Abstract
Idiopathic or alcohol-induced increases in the expression and function of the Group1 metabotropic glutamate receptor subtype 1 (mGluR1) within the extended amygdala are theorized to contribute to an individual’s propensity to consume excessive amounts of alcohol. In the past, the detailed study of the functional relevance of mGluR1 for alcoholism-related behaviors in animal models was hampered by the poor solubility and non-specific side effects of available inhibitors; however, the advent of the highly potent and soluble mGluR1 negative allosteric modulator JNJ-16259685 [(3,4-Dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone] has instigated a re-examination of the role for this mGluR subtype in mediating the behavioral effects of alcohol. In this regard, systemic pretreatment with JNJ-16259685 was proven effective at reducing alcohol reinforcement and motivation for the drug. mGluR1 is a Gαq/o-coupled receptor, the stimulation of which activates phospholipase C (PLC). Thus, the present study investigated potential neuroanatomical substrates and intracellular molecules involved in the ability of JNJ-16259685 to reduce alcohol intake. JNJ-16259685 (0–30 pg/side) was infused into the shell subregion of the nucleus accumbens (NAC) of C57BL/6J and Homer2 knock-out (KO) mice, either alone or in combination with the PLC inhibitor U-73122 (5.8 fg/side). Alcohol intake was then assessed under Drinking-in-the-Dark (DID) procedures. Intra-NAC JNJ-16259685 infusion dose-dependently reduced alcohol consumption by C57BL/6J mice; this effect was not additive with that produced by U-73122, nor was it present in Homer2 KO animals. These data provide novel evidence in support of a critical role for mGluR1-PLC signaling, scaffolded by Homer2, within the NAC shell, in maintaining alcohol consumption under limited access procedures. Such findings have relevance for both the pharmacotherapeutics and pharmacogenetics of risky alcohol drinking and alcoholism.
Keywords: ethanol, binge drinking, mGluR1, nucleus accumbens, Homer2, phospholipase C
1. Introduction
Behavioral pharmacological and neuropharmacological data indicate that inhibition of Group1 metabotropic glutamate receptors (mGluRs; subtypes mGluR1 and mGluR5) may be a pharmacotherapeutic strategy for alcoholism and perhaps related affective disorders. In vitro, alcohol inhibits mGluR5 function (Minami et al., 1998) and the vast majority of research concerning Group1 mGluR-directed therapies investigates alcohol-mGluR5 interactions (c.f., Bird & Lawrence, 2009; Cleva et al., 2010; Olive, 2009). While alcohol is reported to not interact directly with the mGluR1 (Minami et al., 1998), this receptor subtype can regulate alcohol reward/reinforcement when assessed in laboratory rodents. For example, systemic pretreatment of C57BL/6J (B6) mice with the mGluR1α neutral antagonist CPCCOEt [7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester] lowers the efficacy of alcohol to maintain instrumental behavior, engender intake under continuous access procedures, elicit a conditioned place-preference and sensitize alcohol-induced dopamine and glutamate release within the nucleus accumbens (NAC) (Lominac et al., 2006). However, other reports have failed to detect statistically significant effects of comparable doses of CPCCOEt upon alcohol-induced changes in behavior in either alcohol-preferring P rats or B6 mice (e.g., Hodge et al., 2006; Schroeder et al., 2005). Likewise, an intra-NAC infusion of CPCCOEt is only moderately effective at reducing excessive alcohol intake by B6 mice in models of binge alcohol drinking (Cozzoli et al. 2009; 2012). While these variable findings argue against mGluR1 signaling as a mediator of alcohol’s rewarding/reinforcing properties, the poor solubility of CPCCOEt limits full dose-response analyses of behavior and precludes definitive statements regarding the potential functional relevance of mGluR1 for behavior in animal models of alcohol abuse and alcoholism (see Cozzoli et al. 2009). The advent of the highly potent and considerably more soluble mGluR1 negative allosteric modulator JNJ-16259685 (c.f., Niswender & Pin, 2010) has instigated a re-examination of the functional relevance of mGluR1 for alcoholism-related behaviors. To date, the studies employing systemic and intra-NAC JNJ-16259685 pretreatment support its efficacy at reducing both alcohol reinforcement and motivation for alcohol in P rats and localizes this effect, at least in part, to the NAC (Besheer et al., 2008a, 2008b). More recent studies have indicated the central nucleus of the amygdala (CeA) as another neural locus for the “anti-reward” effects of JNJ-16259685, as the local infusion into either inbred B6 mice or mice on a mixed B29 genetic background reduced alcohol intake under 2-hr Drinking-in-the-Dark (DID) procedures, with an approximately 30% reduction in intake achieved by maximally effective doses (Cozzoli et al., 2013). Together, the above data indicate mGluR1 as a viable therapeutic target for the management of heavy alcohol drinking.
mGluR1 and mGluR5 are Gq/11-coupled receptors, the activation of which can stimulate phospholipase C (PLC) activity (c.f., Niswender & Pin, 2010). The cellular localization of these receptor subtypes, as well as their signaling efficiency, is highly dependent upon members of the Homer family of post-synaptic scaffolding molecules (e.g., Fagni et al., 2002) that are well-characterized to regulate behavioral sensitivity to alcohol, as well as other drugs of abuse (c.f., Szumlinski et al., 2008a). Interestingly, a recent report by our group demonstrated that the local infusion of the PLC inhibitor, U-73122, into the CeA reduced dose-dependently alcohol intake under DID procedures (Cozzoli et al., 2013). Notably, the U-73122 dose-intake response function was relatively flat and even when infused at doses 10,000-fold greater than that of the minimally effective dose for drinking reduction, intra-CeA U-73122 did not abolish alcohol intake by either B6 or B29 hybrid mice. Rather, akin to the effects of either mGluR1 or mGluR5 blockade, CeA infusions of a high dose of U-73122 attenuated by approximately 40% the amount of alcohol consumed by mice. However, when mice were co-infused intra-CeA with maximally effective doses of JNJ-16259685 and U-73122, the effects of the antagonists were greater than an infusion of U-73122 alone, suggesting that another affector molecule was activating PLC to impinge upon drinking behavior. This affector is likely to be mGluR5, as the effects of co-infusing U-73122 and a maximally effective dose of the mGluR5 negative allosteric modulator MTEP into the CeA were not additive, suggesting that, within the CeA, PLC operates within a pathway interdependent upon mGluR5, but not mGluR1, that maintains heavy drinking behavior. Despite this functional disconnect, the inhibitory effects of MTEP, JNJ-16259685 and U73122 upon alcohol intake were absent when infused into the CeA of Homer2 knock-out (KO) mice (Cozzoli et al., 2013). These data were the first to indicate a critical role for Homer2 in regulating PLC activity in vivo and indicated that, at least within the CeA, Homer2 scaffolding is essential to observe the inhibitory effects of mGluR5, mGluR1 and PLC inhibitors upon voluntary alcohol intake.
