Intracranial atherosclerosis (ICAS) is an important cause of ischemic stroke throughout the world, accounting for around 30-50% and 10% of ischemic stroke and transient ischemic attack (TIA) in Asians and Caucasians, respectively.1 Several imaging modalities, such as transcranial Doppler (TCD), magnetic resonance angiography (MRA), computed tomographic angiography (CTA), and digital subtraction angiography (DSA), are commonly used in routine clinical practice to detect and assess ICAS, as well as in selection criteria of clinical trials.2-4 Although some of these imaging modalities yield flow information, such as TCD revealing velocity data or waveform turbulence and time-of-flight MRA (TOF-MRA) depicting arterial patterns based on blood flow, most attention has been drawn to the maximal percent stenosis of the arterial lumen. The focus on severity of stenosis has been reinforced since “severe” (70-99%) atherosclerotic stenosis was demonstrated as an independent predictor for stroke recurrence in the territory of the stenotic artery, with the risk of around 20% at 1 year, in the Warfarin versus Aspirin for Symptomatic Intracranial Disease (WASID) trial.5 However, those patients with a traditionally considered “moderate” (50-69%) atherosclerotic stenosis were also at considerable risk of recurrent stroke, approximately 10% at 1 year in the WASID study.5, 6 In more recent studies, the role of percent stenosis in predicting subsequent stroke risk has been superseded by collateral flow and hemodynamics in the same patient cohort.7, 8 Characterization of the atherosclerotic lesion is also poorly represented by percentage of stenosis measured at the narrowest vessel diameter alone. Beyond the maximal luminal stenosis, many other features may reflect the characteristics of ICAS, such as plaque morphology and components, which might also be promising markers in risk stratification of patients with symptomatic ICAS.9 On the other hand, from the view of intracranial stenosis, it could also be attributed to causes other than atherosclerosis, such as moyamoya disease and arterial dissection (not covered in the current review). Therefore, the designation of intracranial atherosclerosis as intracranial stenosis is insufficient and misleading with respect to the diagnosis, characterization of such lesions, and risk stratification for the prevention of subsequent stroke.
In this article, we redefine the diagnosis and evaluation of intracranial atherosclerosis, removing the focus on luminal stenosis alone, drawing upon data from recent imaging studies and we reconsider practical implications of this renewed emphasis on atherosclerotic plaque morphology and hemodynamic impact on downstream brain tissue. Our survey of currently available diagnostic techniques emphasizes potential surrogate markers for risk stratification in symptomatic ICAS.
Diagnostic Modalities for Intracranial Atherosclerosis
Transcranial Doppler and Transcranial Color-Coded Duplex
TCD is a safe, noninvasive and inexpensive method to diagnose ICAS. Although the accuracy of TCD in grading percent stenosis has varied amongst prior studies, it is superior in providing real-time flow information and evidence for direction of flow, collateralization, embolization and steal phenomenon, as compared with static images of CTA and MRA.10 For instance, TCD detection of microembolic signals (MES) has been associated with specific infarct patterns on diffusion-weighted MR images (DWI).11 Persistence of MES may also indicate subsequent worsening of neurological deficits during the acute phase of ischemic stroke.12 Furthermore, MES have been reported as an independent predictor for stroke recurrence in patients with symptomatic ICAS.12 In addition, TCD vasomotor reactivity quantification may reflect the capacity of cerebral autoregulation, often globally impaired in patients with ICAS and a possible risk factor for stroke.12 Most recently, the use of transcranial color-coded duplex (TCCD) has advanced the diagnostic accuracy of ICAS by incorporating anatomical definition of the arterial lumen and has been increasingly applied to evaluate cerebral arteries in studies of revascularization therapies.13
Magnetic Resonance Angiography
