305. doi: 10.1016/s0079-6123(08)63056-3. [DOI] [PubMed] [Google Scholar]
- 2.Becker LK, Etelvino GM, Walther T, Santos RAS, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart Circ Physiol. 2007;293:H1416–H1424. doi: 10.1152/ajpheart.00141.2007. [DOI] [PubMed] [Google Scholar]
- 3.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 4.Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, Lohse MJ, Hein L. Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106:2491–2496. doi: 10.1161/01.cir.0000036600.39600.66. [DOI] [PubMed] [Google Scholar]
- 5.Brooks VL. Interactions between angiotensin II and the sympathetic nervous system in the long-term control of arterial pressure. Clin Exp Pharmacol Physiol. 1997;24:83–90. doi: 10.1111/j.1440-1681.1997.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 6.Bruner CA, Kuslikis BI, Fink GD. Effect of inhibition of central angiotensin pressor mechanisms on blood pressure in spontaneously hypertensive rats. Journal of Cardiovascular Pharmacology. 1987;9:298–304. doi: 10.1097/00005344-198703000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Campagnole-Santos MJ, Heringer SB, Batista EN, Khosla MC, Santos RA. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol Heart Circ Physiol. 1992;263:R89–R94. doi: 10.1152/ajpregu.1992.263.1.R89. [DOI] [PubMed] [Google Scholar]
- 8.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 9.Costa MA, Verrilli MA, Gomez KA, Nakagawa P, Peña C, Arranz C, Gironacci MM. Angiotensin-(1–7) upregulates cardiac nitric oxide synthase in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H1205–H1211. doi: 10.1152/ajpheart.00850.2009. [DOI] [PubMed] [Google Scholar]
- 10.Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol. 1995;361:225–248. doi: 10.1002/cne.903610204. [DOI] [PubMed] [Google Scholar]
- 11.D’Amico M, Di Filippo CC, Berrino L, Rossi FF. AT1 receptors mediate pressor responses induced by angiotensin II in the periaqueductal gray area of rats. Life Sci. 1997;61:PL17–PL20. doi: 10.1016/s0024-3205(97)00362-7. [DOI] [PubMed] [Google Scholar]
- 12.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 13.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106:373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrario CM, Trask AJ, Jessup JA. Advances in the biochemical and functional roles of angiotensin converting enzyme 2 and angiotensin-(1–7) in the regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keay KA, Feil K, Gordon BD, Herbert H, Bandler R. Spinal afferents to functionally distinct periaqueductal gray columns in the rat - An anterograde and retrograde tracing study. J Comp Neurol. 1997;385:207–229. doi: 10.1002/(sici)1096-9861(19970825)385:2<207::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 18.Lovick TA, Adamec R. The Periaqueductal Gray (PAG) Neural Plasticity. 2009 doi: 10.1155/2009/360907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGaraughty S, Chu KL, Bitner RS, Martino B, El Kouhen R, Han P, Nikkel AL, Burgard EC, Faltynek CR, Jarvis F. Capsaicin infused into the PAG affects rat tail flick responses to noxious heat and alters neuronal firing in the RVM. J Neurophysiol. 2003;90:2702–2710. doi: 10.1152/jn.00433.2003. [DOI] [PubMed] [Google Scholar]
- 20.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977;57:313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 21.Phillips MI, Shen L, Richards EM, Raizada MK. Immunohistochemical mapping of angiotensin AT1 receptors in the brain. Regul Pept. 1993;44:95–107. doi: 10.1016/0167-0115(93)90233-x. [DOI] [PubMed] [Google Scholar]
- 22.Prado WA, Pelegrini-da-Silva A, Martins AR. Microinjection of renin-angiotensin system peptides in discrete sites within the rat periaqueductal gray matter elicits antinociception. Brain Res. 2003;972:207–215. doi: 10.1016/s0006-8993(03)02541-1. [DOI] [PubMed] [Google Scholar]
- 23.Santos RAS, Silva ACS, Maric C. Angiotensin-(1–7) is an endogenous ligand for the G 425 protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sewards TV, Sewards MA. The awareness of thirst: proposed neural correlates. Consciousness & Cognition. 2000;9:463–487. doi: 10.1006/ccog.2000.0462. [DOI] [PubMed] [Google Scholar]
- 25.Steckelings UM, Obermuller N, Bottari SP, Qadri F, Veltmar A, Unger T. Brain angiotensin: receptors, actions and possible role in hypertension. Pharmacol Toxicol. 1992;70:S23–S27. doi: 10.1111/j.1600-0773.1992.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 26.Verberne AJM, Guyenet PG. Midbrain central gray: Influence on medullary sympathoexcitatory neurons and the baroreflex in rats. Am J Physiol. 1992;263:R24–R33. doi: 10.1152/ajpregu.1992.263.1.R24. [DOI] [PubMed] [Google Scholar]
- 27.Xing J, Koba S, Kehoe V, Gao Z, Rice K, King N, Sinoway L, Li J. Interstitial norepinephrine concentrations in skeletal muscle of ischemic heart failure. Am J Physiol. 2007;293:H1190–H1195. doi: 10.1152/ajpheart.00231.2007. [DOI] [PubMed] [Google Scholar]
- 28.Xing J, Kong J, Lu J, Li J. Angiotensin-(1-7) inhibits neuronal activity of dorsolateral periaqueductal gray via a nitric oxide pathway. Neurosci Lett. 2012;522:156–161. doi: 10.1016/j.neulet.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xing J, Lu J, Li J. Angiotensin II inhibits GABAergic synaptic transmission in dorsolateral periaqueductal gray neurons. Neurosci Lett. 2009;455:8–13. doi: 10.1016/j.neulet.2009.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- 31.Yang R, Yin J, Li Y, Zimmerman MC, Schultz HD. Angiotensin-(1–7) increases neuronal potassium current via a nitric oxide-dependent mechanism. Am J Physiol Cell Physiol. 2011;300:C58–C64. doi: 10.1152/ajpcell.00369.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimura R, Sato T, Kawada T, Shishido T, Inagaki M, Miyano H, Nakahara T, Miyashita H, Takaki H, Tatewaki T, Yanagiya Y, Sugimachi M, Sunagawa K. Increased brain angiotensin receptor in rats with chronic high-output heart failure. J Card Fail. 2000;6:66–72. doi: 10.1016/s1071-9164(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 33.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol. 2004;84:217–232. doi: 10.1016/j.pbiomolbio.2003.11.010. [DOI] [PubMed] [Google Scholar]