The shell subregion of the NAC shares cytoarchitechtural features and interconnections with the CeA (e.g., Cassell et al., 1999) and neuropharmacological evidence has already provided support for a key role for mGluR5/Homer2-mediated signaling within the NAC shell in regulating binge alcohol intake under both Scheduled High Alcohol Consumption (SHAC) and DID procedures (Cozzoli et al., 2009, 2012). Interestingly, while mice in early withdrawal from a history of drinking under SHAC procedures exhibit elevated mGluR5 levels within the NAC (Cozzoli et al., 2009), mice drinking alcohol under DID procedures exhibit no change in this receptor subtype (Cozzoli et al., 2012). In contrast, mice with a history of drinking under either SHAC or DID procedures exhibit a marked (1.5–2-fold) increase the protein expression of mGluR1 within the NAC, concomitant with increased Homer2 levels (Cozzoli et al., 2009, 2012). Of note, alcohol-induced increases in NAC mGluR1/Homer2 expression are not unique to animals consuming alcohol under limited access procedures, as this neuroadaptation is observed in alcohol-injected mice (Goulding et al., 2011; Szumlinski et al., 2005; 2008b), as well as in rodents with a history of alcohol consumption under continuous access procedures (Obara et al., 2009; Szumlinski et al., 2008b). While the long-term impact of a chronic history of limited-access alcohol intake upon mGluR1 signaling is not fully characterized, increases in NAC mGluR1 expression and that of its scaffolding protein Homer2 persist for at least 2 weeks in alcohol-abstinent rodents with a chronic (1-month) history of continuous alcohol access (Obara et al. 2009; Szumlinski et al., 2008). Based on the above immunoblotting results, as well as the extant behavioral pharmacological data concerning mGluR1 antagonism (Besheer et al., 2008a, 2008b; Cozzoli et al., 2013; Lominac et al., 2006), we theorize that increased mGluR1/Homer2 signaling within the NAC is a pharmacodynamic response to alcohol that likely contributes to the propensity to drink alcohol in excess.
Indeed, basal mGluR1 expression within the NAC is a biochemical correlate of genetic vulnerability to binge drink in mice selectively bred for high versus low alcohol consumption under DID or SHAC procedures, as well as between wild-type and transgenic mice with divergent alcohol intake under these two limited-access procedures (Cozzoli et al., 2009; 2012). Moreover, a recent meta-analysis revealed a significant association between the gene encoding PLCL1 with heavy drinking (Kapoor et al., 2013). Thus, we sought to extend our recent behavioral pharmacological results from our studies of the CeA (Cozzoli et al., 2013) to the NAC shell and determined the effects of the local infusion of mGluR1 and PLC inhibitors, as well as their combination, upon alcohol intake under modified DID procedures in inbred B6 mice. To investigate the potential role for Homer2 in mediating the effects of mGluR1 and PLC inhibitors, we assayed for the effects of intra-NAC infusions of mGluR1 and PLC inhibitors also in mice with null mutations of Homer2.
2. Materials and Methods
2.1 Animals
The studies employed adult (8 weeks of age), male, C57BL/6J (B6) mice that were obtained from Jackson Laboratories (Sacramento, CA). Follow-up studies also employed adult (7–10 weeks old) male C57BL/6J X 129Xi/SvJ mice with null mutations of Homer2 (Homer2 KO) and their wild-type (WT) counterparts (consistent with Cozzoli et al, 2009; 2012, 2013; see Rong et al., 2003 for details). All mice were individually housed and maintained in polyethylene cages in colony rooms, controlled for temperature (25°C) and humidity (71%), under a reversed light cycle (lights off: 1100 h) in an AAALAC-accredited vivarium. Food and water were available ad libitum throughout the study, unless indicated. B6 mice were allowed to acclimatize to the colony room for at least 2 days following arrival, prior to surgical procedures (see below). All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of California Santa Barbara and were consistent with the guidelines of the NIH Guide for Care and Use of Laboratory Animals.
2.2 Surgical Procedures
The surgical procedures to implant bilateral guide cannulae above the NAC shell subregion were identical to those employed in previous reports (e.g., Cozzoli et al, 2012). Under isoflurane anesthesia, guide cannulae (20-gauge, 10 mm long) were implanted 2 mm over the NAC shell using the following coordinates from the mouse brain atlas of Paxinos and Franklin (2004): AP: +1.3; ML: ±0.5 mm; DV: −2.3 mm from Bregma. To prevent continuous externalization, dummy cannulae (24 gauge; 10 mm long) were placed inside the guide cannulae. These guide cannulae were only removed before intracranial infusion (see below) and were immediately reinserted following microinfusion procedures Animals were allowed a minimum of 5 days recovery from surgery prior to commencing our modified DID drinking procedures.
2.3 Drinking-in-the-Dark (DID) Procedures
The DID procedures to entice high alcohol intake (3.5–5.0 g/kg/2hr) over a limited period of time (2 hrs) were similar to those employed previously by our group (e.g., Cozzoli et al, 2012, 2013) and are a modification of DID procedures originally described in Rhodes et al (2005). Briefly, 3 hours after lights out (i.e. 14:00 h), the home cage water bottle was replaced by a sipper tube (5 ml plastic pipette, fitted with a sipper spout; see Finn et al., 2005), containing 20% alcohol (v/v) or 5% sucrose (w/v) in tap water. In all studies, mice were allowed to drink from a single sipper tube for a total of 2 hrs/day and then the home cage water bottle was returned. As conducted previously (Cozzoli et al, 2012, 2013), mice underwent our modified DID procedures daily, 7 days a week, until the completion of the study. On each day, the volume of alcohol/sucrose consumed was determined by weighing the sipper tubes immediately before and after the drinking session. Fresh alcohol/sucrose solutions were prepared weekly and mice were weighed once a week. The alcohol/sucrose intake of the animals (in g) was expressed as a function of the animal’s body weight (in kg). It has been our experience (Cozzoli et al. 2012, 2013) that B6 mice drinking under these daily DID procedures exhibit consistently high intakes across days that are within the range reported by our laboratory and others to elicit blood alcohol concentrations (BACs) of 80 mg% or higher (e.g., Cozzoli et al., 2013; Moore and Boehm, 2009; Rhodes et al., 2005). Unfortunately, at the time of study, technical difficulties with our Analox Analyzer precluded reliable determinations of BACs in the animals. Thus, BACs were estimated from recorded intakes based on the results of published correlational analyses in B6 mice (Rhodes et al., 2005), as conducted previously (Cozzoli et al., 2009).
2.4 Intracranial Drug Infusion Procedures
2.4.1. Experimental design
Bilaterally cannulated B6, Homer2 WT and Homer2 KO mice were trained to consume 20% alcohol under our 2-hr DID procedures until stable intake was established (<10% variability across 3 consecutive presentations). Stable intakes were achieved typically within the first 4 days of drinking. The entire study was conducted in replicates of 8–14 mice each. Mice from each replicate received a maximum of 5 microinfusions (0.25 μl/side infusion), with microinfusions spaced at least 3 days apart, in order to assay for carry-over effects and to ensure that intake returned to pre-infusion levels prior to subsequent testing. The first infusion was intended to habituate the animals to the microinjection procedures and consisted of an injection of 0.1% DMSO (dimethyl sulfoxide) in water vehicle. The amount of alcohol consumed was recorded during this habituation session, but not employed in the final statistical analyses of the data as typically intakes were low (i.e., <2.5 g/kg) as a result of handling/injection stress.
It is our experience that B6 mice consume somewhere between 3.5 to 6.5 g/kg alcohol under our 2-hr DID procedures, while the intakes of Homer2 WT/KO mice tend to be a bit lower, within the range of 3.0 to 4.5 g/kg alcohol (see Cozzoli et al., 2012, 2013). Thus, B6 or Homer2 WT/KO mice that exhibited very low alcohol intake (i.e., <1.0 g/kg) upon this habituation infusion were considered hyper-reactive to microinjection stress and discarded immediately from the study (n=0–2/replicate). As we have yet to observe significant carry-over effects of any of our intracranial manipulations upon alcohol drinking under SHAC or DID procedures in either B6 or Homer2 WT/KO mice (Cozzoli et al., 2009, 2012, 2013), any mice that exhibited a reduction in alcohol intake upon microinfusion (including the habituation infusion) but failed to recover their intakes back to their pre-habituation baseline during the time intervening between microinfusions (± 0.5 g/kg) were also discarded from further experimentation (0–2 mice/replicate).