TOF-MRA and contrast-enhanced MRA (CE-MRA) are now commonly used for assessment of the intracranial vasculature. TOF-MRA, based on the contrast mechanism known as flow-related enhancement, accentuates hemodynamic features and therefore generally overestimates the degree of stenosis, especially in cases with low flow distal to the ICAS. But the flow information carried by TOF-MRA is likely to play a role in the assessment of hemodynamic impact of the lesion, which is discussed in detail below.8, 14 CE-MRA, acquired with a combined head and neck coil, permits simultaneous imaging of the entire supra-aortic vasculature, from extracranial segments to distal intracranial branches.15 It may provide better morphological visualization as compared with TOF-MRA, especially for a high-degree stenosis with low flow, but its sensitivity to detect intracranial lesions is lower than that of extracranial lesions.15 Quantitative MRA (QMRA), based on a phase-contrast technique and employing TOF-MRA to facilitate vessel localization, is a relatively novel application of MRA to measure blood flow through vessels of interest.16 Quantification of blood flow by QMRA has been found promising in identifying patients at high risk of stroke recurrence, assessing intracranial in-stent stenosis, and revealing pathophysiology in various cerebrovascular disorders.17, 18
High-Resolution Magnetic Resonance Imaging
Recent research using modern magnetic resonance imaging (MRI) techniques such as high-resolution MRI (HR-MRI), and other advanced technology such as the computational fluid dynamics (CFD) technique as detailed below, produce important information to improve understanding of pathophysiology and diagnosis of intracranial atherosclerotic disease. Translation of HR-MRI from the depiction of coronary and carotid plaques to intracranial applications (Figure 1), has enabled imaging of intracranial plaque and the adjacent arterial wall, possibly identifying intracranial plaques due to atherosclerosis or other causes, revealing plaque morphology and constituents, including intraplaque hemorrhage, lipid core and fibrous cap.9 Imaging features of intracranial plaque on HR-MRI (7 Tesla) have been reported to be closely correlated with plaque components by histopathologic analysis in a postmortem, in vitro study.19 HR-MRI was also recently been reported to be helpful in guiding endovascular intervention of atherosclerotic disease of the basilar artery.9 Further studies exploring the relationships between plaque features by HR-MRI and subsequent stroke risk, may provide additional insight regarding the plaque stability and corresponding intervention strategy in patients with symptomatic ICAS.
Figure 1.
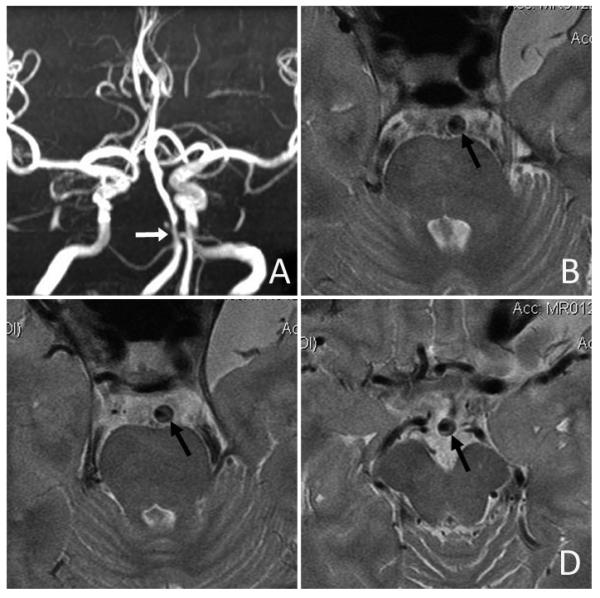
Intracranial plaque and arterial wall imaging by high-resolution MRI. An ICAS lesion located at proximal basilar artery with severe luminal stenosis was identified on time-of-flight MRA (white arrow in Panel A). High-resolution MRI revealed an eccentric atherosclerotic plaque along the anterolateral and posterolateral walls of basilar artery (black arrows in Panels B, C and D). (Courtesy of Professor WH Xu of Peking Union Medical College Hospital, Beijing, China.)
Computed Tomographic Angiography
As a minimally invasive imaging modality, CTA provides better delineation of the anatomy of intracranial arteries, thus yielding higher diagnostic accuracy of the luminal stenosis of ICAS as compared with TCD and MRA, with DSA as the reference standard, though the visualization of petrous and cavernous segments of ICA by CTA may be affected by bony artifacts. Unlike TOF-MRA, the nature of CTA is not based on flow in the vessel, which is an advantage in depicting the vessel morphology but a disadvantage in the way that it tends to eliminate any temporal information about blood flow. Recently, CTA has been increasingly used in evaluating collateralization in ICAS, including leptomeningeal collateral routes, which have been correlated with risk of recurrent events.20, 21 CTA images are a good source for geometric reconstruction in preparation for blood flow simulation by CFD techniques, discussed below.
Digital Subtraction Angiography
DSA is currently considered as the reference standard for diagnosing intracranial vascular diseases including ICAS, due to its superb spatial and contrast resolution to depict the vessels, as well as its ability to reveal temporal information on antegrade and collateral flow.10 It is almost always used as the reference standard in studies testing the accuracy of other imaging modalities to grade the luminal stenosis of ICAS. For the evaluation of collaterals, DSA may clearly reveal patent segments and the direction of flow across segments of the circle of Willis, and it has been demonstrated of good to very good inter-/intra- observer agreement to grade leptomeningeal collateralization, though grading methods have varied.21 However, as an invasive method, DSA could lead to periprocedural complications, including transient or even permanent neurological deficits.