In the earliest replicates of study, we tested the effects of blocking mGluR1 in the NAC shell with the negative allosteric modulator JNJ-16259685 [0, 5, 15 and 30 pg/side; Tocris Bioscience, Ellisville, MO (Cozzoli et al., 2013; Xie et al., 2010)] upon the alcohol intake of B6 mice under our 2-hr DID procedures. JNJ-16259685 was dissolved in 0.1% DMSO and 0.1% DMSO served as our vehicle control for all mice in this report. In our previous study of the CeA (Cozzoli et al., 2013), PLC inhibition reduced alcohol intake under DID procedures and this effect was not additive with that produced by mGluR1 blockade, indicating the involvement of inter-dependent signaling pathways. As the effects of blocking PLC within the NAC have yet to be assessed upon alcohol drinking, we also tested conducted a dose-response study to examine the effects of an intra-NAC infusion of the PLC inhibitor U-73122 [1-[6-((17β-3-Methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione; 0, 5.8 and 58 fg/side; EMD Millipore, Billerica, MA], as well as the co-infusion of the highest dose of JNJ-16259685 (30 pg/side) with the 58 fg/side dose of the PLC inhibitor U-73122. The 58 fg/side dose of U-73122 was selected for co-infusion as this dose produced a greater than 50% reduction in alcohol intake during early replicates of this study (n=6) that was not statistically different from that produced by the 5.8 fg/side dose and this dose is 100-fold greater than that reported to be maximally effective at reducing alcohol intake under DID procedures when infused intra-CeA (Cozzoli et al., 2013).
As alcohol experience is reported to elevate mGluR1 levels within the NAC shell (Cozzoli et al., 2012; Goulding et al., 2011; Obara et al., 2010; Szumlinski et al., 2008), the intracranial treatments were administered in a pseudo-counterbalanced fashion such that the effects of each of the different intracranial treatments were assayed early, mid-way and later during the 2.5–3 weeks of bottle presentation, as often as possible (see Section 2.5 below). Moreover, for each replicate of mice tested, some animals (number varying depending on the total number of animals in a particular replicate) were infused with vehicle on each microinjection day to ensure that any reduction in drinking observed in response to intra-NAC infusion of the test compound(s) reflected a pharmacological response to the compound(s) and not some non-specific reduction in intake as a consequence of the repeated microinjection procedures. Over the course of the 4 experimental microinjections, no animal received a particular intracranial treatment more than once.
Upon completion of the habituation infusion, B6 animals were slated to receive an intra-NAC infusion of vehicle + 3 experimental manipulations, which could include 1 of 3 doses of JNJ-16259685, 1 of 2 doses of U-73122 and, in later replicates (after the efficacy of intra-NAC U-73122 to reduce alcohol intake was established; n=6), the combination treatment. Upon completion of the habituation infusion, Homer2 WT and KO mice were slated to receive vehicle, the dose of JNJ-1625985 that was minimally effective at reducing alcohol intake in B6 mice (15 pg/side), and the 2 doses of U-73122. Both doses of U-73122 were examined in WT and KO mice to determine whether or not genotypic differences might exist in the sensitivity of the animals to the inhibitory effects of this drug upon drinking.
2.4.2. Microinfusion procedures
As conducted previously, microinfusions were delivered via a 33-gauge injector (12 mm long) at a rate of 0.25μl/min for a total volume of 0.25μl/side, and the injectors remained in place for an additional 60 sec prior to their careful removal. Immediately after infusion, dummy cannulae were re-inserted, mice were returned to their home cage and presented with the 20% alcohol-containing sipper tube for 2 hrs. To assess for nonspecific effects of intra-NAC JNJ-16259685 and U-73122 infusion, their effects were assayed upon 5% sucrose intake during a 2-hr session in separate groups of mice. Standard cresyl violet histochemical procedures were used to verify injector cannulae localization in the NAC shell (see Figure 3) and to examine for signs of tissue damage. Importantly, the repeated microinfusion procedures did not produce any overt signs of distress in the animals, as indexed by coat condition and changes in body weight. On average, the B6 mice that completed the 31–35 days of testing (i.e., received 5 microinfusions) gained 0.5 ± 0.04 g over the course of the study, while no changes in body weight were noted for either Homer2 WT or KO mice that were tested for 10–15 days (depending upon the study).
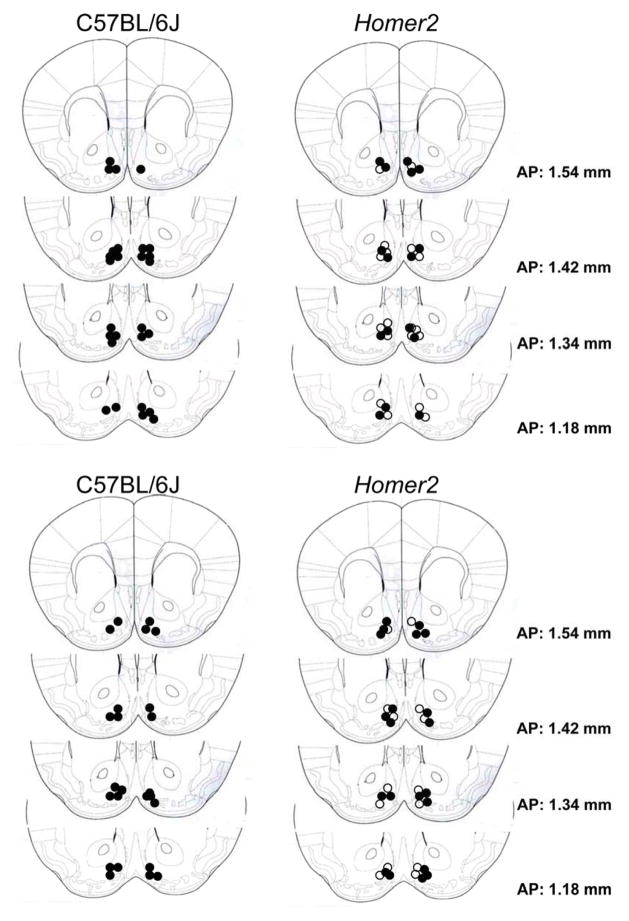
Figure 3.
Representative placements of the tips of the microinjection cannulae aimed at the shell of the nucleus accumbens from one replicate each of C57BL/6J (left) and Homer2 wild-type (WT) and knock-out (KO) mice (right; open=WT; closed=KO) infused with JNJ-16259685 (top) and U-73122 (bottom).
2.5 Statistical Analyses
It was intended that all mice within the JNJ-16259685 study receive all doses of the mGluR1 antagonist, while those in the U-73122 study receive all doses of this inhibitor, in addition to an infusion of JNJ-16259685 + U-73122. However, issues associated with spurious loss of cranial implant or, more commonly, guide cannulae patency, and an earthquake mid-way through testing of a replicate of both B6 and Homer2 WT/KO animals (affecting 14 B6 mice following their 3rd microinjection, in addition to 12 WT and 6 KO animals following their 2nd microinjection), approximately 30% of the B6 and 50% of the WT/KO animals did not receive all intended intracranial treatments within a study. Moreover, in the earliest replicates of B6 mice (total number of mice at the outset of testing = 36), the response to infusion of the 15 pg/side dose of JNJ-16259685 was unusually variable (sem > 2.25 g/kg), which contrasted with our previous findings for the CeA (Cozzoli et al., 2013). This variability likely reflected differences in laboratory personnel conducting the study, seasonality of testing, and/or regional differences in JNJ-16259685 responsiveness that required further flushing out. As such, we included this dose also in some later replicates of B6 mice originally slated for U-73122 testing (accounting for the relatively high sample size of this treatment condition). Consequently, mice treated in this manner received only the one dose of JNJ-16259685 and did not receive all doses of U-73122, prohibiting a within-subjects analysis of drinking behavior. Finally, we could not include the inhibitor co-infusion condition in the earlier replicates of the U-73122 dose-response study in B6 mice because we had yet to identify the efficacy of intra-NAC infusions to lower drinking in our model and yet to identify the most appropriate dose in this regard. In the end, only approximately 10% of the total number of B6 mice tested received all doses of either inhibitor, while approximately 50% of the WT/KO mice in each study received all doses of either inhibitor. Thus, we reduced the total numbers of animals required for this investigation by including all data from mice that were successfully microinjected with any compound(s) (incl. return to baseline levels of drinking between intracranial treatments), and we approached the Treatment factor as a between-subjects factor. Thus, the data from studies of B6 mice were analyzed using a one-way, between-subjects, ANOVA, followed by LSD post-hoc tests, when appropriate. The data from studies of WT/KO mice were analyzed using a between-subjects Treatment X Genotype ANOVA and a significant interaction was deconstructed along the Genotype factor, followed by LSD post-hoc tests, when appropriate. While it is recognized that analyzing the data using a between-subjects versus within-subjects approach reduces the degrees of freedom of the analysis and thus, lowers the power to detect group differences, we rationalized that the sample sizes realized when we approached the data in this manner were sufficiently large to mitigate this issue. However, it should be noted that because we were successful at testing the effects of vehicle vs. JNJ-16259685 and vehicle vs. U-73122 upon sucrose intake in B6 mice, these data were analyzed using paired t-tests, separately for each experiment, as appropriate for the intended experimental design. Also, we compared the pre-testing body weights of the B6 animals that received all 5 microinfusions (i.e. animals that were tested over 31–35 days) to that obtained during the last week of testing as well as the change in body weight exhibited by Homer2 WT/KO mice from the start to end of testing (~10–15 days, depending upon the replicate), using paired t-tests. α=0.05 for all analyses.
3. Results
3.1 Intra-NAC JNJ-16259685 reduces dose-dependently binge alcohol intake in a manner dependent upon Homer2 expression
At the outset of testing for the effects of intra-NAC infusions of JNJ-16259685 upon alcohol intake under our modified 2-hr DID procedures, the average alcohol consumption exhibited by B6 mice was 4.67 ± 0.19 g/kg (N=63). Importantly, the amount of alcohol consumed by B6 following an infusion of 0.1% DMSO vehicle into the NAC shell, administered at various time-points throughout neuropharmacological testing (4.70 ± 0.45 g/kg, N=30) did not vary from this pre-injection baseline (within-subjects ANOVA, p>0.90). Moreover, the alcohol intake exhibited by B6 mice when infused with vehicle did not vary as a function of the number of prior microinjections/number of days engaged in alcohol drinking prior to infusion (Day effect, p>0.65).
As illustrated in Figure 1A, an infusion of the mGluR1 antagonist JNJ-16259685 (0–30 pg/side) reduced dose-dependently alcohol consumption by B6 mice [F(3,103)=5.26, p=0.002]. LSD post-hoc tests indicated that, relative to VEH-infused controls, 5 pg/side JNJ-16259685 reduced only moderately alcohol intake (p=0.14), while the 2 highest doses lowered significantly the amount of alcohol consumed (0 vs. 15 pg: p<0.0001; 0 vs. 30 pg, p=0.02) and did so to a similar extent (i.e., by ~30%; 15 pg vs. 30 pg: p=0.48). Consistent with our prior reports (Cozzoli et al. 2012, 2013), with the exception of 4 animals, there was no evidence for any carry-over inhibitory effects upon, or rebound increases in, intake the day following any infusion (data not shown). These data indicate that inhibition of mGluR1 within the NAC shell produces an acute reduction in high alcohol intake under limited access conditions in B6 mice.
Figure 1.
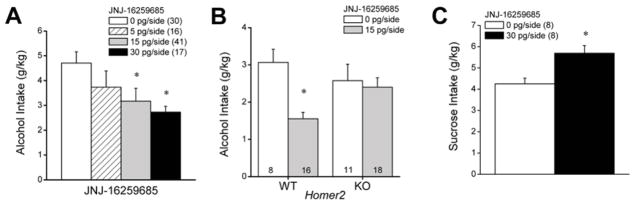
Summary of the effects of intra-NAC infusions of the mGlu1 receptor negative allosteric modulator JNJ-16259685 upon alcohol and sucrose intake under DID procedures. (A) In C57BL/6J mice, the local infusion of JNJ-16259685 dose-dependently reduced alcohol intake. (B) Intra-NAC infusion of 15 pg/side JNJ-16259685 significantly lowered alcohol intake in Homer2 wild-type (WT) mice, but failed to influence intake in knock-out (KO) animals. (C) In contrast to its effects upon alcohol intake, intra-NAC infusion of 30 pg/side JNJ-16259685 increased the intake of a 5% sucrose solution. *p<0.05 vs. 0 pg/side JNJ-16259685 (LSD post-hoc tests in panels A & B; paired t-tests in panel C). Sample sizes employed in the statistical analyses of the data from each study are indicated in parentheses within their respective panels.
We observed no differences in alcohol intake under our modified DID procedures between WT and Homer2 KO mice prior to neuropharmacological testing (WT intake = 2.86 ± 0.38 g/kg, n=20; KO intake = 3.31 ± 0.36 g/kg, n=20; t-test, p>0.05). As depicted in Figure 1B, we also observed no genotypic differences in alcohol intake in response to an intra-NAC infusion of 0.1% DMSO vehicle (WT intake = 3.07 ± 0.36 g/kg, n=8; KO intake: 2.58 ± 0.44 g/kg, n=11; Microinjection X Genotype ANOVA: no main effects or interaction, p’s>0.05). Moreover, as observed in the study of B6 mice (see above): (1) the vehicle infusion did not alter significantly alcohol intake in either genotype, relative to their baseline (paired t-tests, p>0.15; N=19) and (2) there was no obvious differences in the levels of alcohol intake between animals who received their vehicle infusion on the 2nd versus 3rd microinjection day (unpaired t-tests, p>0.86; N=19). However, when the data pertaining to the infusion of JNJ-16259685 (0 vs. 15 pg/side) was compared, we detected a significant Drug X Genotype interaction [F(1,52)=5.39, p=0.02]. As illustrated in Figure 1B, JNJ-16259685 reduced alcohol drinking in Homer2 WT mice by ~50% [F(1,28)=18.69, p<0.0001], but failed to do so in KO animals (one-way ANOVA, p=0.72). Based on these data, we conclude that Homer2 expression is necessary for the capacity of JNJ-16259685 to reduce alcohol drinking under limited-access procedures.