Perfusion Imaging
Hypoperfusion serves as a common cause for ischemic stroke in patients with ICAS.22 CT perfusion (CTP) and perfusion-weighted MR imaging (PWI), have been used to detect hypoperfused territories in acute ischemic stroke during the past decade, which allows for the identification of potentially salvageable tissue, or the ischemic penumbra.23 Mismatch between the hypoperfused tissue and the infarct core on multimodal CT and MR images has been used as an indicator for reperfusion therapies in clinical trials, which is altering the traditional concept of time windows.23 Recently, selective arterial spin-labeling (ASL) MR imaging has been used to reveal the perfusion territories and measure cerebral blood flow of individual cerebral arteries.24 This technique also enables visualization and quantification of the actual collateral flow information in the setting of ICAS.24 These perfusion imaging methods may help reveal the underlining pathophysiology of stroke and reflect the hemodynamic impact of ICAS, which may aid clinical decision making.
Computational Fluid Dynamics of ICAS
Beyond the methods detailed above to evaluate the presence of ICAS, CFD techniques can also be applied to the study of ICAS in order to investigate hemodynamic impact of a specific lesion.25-27 Three-dimensional geometry of the diseased vessels may be reconstructed from angiographic images for simulation of blood flow, through which hemodynamic features of the lesion, including pressure gradients across the atherosclerotic plaque, or fractional flow, may be analyzed.25 CFD simulation based on biplanar DSA images revealed low fractional flow in only 40% of the “severe” (70-99%) stenoses in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial,25 again underscoring the considerable need for more comprehensive evaluation of ICAS rather than arbitrarily grading it as “moderate” or “severe” based on the maximal luminal stenosis.
Stenosis versus Lesion Identification
Despite the above mentioned methods to profile different aspects of the lesions in patients with symptomatic ICAS, percentage of luminal stenosis has been the leading or only indicator for patient selection in successive clinical trials, and those with 70-99% stenosis have been almost exclusively targeted as the “high-risk” population in recent studies.2, 3 Mounting evidence on the importance of collaterals, hemodynamic impact and other factors in determining subsequent stroke risk in patients with symptomatic ICAS,7, 8, 25 suggest that the diagnosis and evaluation of intracranial atherosclerotic disease should be redirected from grading of stenosis to hemodynamic and emboligenic lesion characterization, to optimally identify those who are truly at “high risk”.
Overall Problem with Studies on Diagnostic Tests Focusing on “Stenosis” rather than Other Measures
Noninvasive imaging modalities of TCD, MRA, and CTA, though widely used to detect ICAS, their diagnostic abilities have not been rigorously tested in large, prospective studies. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial, as a companion study to WASID and the first prospective, multicenter study to test the diagnostic ability of these imaging modalities against DSA, focused solely on the maximal degree of luminal stenosis as many other preceding studies did, which is only one amongst many aspects to properly characterize the lesions.10 This might be one of the most important reasons why this study showed poor consistency across these imaging modalities. More recently, the predictive ability of percent stenosis measured via TOF-MRA for the risk of stroke recurrence, has been surpassed by that of the hemodynamic impact of ICAS derived from TOF-MRA as detailed below, in subsequent analyses of the MRA archives of SONIA study.8 Thus, the degree of stenosis may not be an appropriate target for studies investigating diagnostic tests for ICAS, which needs to be addressed in future studies.
Role of Collateralization in ICAS
Collateral status has been demonstrated to correlate with acute and final infarct volume and infarct volume expansion in acute ischemic stroke patients.28, 29 Collateral perfusion is also predictive of response to intravenous thrombolysis and endovascular therapies, with good collaterals reducing hemorrhagic transformation while enhancing revascularization rate.30, 31 Most importantly, the degree of collateral circulation has been found to be significantly correlated with functional outcomes of patients with symptomatic ICAS.20, 32 In the WASID trial, collateral status was identified as an independent predictor for stroke recurrence in the symptomatic arterial territory, altering the stroke risk that otherwise would be different if only predicted based on the severity of luminal stenosis.7 More extensive collaterals reduced the risk of recurrent territorial stroke in 70 to 99% stenoses (none versus good collaterals: HR 4.60, 95% CI 1.03–20.56; poor versus good collaterals: HR 5.90, 95% CI 1.25–27.81; p=0.0427), while increased that risk in 50-69% stenoses (none versus good collaterals: HR 0.18, 95% CI 0.04–0.82; poor versus good collaterals: HR 1.78, 95% CI 0.37–8.57; p < 0.0001). Collateral flow therefore is one of the most essential mediators in cerebral ischemia due to ICAS, and hence an important indicator in risk prediction and treatment allocation in patients with symptomatic ICAS. However, it has not been systematically investigated, and little is known about the process of collateral recruitment or arteriogenesis in progressive ICAS, which warrant further studies.