3.2. Intra-NAC JNJ-16259685 augments sucrose intake
The average intake of 5% sucrose solution exhibited by B6 mice prior to neuropharmacological testing was 3.87 ± 0.24 g/kg (n=8) during the 2-h period. While an intra-NAC infusion of 0.1% DMSO vehicle did not influence sucrose intake (paired t-tests, p>0.05), infusion of the 30 pg/side dose of JNJ-16259685 elevated, rather than reduced, sucrose drinking under our 2-hr DID procedures (Figure 1C) [paired t-tests, t(7)=8.68, p<0.0001]. These data argue that the reduction in alcohol intake produced by intra-NAC infusion of JNJ-16259685 does not reflect decrements in the physical capacity to drink, general motivational processes and/or motor function.
3.3 Intra-NAC U-73122 dose-dependently reduces binge alcohol intake in a manner inter-dependent upon mGlu1 receptors, but not Homer2
At the outset of testing for the effects of intra-NAC infusions of U-73122 upon alcohol intake, the average alcohol consumption exhibited by B6 mice was 3.76 ± 0.16 g/kg (N=30), which was very similar to that exhibited by mice infused with the 0.1% DMSO vehicle at various time-points throughout neuropharmacological testing (3.75 ± 0.39 g/kg, N=27). As illustrated in Figure 2A, infusion of the PLC inhibitor U-73122 (0–58 fg/side) into the NAC shell markedly reduced alcohol consumption under our DID procedures [F(3,70)=12.17, p<0.0001] and LSD post-hoc tests confirmed that all intracranial drug treatments reduced alcohol intake, relative to VEH-infused controls (all p’s <0.0001). As reported for the CeA (Cozzoli et al., 2013), the magnitude of the reduction in alcohol intake (~50%) was similar for both the 5.8 and 58 fg/side doses of U-73122 when infused alone (p=0.31) and the magnitude of the reduction in alcohol intake produced by the 58 fg/side dose of U-73122 did not vary upon JNJ-16256985 co-infusion (p=0.86). These data indicate that PLC activation within the NAC shell is necessary for the manifestation of heavy drinking under limited access conditions. Moreover, as co-infusion of JNJ-16256985 did not augment further the inhibitory effect of a high dose of U-73122, these data indicate that PLC operates to regulate heavy drinking in a pathway inter-dependent upon mGluR1 activity.
Figure 2.
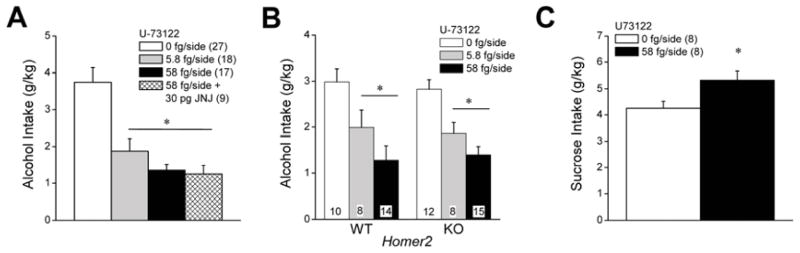
Summary of the effects of intra-NAC infusions of the PLC inhibitor U-73122 upon alcohol and sucrose intake under DID procedures. (A) In C57BL/6J mice, the local infusion of U-73122 reduced binge alcohol intake in a dose-independent manner and co-infusion of 58 fg/side U-73122 with 30 pg/side JNJ-16259685 did not alter the magnitude of the reduction in binge drinking produced by the 58 fg/side dose of U-73122 alone. (B) Intra-NAC infusion of 58 fg/side U-73122 significantly lowered alcohol intake also in Homer2 WT, but not KO, mice. (C) In contrast, intra-NAC infusion of 58 fg/side U-73122 increased the intake of a 5% sucrose solution. *p<0.05 vs. 0 pg/side U-73122 (LSD post-hoc tests in panels A & B; paired t-tests in panel C). Sample sizes employed in the statistical analyses of the data from each study are indicated in parentheses within their respective panels.
In the follow-up study of the effects of U-73122 upon the alcohol intake of Homer2 WT and KO mice, we again observed no genotypic differences in pre-microinjection baseline drinking (WT intake = 3.01 ± 0.42 g/kg, n=14; KO intake = 2.86 ± 0.52 g/kg, n=15; t-test, p>0.05). As depicted in Figure 2B, we also observed no genotypic differences in alcohol intake in response to an intra-NAC infusion of 0.1% DMSO vehicle (WT intake = 2.99 ± 0.27 g/kg, n=10; KO intake: 2.82 ± 0.21 g/kg, n=12; Microinjection X Genotype ANOVA: no main effects or interaction, p’s>0.05). Interestingly, an intra-NAC infusion of either 5.8 or 58 fg/side U-73122 lowered alcohol intake by ~50% in both genotypes (Figure 2B), with no evidence for a genotypic difference in the magnitude of this reduction [U073122 effect: F(1,67)=21.92, p<0.0001; no main effect of Genotype or Genotype X U-73122 interaction: p’s>0.90]. As observed in the JNJ-16259685 study (Figure 1C), an intra-NAC infusion of the 58 fg/side dose of U-73122 also increased significantly sucrose consumption under our DID procedures in B6 mice (Figure 2C) [paired t-tests, t(7)=7.06, p<0.0001]. These data provide the first evidence that mGluR1-mediated stimulation of PLC activity within the NAC shell is required for the manifestation of binge alcohol drinking, in a manner independent of Homer2, and that the capacity of PLC blockade to attenuate alcohol intake does not reflect deficits in motivational or motor processes.
4. Discussion
In the present study, site-directed microinfusions of the selective and highly potent mGluR1 negative allosteric modulator JNJ-16259685 (0–30 pg/side) and the PLC inhibitor U-73122 (0–58 fg/side) into the NAC shell reduced voluntary alcohol drinking under modified (2-hr) DID procedures by 30–50% and this effect was apparent in both high alcohol-consuming, inbred B6 mice and more moderate drinking, hybrid B29 mice. The effects of intra-NAC infusions of JNJ-16259685, but not those of U-73122, required intact Homer2 expression as they were absent in Homer2 KO mice. Finally, the magnitude of the reduction in alcohol drinking produced by an intra-NAC infusion of a maximally effective dose of U-73122 was unaffected by co-infusion of a high dose of JNJ-16259685. These data suggest that, in contrast to the CeA (Cozzoli et al., 2013), PLC in the NAC shell regulates alcohol intake under limited access procedures by operating within a signaling pathway interdependent with mGluR1. These findings extend the existing literature supporting a critical role for mGluR1 in alcoholism-related behavior (Besheer et al., 2008a, 2008b; Cozzoli et al., 2013; Lominac et al., 2006) and provide further support for PLC activity within extended amygdala structures as a neural substrate underpinning heavy alcohol drinking.
4.1 mGluR1 activity, Homer2 scaffolding and alcohol intake
As reported previously by our group (Cozzoli et al., 2009; 2012, 2013), differences were not observed between Homer2 WT and KO mice regarding the amount of alcohol consumed under limited-access conditions; WT-KO differences were not noted prior to microinfusion procedures nor were they apparent upon an intra-NAC infusion of DMSO vehicle. These data contrast strikingly with the pronounced effects of Homer2 deletion upon alcohol intake under continuous access and operant procedures, in which KO mice exhibit very low levels of alcohol consumption and/or preference, particularly for alcohol concentrations greater than 6% (v/v) (Szumlinski et al., 2005). As virus-mediated knock-down of Homer2b within either the NAC shell or the CeA reduces binge alcohol intake by B6 mice (Cozzoli et al., 2009, 2012, 2013), we proposed that some compensatory mechanism(s) secondary to gene deletion masked the binge drinking phenotype of the KO (e.g., Cozzoli et al., 2012). However, the results of a recent, large, meta-analysis of over 100 different transgenic and inbred strains also failed to detect a correlation between alcohol intake under conventional DID procedures (i.e., 3 days of 2-hr access, followed by 1 day of 4-hr access to 20% alcohol; see Rhodes et al., 2005) and behavior in other more traditional alcoholism-related paradigms (incl. continuous alcohol access) (Blednov et al., 2012). In retrospect, this finding is perhaps not so surprising as selective breeding of HS/Npt mice, on a heterogeneous genetic background, for high blood alcohol concentrations (an index of self-intoxication) under conventional DID indicates that the heritability of this trait is very low, with a realized approximate heritability of h2=0.096 (Crabbe et al., 2009). Moreover, the response to selection for high levels of self-intoxication under conventional DID procedures exhibited a linear increase even after 11 generations of selective breeding (Crabbe et al., 2009). This phenomenon suggests additive genetic variability in the population contributes to this phenotype (see Crabbe et al., 2009), which is not likely to manifest when studying single gene mutant animals. Thus, while some compensatory change(s) may mask the effects of Homer2 deletion upon alcohol intake under our modified DID procedures, the failure to detect WT-KO differences more likely reflect the complexity of the genetic interactions underpinning heaving drinking/high levels of self-intoxication under limited access conditions.
Although there were no genotypic difference in alcohol intake between vehicle-treated Homer2 WT and KO mice, an intra-NAC infusion of a minimally effective dose of JNJ-16259685 (15 pg/side) failed to influence alcohol drinking in Homer2 KO animals (Fig. 1B). Thus, the inhibition of drinking produced by JNJ-16259685 binding in the NAC shell depends upon intact Homer2 expression or Homer2 is required for full sensitivity of the receptor to the effects of mGluR1 negative allosteric modulators (inverse agonists). Indeed, constitutively expressed Homer proteins, notably Homer3, are reported to negatively regulate the constitutive activity of Group1 mGluRs in cell culture (including inositol phosphate accumulation) and to influence receptor sensitivity to both neutral antagonists and negative allosteric modulators of mGluR1/5 (Ango et al., 2001). The role for Homer2 in regulating constitutive receptor activity has yet to be assayed in vitro, let alone in vivo. However, Homer2 is required for both the in vivo stimulation of glutamate release within the NAC shell by the non-selective mGluR1/5 agonist DHPG (Szumlinski et al., 2004) as well as for the capacity of an intra-NAC infusion of the mGluR5 negative allosteric modulators MPEP and/or MTEP to reduce binge alcohol drinking under both SHAC and DID procedures (Cozzoli et al., 2009, 2012). These findings, coupled with evidence that intra-NAC infusions of non-competitive, neutral, mGluR1 antagonist CPCCOEt (Litsching et al., 1999) fail to significantly reduce alcohol intake under limited access procedures (e.g., Cozzoli et al., 2009, 2012), suggest that both mGluR1 and mGluR5 within the NAC shell may be constitutively active in vivo and that Homer2 may play a critical role in regulating this constitutive activity.
However, repeated alcohol experience up-regulates Homer2 expression within NAC subregions (Cozzoli et al., 2009, 2012; Goulding et al., 2011; Obara et al., 2010; Szumlinski et al., 2008). Based upon the available data for Homer3 (Ango et al., 2001), this neuroadaptation should blunt constitutive receptor activity and reduce an animal’s sensitivity to negative allosteric modulators, such as MPEP, MTEP and JNJ-16259685. It should be noted that in the present study, as well as those published previously by our group (Cozzoli et al., 2009, 2012, 2013), inhibitor infusion was pseudo-counterbalanced across drinking days, such that the effects of each drug dose were assayed in subsets of animals near the start, mid-way through, and towards the end of their drinking histories. Unfortunately, due to subject attrition, we haven’t sufficient statistical power to decipher whether or not the effects of intra-NAC JNJ-16259685 (or U-73122, for that matter) varied as a function of drinking history. However, we do know from much earlier work that tolerance does not develop to the inhibitory effect of MPEP or CPCCOEt upon alcohol consumption/preference in a continuous access 4-bottle-choice paradigm, at least when the drug is administered systemically for 5 consecutive days to alcohol-experienced mice (Lominac et al., 2006). Thus, if an alcohol-induced increase in Homer2 expression was influencing constitutive mGluR activity within the NAC, such data would indicate that this effect does not contribute to the development of tolerance to the “therapeutic” efficacy of systemic antagonist treatment.
Alternatively, another hypothesis worthy of future exploration may be that an alcohol-induced increase in Homer2 expression may facilitate constitutive receptor activity, sensitizing mGluR1/5 to inhibition by negative allosteric modulators. Three genes (Homer1, 2, 3) encode the Homer family of proteins in mammals (c.f., Shiraishi-Yamaguichi & Furuichi, 2009) and so very little is known regarding the cellular consequences of specific mGluR-Homer interactions in vivo. While it was once presumed that constitutively expressed Homer1, Homer2 and Homer3 proteins were redundant with respect to their functional interactions with Group1 mGluRs (e.g., Worley et al. 2007), Homer2 exhibits higher affinity for mGluR1 than Homer1 and can occlude mGluR-Homer1 interactions (Kammermeier et al., 2000) and constitutively expressed Homer1 versus Homer2 isoforms within medial prefrontal cortex play opposing roles in regulating cocaine preference and –seeking behavior (Ary et al., 2013; Ben-Shahar et al., 2013). Given the consistencies in findings with respect to the inhibitory effects of the mGluR1 negative allosteric modulator JNJ-16259685 upon indices of alcohol reward/reinforcement (Besheer et al., 2008a, 2008b; Cozzoli et al., 2013; present study), more detailed studies of the molecular interactions underpinning receptor pharmacology is warranted to move forward in our understanding of the neurobiology relevant to alcoholism treatment.
4.2. PLC as a target for pharmacotherapeutic intervention in alcoholism
Group1 mGluRs are Gαq/11-coupled receptors, the activation of which directly stimulates PLC activity (e.g., Niswender and Pin, 2010). While there has been considerable focus in the alcohol literature on mGluR5 activation of PKC isoforms as important signaling mechanisms underpinning sensitivity to alcohol reward, intoxication/sedation and neuropathic pain (e.g. Newton & Messing, 2010), little attention has been placed on characterizing the functional relevance of intervening molecules in alcoholism-related behavior, which may have ramifications for drug discovery. To the best of our knowledge, only one study to date has examined directly the role for PLC in regulating alcoholism-related behavior and demonstrated that the local infusion of the PLC inhibitor U-73122 into the CeA attenuated alcohol intake under our modified (2-hr) DID procedures by ~40–50% (Cozzoli et al., 2013). The intra-CeA U-73122 dose-alcohol intake function was surprisingly flat over a 10,000-fold dose range (0.58 fg/side-58,000 fg/side), suggesting that U-73122 was very potent in this regard. While we did not conduct a full dose-response analysis of U-73122’s effects herein, we did not observe significant differences between the attenuation of drinking by the 5.8 and 58 fg/side U-73122 doses in either B6 mice or in mice on a mixed B29 background, although the latter strain tended to exhibit a more step-wise function (see Fig. 2B). PLC expression within the CeA is up-regulated in mice with a month-long history of drinking 20% alcohol under our 2-hr DID procedures (Cozzoli et al., 2013). While it remains to be determined whether or not PLC expression within NAC subregions is similarly influenced by alcohol drinking history, the fact that U-73122 infusion into the NAC shell attenuated drinking in a manner akin to that reported for infusion into the CeA (Cozzoli et al., 2013), points to PLC signaling throughout the extended amygdala as an important molecular substrate regulating alcohol intake under limited access procedures. These neuropharmacological data are timely in the face of very recent evidence for an association between polymorphisms within PLCL1 and drinks per day that arose from a meta-analysis of human data (Kapoor et al., 2013) and they are important as they provide evidence for a clear cause-effect relation between PLC activity and heavy drinking that cannot be derived from correlational studies in humans.