Role of Fractional Flow in ICAS
Translation of recent advances in the cardiology field has inspired vascular neurologists to apply novel measures to characterization of ICAS. The paradigm shift in ischemia-related coronary artery disease (CAD), from anatomical measures of percent stenosis to hemodynamic impact of lesions, may be paralleled in the diagnostic approach and decision-making of ICAS. Fractional flow reserve (FFR), measured as the ratio of pressures distal and proximal to a coronary lesion under induced hyperemia by floating a pressure wire during percutaneous coronary angiography, has become the gold standard to assess the hemodynamic significance of CAD.33 Large clinical trials on FFR have demonstrated the unreliability of percent stenosis as an indicator to define a hemodynamically significant CAD, especially in cases with anatomically moderate stenoses.34 For instance, in the Fractional Flow Reserve versus Angiography in Multivessel Evaluation (FAME) study, 35% of lesions with an angiographically moderate severity (50-70% stenosis) were found to be functionally significant.35 Moreover, FFR-guided coronary revascularization has been demonstrated safe and superior to angiography-guided strategy in reducing major cardiac events and composite adverse events.36 More recently, the CFD technique has been applied to coronary CTA to noninvasively quantify FFR.34 The noninvasive FFR has been proven of good diagnostic accuracy for the hemodynamic significance of CAD, with invasive FFR as the reference.34 Although cerebral arteries in many ways differ from coronary arteries, these findings in cardiology further aroused the need for neurologists to divert the solely focus on the degree of stenosis to more sound and reasonable ways to evaluate ICAS.
Similar to the application of noninvasive FFR in the coronary arteries, the CFD technique may also be used to assess fractional flow across ICAS lesions. As mentioned above, CFD modeling based on the SAMMPRIS angiography disclosed hemodynamic effects of ICAS, with decreased pressure identified distal to the ICAS lesions.25 Besides, we have performed a pilot study of 10 cases on CFD modeling of ICAS based on intracranial CTA, which have confirmed the feasibility to reconstruct CFD models out of routinely obtained intracranial CTA source images (Figure 2). For CFD modeling of an ICAS lesion based on CTA, three-dimensional geometry of a target arterial segment containing the lesion could be extracted and reconstructed from the CTA source images, which could then be meshed for simulation of the blood flow across the lesion. The simulated CFD models showed decreased pressure and increased flow velocity in situ and beyond the ICAS lesions, as shown in Figure 2. Although the CFD-based fractional flow in ICAS has yet to be correlated with subsequent stroke risk, it provides a fertile ground for next steps in the clinical research on the diagnosis and optimal treatment of ICAS.
Figure 2.
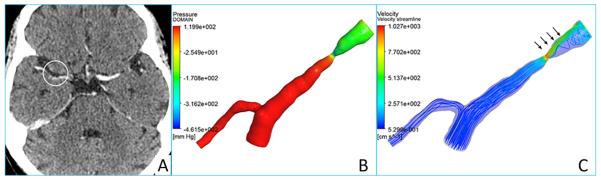
CTA source image (A) showing a right middle cerebral artery stenosis, and the reconstructed CFD models illustrating pressure (B) and flow velocity (C) changes across the lesion. Decreased pressure (B) and increased flow velocity (C) in situ and downstream to the lesion are highlighted with arrows on the CFD models.
Besides the CFD-based evaluation of fractional flow, we developed another method including use of the TOF-MRA termed signal intensity ratio (SIR), based on its flow-related signal contrast mechanism, to systematically gauge the hemodynamic effects of an ICAS. SIR of an ICAS was measured as the ratio of distal and proximal signal intensities within the vessel lumen, adjusted by the background signal intensity on the maximum intensity projection images (Figure 3).14 We have preliminarily explored the clinical significance of SIR.8, 37 It was found to be significantly related to acute infarct volume on DWI in a preliminary study.37 Moreover, it was identified as an independent predictor for recurrent stroke in the territory of the diseased artery in the SONIA-WASID cohort.8 Therefore, SIR by TOF-MRA, as a noninvasive, easy-to-perform and highly reproducible method, may be a useful tool to differentiate high-risk ICAS.26, 38
Figure 3.