Another strength of the animal model is the ability to employ neuropharmacological and transgenic strategies to dissect cellular and molecular mechanisms that might contribute to disease pathology or recovery. Homer proteins are reported to regulate the downstream signaling pathways activated upon stimulation of mGluR1/5, including their ability to stimulate PLC activity (e.g., Ango et al., 2001; c.f., Shiraishi-Yamaguchi and Furuichi, 2009). Consistent with this, the effects of PLC inhibition within the CeA are absent in the Homer2 KO mouse (Cozzoli et al., 2013), suggesting an important role for this scaffolding protein in the regulation of PLC activity within this structure. To our surprise, the U-73122 dose-response function exhibited by Homer2 KO mice in the present study was nearly identical to that exhibited by their WT counterparts (Fig. 2B). Thus, Homer2 deletion does not impact U-73122 sensitivity within the NAC shell. The distinct effects of Homer2 deletion upon U-73122 sensitivity between the CeA and the NAC shell is very interesting and suggests that despite evidence that Homer2 is up-regulated approximately 2-fold in both brain regions as a consequence of heavy drinking (Cozzoli et al., 2012, 2013), the intracellular consequences of this adaptation may be region-specific. Indeed, a positive relation exists between basal extracellular glutamate and Homer2 expression within the NAC shell (e.g., Goulding et al., 2011; Szumlinski et al., 2004, 2005, 2006, 2008), while an inverse relation between these two variables exists within the medial prefrontal cortex (Ary et al., 2013) and the details of the molecular interactions that contribute to these region-specific interactions are completely unknown. The question as to why the alcohol drinking behavior of Homer2 KO mice is differentially sensitive to U-73122 infusion into the CeA versus the NAC shell is difficult to address using in vivo preparations. Nevertheless, the intact capacity of intra-NAC infusions of U-73122 to lower alcohol intake in Homer2 KO mice is a finding consistent with in vitro evidence that neither long nor short Homer protein isoforms regulate mGluR1 coupling to either Gαq/11 or Gαi/o (Kammermeier et al., 2007), which stimulate the activity of PLCβ1/3 directly or via the liberation of βγ subunits, respectively (c.f., Niswender and Pin, 2011). Indeed, the scaffolding function exhibited by long Homer isoforms is purported to facilitate the efficiency through which IP3 induces calcium release from internal stores upon Group1 mGluR/PLC activation and not necessarily the capacity of the receptors stimulate PLC activity (c.f., Worley et al., 2007).
While it is clear from the present results that intact Homer2 expression is not required in order to observe an attenuating effect of intra-NAC U-73122 upon alcohol intake under our DID procedures, the inhibitory effects of infusing a maximally effective dose of U-73122 upon alcohol intake were not additive with those produced by co-infusion of a maximally effective dose of JNJ-16259685 (Fig. 2A). Thus, mGluR1 activity (constitutive or otherwise) is required in order to observe the inhibitory effects of a PLC inhibitor upon limited access drinking, a finding indicating that mGluR1 and PLC operate within an inter-dependent pathway to maintain alcohol intake. As mentioned at the outset of this discussion, an intra-NAC infusion of neither U-73122 nor JNJ-16259685 alone completely abolished drinking. Consistent with our findings for the CeA (Cozzoli et al., 2013), intra-NAC infusion of either drug reduced intake by only 30–50%, depending upon the background strain of the mice. As such, it is not likely that the failure to observe an additive effect of inhibitor co-infusion reflected a floor effect upon behavior. In further support of this argument, the co-infusion of 58 fg/side U-73122 and 15 pg/side JNJ-16259685 into the CeA reduced alcohol intake to a greater degree than that produced by an intra-CeA infusion of 58 fg/side U-73122 alone (Cozzoli et al., 2013). Such findings demonstrate: (1) that alcohol intake under our modified DID procedures is sensitive to the additive effects of neuropharmacological manipulations and (2) in contrast to the NAC shell, PLC activity within the CeA influences alcohol drinking via some upstream molecule other than mGluR1. This upstream molecule appears to be mGluR5 as the effects of intra-CeA U-73122 co-infusion with a maximally effective dose of the mGluR5 negative allosteric modulator MTEP was not found to be additive with those produced by U-73122 alone (Cozzoli et al., 2013). Thus, not only do differences exist between the NAC shell and CeA regarding the importance of Homer2 for regulating PLC activity of relevance to drinking, but there also appears to be regional distinctions with respect to the relative role for different Group1 mGluR subtypes in the activation of PLC that maintains alcohol intake under limited access conditions. The downstream effectors stimulated within the NAC shell by the activation of mGluR1-PLC signaling that contribute to heavy drinking remain to be elucidated but likely involve those classically activated upon hydrolysis of phosphoinositides by PLC and the generation of IP3-dependent calcium signaling and DAG, in particular PKCs. Indeed, we have evidence implicating mGluR1 stimulation of PKCε activity within the NAC shell as also important for alcohol intake under DID procedures (Cozzoli et al., unpublished observations), that is consistent with extant data derived from the study of an animal model of alcohol reinforcement (Gass and Olive, 2009).
From a therapeutic perspective, the fact that mGluR5, mGluR1 and PLC inhibitors are more or less equally effective at reducing alcohol drinking, whether infused into the NAC or into the CeA (Cozzoli et al., 2012, 2013; present study), argues that either receptor, or the downstream lipase, have the potential for medications development as a strategy for curbing alcohol intake in heaving drinkers. While we have yet to assay for the effects of co-inhibiting mGluR5 with PLC within the NAC, the fact that mGluR1 and PLC inhibitors exert a more pronounced reduction in alcohol drinking when infused into the CeA than either inhibitor alone (Cozzoli et al., 2013), suggests that the simultaneous targeting both mGluR1 and PLC, or alternatively, co-treatment with negative allosteric modulators of both mGluR1 and mGluR5 (or treatment with a less subtype-selective negative allosteric modulator) would be more efficacious a reducing heaving drinking than targeting an mGluR alone.