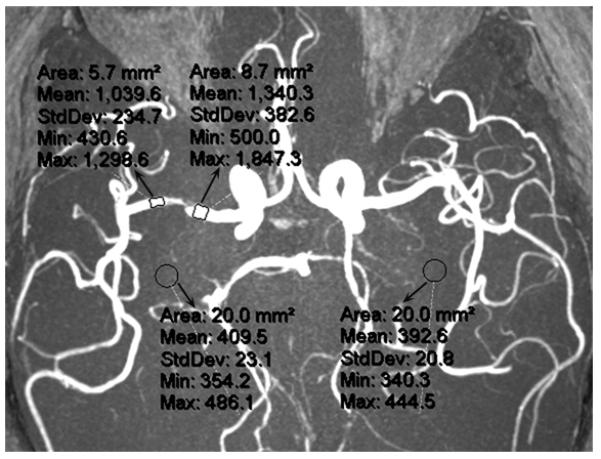
The method for measurement of SIR of an ICAS on a MRA maximum intensity projection. SIR of the lesion at right middle cerebral artery is calculated as the ratio of mean signal intensities distal (1,039.6) and proximal (1,340.3) to the lesion, adjusted by the mean background signal intensity (401.1; mean of 409.5 and 392.6), which is (1,039.6 – 401.1) / (1,340.3 – 401.1) = 0.68.
Practical Considerations for ICAS
Although ICAS has been established as a prominent cause of ischemic stroke and TIA, it has been relatively understudied during the past decades. Compared with numerous studies on symptomatic and asymptomatic carotid artery disease, the relatively limited interest on ICAS has hindered progress in relevant research and clinical areas. Insufficient evidence on specific treatment effects in large clinical studies has produced considerable gaps between research and practical risk stratification and decision-making in patients with ICAS. This may partly explain why the clinical diagnosis and treatment of ICAS continues to be based solely on luminal stenosis despite recent findings for many other potential predictors of subsequent stroke risk. In coming years, more diagnostic test data concerning the above imaging modalities will further identify denominators of cases with high risks, and validate the roles of perfusion imaging and other novel imaging methods in the evaluation of hemodynamic effects of ICAS, which will allow us to truly identify “high-risk” intracranial atherosclerosis but not highly selected and biased cases of intracranial stenosis. In the clinical diagnosis and assessment of ICAS, as well as in future relevant clinical studies, the following practical considerations are important to address this common disease worldwide.
Need for Noninvasive Detection of ICAS
Despite the utility and accuracy in evaluating anatomic severity, antegrade and collateral flow in ICAS, the invasive nature and potential periprocedural risk of DSA prevent its extensive and repeated use in indicated patients, and the high costs and dependence on experienced operators further limit its use as a routine examination for intracranial arteries in all ischemic stroke patients. Therefore, noninvasive methods such as TCD, MRA, and CTA are still commonly used for the diagnosis of ICAS in clinical scenarios. Although these currently available noninvasive imaging modalities also have limitations, detailed below, comprehensive interpretation of noninvasively obtained intracranial vascular images, in combination with perfusion imaging and other methods reflecting different aspects of ICAS, may avoid the need to proceed with an invasive angiography procedure.
Limitations of Noninvasive Diagnostic Modalities and Need for Reasonable Interpretation
Based on the inherent nature of TCD, TOF-MRA, and CTA, they address different aspects of the lesion when characterizing ICAS, yet the severity of stenosis remains the primary focus despite enormous information on flow and other aspects of ICAS often ignored by clinicians. Paradoxically, the percentage of stenosis itself is not concordant amongst these imaging modalities.10 Furthermore, each of these noninvasive methods has its specific limitations. TCD, as a low-cost and useful tool to provide real-time cerebral flow information, requires thorough skill training and is highly operator-dependent, which therefore is still underused throughout the world around 30 years after its first use in cerebrovascular diseases.12 TOF-MRA and CTA, as the most commonly used noninvasive methods to depict the morphology of major intracranial arteries, respectively based on the blood flow and vascular geometry, could complement each other with respect to the evaluation of hemodynamic and lumen-narrowing effects of ICAS, yet may incorrectly reflect severity of the lesion if interpreted alone in the evaluation of ICAS. Additionally, though CTA provides a reliable method to assess the leptomeningeal collateral circulation, inconsistency in the scaling methods impedes generalization of recent findings on the correlations between noninvasive leptomeningeal collateral grading and the clinical outcomes.21 Therefore, application of these imaging methods and interpretation of the results should be based on the unique characteristics of each modality, to permit a comprehensive assessment of the lesion.