4.3. Non-specific effects of intra-NAC mGluR1 and PLC blockade
A major issue related to the discovery of glutamate-based pharmacotherapeutics for the treatment of any neuropsychiatric disorder pertains to non-specific drug effects that negatively impact cognitive, motivational and motor processes (c.f., Olive et al., 2012). Systemic JNJ-16259685 pretreatment is reported to impair spontaneous motor activity in rats at doses that are minimally effective at reducing alcohol reinforcement (Besheer et al., 2008a; 2008b), although other reports have failed to detect any effects of mGluR1 blockade upon indices of motor activity in mice or in rats (e.g., Hodge et al. 2006; Lominac et al. 2006; Schroder et al. 2005). Moreover, doses of JNJ-16259685 that were minimally effective at reducing alcohol reinforcement failed to influence sucrose reinforcement in earlier reports (Besheer et al., 2008a; 2008b) and we failed to detect any evidence for negative side-effects of intra-NAC pretreatment with a high dose of either JNJ-16259685 (30 pg/side) or U-73122 (58 fg/side) upon the consumption of a 5% sucrose solution under DID procedures. In fact, both compounds elevated significantly sucrose intake under our 2-hr limited-access procedures when infused intra-NAC (Fig. 1C & 2C). The potentiation of sucrose consumption under DID procedures following intra-NAC infusion of JNJ-16259685 or U-73122 is interesting and not observed when these compounds are infused into the CeA (Cozzoli et al., 2013). This suggests perhaps a special role for mGluR1 signaling within the NAC shell in the gating of the motivation to consume non-alcohol versus alcohol rewards. We demonstrated previously that the mGluR1 neutral antagonist CPCCOEt can potentiate the sedative/hypnotic effects of alcohol in mice (Lominac et al., 2006), although another report failed to detect any effect of systemic CPCCOEt upon alcohol’s sedative/hypnotic effects (Hodge et al., 2006). Thus, the selective reduction in alcohol intake observed upon intra-NAC infusions of JNJ-16259685 and/or U-73122 may reflect increased sensitivity to alcohol’s sedative/hypnotic properties, which are reported to be inversely related to the positive subjective effects of alcohol in humans (e.g., Schuckit et al., 2004). Alternatively, it may reflect a shift to the left in the dose-response function for alcohol reward, such that animals require less alcohol to achieve a particular hedonic state. Indeed, it remains to be determined how either systemic or intracranial pretreatment with JNJ-16259685 and/or U-73122 influences the dose-response function for alcohol intake or for alcohol-induced sedation. Nevertheless, the potentiation of sucrose consumption by intra-NAC infusions of either JNJ-16250685 or U-73122 in the present study and the lack of an effect of systemic JNJ-16259685 pretreatment upon sucrose reinforcement (Besheer et al., 2008a; 2008b) argue that the capacity of mGluR1/PLC blockade to reduce alcohol intake does not likely reflect non-specific effects upon general motivation or motor processes that are likely to hinder their potential therapeutic efficacy.
5. Conclusions
The results of the present study indicate that mGlu1 receptor-mediated activation of PLC within the shell subregion of the NAC is an intracelluar signaling pathway involved in the maintenance of alcohol intake by mice under limited access procedures. The present neuropharmacological data, coupled with results from earlier immunoblotting studies, pose idiopathic or alcohol-induced increases in mGlu1 receptor-mediated stimulation of PLC activity as an important signaling pathway in the etiology of alcoholism and provide experimental support for the potential utility of targeting mGlu1 receptors for the pharmacotherapeutic intervention of this disorder.
Highlights.
Blockade of mGluR1 and PLC within the nucleus accumbens reduced binge alcohol drinking by mice.
The effects of mGluR1, but not PLC, blockade depended upon Homer2 expression.
The effects of mGluR1 and PLC blockade were not additive.
Blockade of mGluR1 and PLC within the nucleus accumbens enhanced sucrose drinking.
Acknowledgments
This research was supported by NIH grant AA016650 to KKS.
Footnotes
Conflicts of Interest
None of the authors have any knowledge of potential conflicts of interest regarding the research presented in this report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ary AW, Lominac KD, Wroten MG, Williams AR, Campbell RR, Ben-Shahar O, von Jonquieres G, Klugmann M, Szumlinski KK. Imbalances in prefrontal cortex CC-Homer1 versus –Homer2 expression promote cocaine-seeking behavior. J Neurosci. 33:8101–8113. doi: 10.1523/JNEUROSCI.1727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE, et al. Deficits in ventral prefrontal cortex Group1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci. 2013;33:495–506. doi: 10.1523/JNEUROSCI.3710-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008a;32:209–221. doi: 10.1111/j.1530-0277.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol. 2008b;42:13–20. doi: 10.1016/j.alcohol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr Mol Pharmacol. 2009;2:83–94. doi: 10.2174/1874467210902010083. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Thompson AB, Wroten M, Zhang PW, Xiao B, Hu J-H, Klugmann M, Metten P, Worley PW, Crabbe JC, Szumlinski KK. Accumbens shell metabotropic glutamate receptor 5-associated signaling regulates binge alcohol drinking: Evidence from Drinking-in-the-Dark studies. Alcohol Clin Exp Res. 2012;36:1623–1633. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK. Binge alcohol drinking by mice requires intact Group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.214. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu J-H, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking up-regulates accumbens mGluR5-Homer2-PI3K signaling: Functional implications for alcoholism. J Neuroscience. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber G, Szumlinski KK, Kash TL, Roberto M. New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology. 2012;67C:223–232. doi: 10.1016/j.neuropharm.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC. A procedure to produce high alcohol intake in mice. Psychopharmacology (Berl) 2005;178:471–480. doi: 10.1007/s00213-004-2039-8. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Role of protein kinase C epsilon (PKCε) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl) 2009;204:587–597. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, Szumlinski KK. Accumbens Homer2-mediated signaling: a factor contributing to mouse strain differences in alcohol drinking? Genes Brain Behav. 2011 Feb;10(1):111–26. doi: 10.1111/j.1601-183X.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci. 2000;20:7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Wang JC, Wetherill L, Le N, Bertelsen S, Hinrichs AL, Budde J, Agrawal A, Bucholz K, Dick D, Harari O, Hesselbrock V, Kramer J, Nurnberger JI, Jr, Rice J, Saccone N, Schuckit M, Tischfield J, Porjesz B, Edenberg HJ, Bierut L, Foroud T, Goate A. A meta-analysis of two genome-wide association studies to identify novel loci for maximum number of alcoholic drinks. Hum Genet. 2013;132:1141–1151. doi: 10.1007/s00439-013-1318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litschig S, et al. CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding. Mol Pharmacol. 1999;55:453–461. [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Dep. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Minami K, Gereau RW, 4th, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;53:148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAA Newsletter. 2004 http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf.
- Newton PM, Messing RO. The substrates and binding partners of protein kinase Cepsilon. Biochem J. 2010;427:189–196. doi: 10.1042/BJ20091302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Pin JP. Metabotropic glutamate receptors: Physiology, Pharmacology and Disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK. Subregional selectivity in the effects of alcohol withdrawal upon Homer/glutamate receptor expression in the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33:1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rong R, Ahn J-Y, Huang H, Nagata E, Kalman D, Kapp JA, et al. PI3 kinase enhancer-Homer complex couples mGluR1 to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. Findings across subgroups regarding the level of response to alcohol as a risk factor for alcohol use disorders: a college population of women and Latinos. Alcohol Clin Exp Res. 2004;28:1499–1508. doi: 10.1097/01.alc.0000141814.80716.32. [DOI] [PubMed] [Google Scholar]
- Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Abernathy KE, Oleson EB, Klugmann M, Lominac KD, He D-Y, Ron D, During MT, Kalivas PW. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006;31:768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Rohrer J, Griffin W, III, Klugmann M, Toda S, Champtiaux NP, Berry T, Shealy S, During M, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate vulnerability to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology (Berl) 2007;190:415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang G, Kim JY, Shin DM, Kim MS, Yuan JP, Kiselyov K, Muallem S. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium. 2007;42:363–371. doi: 10.1016/j.ceca.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]