Recurrent Risk in Patients with ICAS of Mild or Moderate Luminal Stenosis
In contrast to intracranial atherosclerotic lesions resulting in 70-99% reduction of the vessel caliber, which are considered “severe” and “high-risk” in relevant studies, ICAS of <50% and 50-69% luminal stenosis are usually regarded nonsignificant, or defined as “mild” and “moderate” lesions, respectively.2, 3 However, in spite of the high risk of stroke recurrence in patients with symptomatic 70-99% stenosis, ICAS of non-severe luminal stenosis may also be at risk of recurrent events. In the WASID and the Chinese IntraCranial AtheroSclerosis (CICAS) studies, nearly half of the recurrent stroke occurred in patients with 50-69% stenosis.5, 6 Few data are available regarding the prognosis of those with ICAS of mild (<50%) stenosis. According to large parallel cardiovascular studies performed in patients with acute coronary syndromes, those with CAD of <50% luminal stenosis also faced a non-negligible risk of death and re-infarction, though relatively lower than that of those with obstructive lesions.39 Among the limited data concerning outcomes of patients with ICAS of <50% luminal stenosis, a considerable risk of recurrent stroke was observed in the CICAS cohort, yet the percentages of luminal stenosis in these “mild” lesions were not specifically reported.6 Therefore, recurrent risks in patients with ICAS of <70% stenosis need to be fully appreciated in future studies, so that high-risk patients would not be missed due to the artificially graded severity of ICAS by the degree of luminal stenosis.
Need for Correlating with Subsequent Clinical Events, NOT Percent Stenosis
Increasing evidence on potential determinants of subsequent stroke risk beyond the severity of stenosis call for changes in the concept of how to diagnose ICAS. The traditional method to grade ICAS by the maximal percentage of luminal stenosis is irrational, in light of the considerable risk of stroke recurrence in patients with ICAS of <70% luminal stenosis and the complex effects of different aspects of ICAS on stroke risk. Thus, the currently defined “high-risk” ICAS based on the angiographic severity of stenosis may be misleading. For this reason, evaluation of ICAS based on its correlations with subsequent clinical events rather than the percent stenosis may be of higher clinical significance, supported by recent findings in the roles of collateral status, plaque characteristics and hemodynamic features in determining recurrent risks in symptomatic ICAS.7, 9, 26
Need to Develop Methods to Evaluate All Stroke Cases with ICAS
Due to the widely adopted philosophy of grading ICAS by the severity of luminal stenosis, successive observational and interventional studies tend to be carried out in restricted populations with a certain degree of stenosis, for instance, the WASID-SONIA and the SAMMPRIS trials specifically focused on patients with symptomatic ICAS of 50-99% and 70-99% luminal stenosis.2, 3, 10 Given the increasingly emerging evidence for other potential indicators altering subsequent stroke risks in symptomatic ICAS, it is in great need to establish more reasonable and generalizable methods for the evaluation of all stroke cases with ICAS, regardless of the degree of stenosis. Future studies with broader considerations of this patient subset will provide abundant information regarding the diagnosis and treatment of all symptomatic ICAS and embrace better understanding of this important cause of ischemic stroke.
Stroke Risk of Asymptomatic ICAS
Identifying asymptomatic intracranial atherosclerotic lesions with high risk of first-ever ischemic stroke or TIA will be a further step in advancing the management of such a patient subset, yet data concerning the stroke risk and prognostic factors in asymptomatic ICAS have been scarce to date.40 Although previous studies reported a relatively benign clinical course of asymptomatic ICAS as compared with symptomatic lesions, these findings have not been verified in large, prospective, population-based studies. Additionally, in patients with symptomatic intracranial atherosclerotic lesions, recurrent stroke may also occur in the territories of concomitant asymptomatic ICAS, for which case little is known about the clinical course and mechanisms. Exploration of the natural history and stroke risks in individuals with asymptomatic ICAS in future studies will undoubtedly facilitate primary prevention of stroke.
Conclusions
Key messages of this review article are summarized in Table 1. In patients with intracranial atherosclerosis, the roles of collateral status, plaque stability, hemodynamic impact and other potential factors may surpass that of percent stenosis in predicting the risk of subsequent recurrent events. The diagnostic emphasis on intracranial atherosclerosis rather than intracranial stenosis is fundamental in risk stratification and rational decision-making with respect to available therapies. Erroneously estimating the risk of stroke recurrence based on the maximal degree of luminal stenosis without consideration of hemodynamic features may be devastating. Currently available imaging methods should be comprehensively evaluated based on specific advantages in each technique rather than focusing solely on the severity of stenosis. After many years with a persistent focus on percent stenosis, redefinition of ICAS based on other diagnostic test information may facilitate the development of novel treatment strategies for patients with ICAS around the world. In coming years, increasing application of perfusion imaging and other novel methods to directly or indirectly evaluate the hemodynamic impact of ICAS will promote a broader understanding of this common cerebrovascular disorder. Future studies correlating various aspects of ICAS with subsequent stroke risk will facilitate diagnosis of high-risk or “severe” intracranial atherosclerotic lesions, instead of an arbitrary measure of maximal luminal stenosis.
Table 1.
Key messages of the review
|
Supplementary Material
Acknowledgements
The authors thank Dr. Weihai Xu, Department of Neurology, Peking Union Medical College Hospital, for providing original figures of high-resolution MRI for intracranial plaque (Figure 1).
Sources of Funding: This work was supported by NIH-NINDS K24NS072272 (DSL) and the S. H. Ho Cardiovascular Disease and Stroke Centre, Institute of Vascular Medicine, the Chinese University of Hong Kong.
Footnotes
Disclosures: None.
References
- 1.Qureshi AI, Feldmann E, Gomez CR, Johnston SC, Kasner SE, Quick DC, et al. Intracranial atherosclerotic disease: an update. Ann Neurol. 2009;66:730–738. doi: 10.1002/ana.21768. [DOI] [PubMed] [Google Scholar]
- 2.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 3.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldmann E. Diagnosis and quantitation of intracranial stenosis. J Neuroimaging. 2009;19(Suppl 1):22S–24S. doi: 10.1111/j.1552-6569.2009.00418.x. [DOI] [PubMed] [Google Scholar]
- 5.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Liu L, Wang Y, Soo Y, Pu Y, Wong KS. A multicenter study of the prevalence and outcomes of intracranial large artery atherosclerosis among stroke and TIA patients in China. Stroke. 2012;43:A120. [Google Scholar]
- 7.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–974. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebeskind DS, Kosinski AS, Lynn MJ, Scalzo F, Fong AK, Fariborz P, et al. Noninvasive fractional flow on MRA predicts stroke risk of intracranial stenosis in SONIA/WASID. Stroke. 2013;44:ATP166. doi: 10.1111/jon.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke. 2013;44:287–292. doi: 10.1161/STROKEAHA.112.664680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, et al. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007;68:2099–2106. doi: 10.1212/01.wnl.0000261488.05906.c1. [DOI] [PubMed] [Google Scholar]
- 11.Wong KS, Gao S, Chan YL, Hansberg T, Lam WW, Droste DW, et al. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74–81. doi: 10.1002/ana.10250. [DOI] [PubMed] [Google Scholar]
- 12.Alexandrov AV, Sloan MA, Tegeler CH, Newell DN, Lumsden A, Garami Z, et al. Practice standards for transcranial Doppler (TCD) ultrasound. Part II. Clinical indications and expected outcomes. J Neuroimaging. 2012;22:215–224. doi: 10.1111/j.1552-6569.2010.00523.x. [DOI] [PubMed] [Google Scholar]
- 13.Nedelmann M, Stolz E, Gerriets T, Baumgartner RW, Malferrari G, Seidel G, et al. Consensus recommendations for transcranial color-coded duplex sonography for the assessment of intracranial arteries in clinical trials on acute stroke. Stroke. 2009;40:3238–3244. doi: 10.1161/STROKEAHA.109.555169. [DOI] [PubMed] [Google Scholar]
- 14.Leng X, Wong LK, Soo Y, Leung T, Zou X, Wang Y, et al. Signal intensity ratio as a novel measure of hemodynamic significance for intracranial atherosclerosis. Letter to the Editor. Int J Stroke. 2013;8:E46. doi: 10.1111/ijs.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Laar PJ, van der Grond J, Mali WP, Hendrikse J. Magnetic resonance evaluation of the cerebral circulation in obstructive arterial disease. Cerebrovasc Dis. 2006;21:297–306. doi: 10.1159/000091534. [DOI] [PubMed] [Google Scholar]
- 16.Zhao M, Amin-Hanjani S, Ruland S, Curcio AP, Ostergren L, Charbel FT. Regional cerebral blood flow using quantitative MR angiography. AJNR Am J Neuroradiol. 2007;28:1470–1473. doi: 10.3174/ajnr.A0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin-Hanjani S, Alaraj A, Calderon-Arnulphi M, Aletich VA, Thulborn KR, Charbel FT. Detection of intracranial in-stent restenosis using quantitative magnetic resonance angiography. Stroke. 2010;41:2534–2538. doi: 10.1161/STROKEAHA.110.594739. [DOI] [PubMed] [Google Scholar]
- 18.Brisman JL, Pile-Spellman J, Konstas AA. Clinical utility of quantitative magnetic resonance angiography in the assessment of the underlying pathophysiology in a variety of cerebrovascular disorders. Eur J Radiol. 2012;81:298–302. doi: 10.1016/j.ejrad.2010.12.079. [DOI] [PubMed] [Google Scholar]
- 19.Majidi S, Sein J, Watanabe M, Hassan AE, Van de Moortele PF, Suri MF, et al. [Accessed October 30, 2013];Intracranial-derived atherosclerosis assessment: an in vitro comparison between virtual histology by intravascular ultrasonography, 7T MRI, and histopathologic findings. AJNR Am J Neuroradiol. 2013 doi: 10.3174/ajnr.A3631. published online ahead of print June 27, 2013. http://www.ncbi.nlm.nih.gov/pubmed/23811977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 21.McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol. 2012;33:576–582. doi: 10.3174/ajnr.A2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplan LR, Wong KS, Gao S, Hennerici MG. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovasc Dis. 2006;21:145–153. doi: 10.1159/000090791. [DOI] [PubMed] [Google Scholar]
- 23.Khan R, Nael K, Erly W. Acute stroke imaging: what clinicians need to know. Am J Med. 2013;126:379–386. doi: 10.1016/j.amjmed.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Donahue MJ, Strother MK, Hendrikse J. Novel MRI approaches for assessing cerebral hemodynamics in ischemic cerebrovascular disease. Stroke. 2012;43:903–915. doi: 10.1161/STROKEAHA.111.635995. [DOI] [PubMed] [Google Scholar]
- 25.Liebeskind DS, Fong AK, Scalzo F, Lynn MJ, Derdeyn CP, Fiorella DJ, et al. The SAMMPRIS Investigators. SAMMPRIS angiography discloses hemodynamic effects of intracranial stenosis: computational fluid dynamics of fractional flow. Stroke. 2013;44:A156. [Google Scholar]
- 26.Liebeskind DS, Feldmann E. Fractional Flow in Cerebrovascular Disorders. Interv Neurol. 2013;1:87–99. doi: 10.1159/000346803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schirmer CM, Malek AM. Prediction of complex flow patterns in intracranial atherosclerotic disease using computational fluid dynamics. Neurosurgery. 2007;61:842–852. doi: 10.1227/01.NEU.0000298914.32248.DC. [DOI] [PubMed] [Google Scholar]
- 28.Angermaier A, Langner S, Kirsch M, Kessler C, Hosten N, Khaw AV. CT-angiographic collateralization predicts final infarct volume after intra-arterial thrombolysis for acute anterior circulation ischemic stroke. Cerebrovasc Dis. 2011;31:177–184. doi: 10.1159/000321868. [DOI] [PubMed] [Google Scholar]
- 29.Shimoyama T, Shibazaki K, Kimura K, Uemura J, Shiromoto T, Watanabe M, et al. Admission hyperglycemia causes infarct volume expansion in patients with ICA or MCA occlusion: association of collateral grade on conventional angiography. Eur J Neurol. 2013;20:109–116. doi: 10.1111/j.1468-1331.2012.03801.x. [DOI] [PubMed] [Google Scholar]
- 30.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calleja AI, Cortijo E, Garcia-Bermejo P, Gomez RD, Perez-Fernandez S, del Monte JM, et al. Collateral circulation on perfusion-computed tomography-source images predicts the response to stroke intravenous thrombolysis. Eur J Neurol. 2013;20:795–802. doi: 10.1111/ene.12063. [DOI] [PubMed] [Google Scholar]
- 32.Souza LCS, Yoo AJ, Chaudhry ZA, Payabvash S, Kemmling A, Schaefer PW, et al. Malignant CTA collateral profile is highly specific for large admission DWI infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol. 2012;33:1331–1336. doi: 10.3174/ajnr.A2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pijls NHJ, DeBruyne B, Peels K, VanderVoort PH, Bonnier H, Bartunek J, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 34.Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve. Scientific basis. J Am Coll Cardiol. 2013;61:2233–2241. doi: 10.1016/j.jacc.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 35.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 36.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 37.Leng X, Wong KS, Soo Y, Leung T, Zou X, Wang Y, et al. Magnetic resonance angiography signal intensity as a marker of hemodynamic impairment in intracranial arterial stenosis. Stroke. 2013;44:A158. doi: 10.1371/journal.pone.0080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leng X, Ip HL, Soo Y, Leung T, Liu L, Feldmann E, et al. [Accessed October 30, 2013];Inter-observer reproducibility of signal intensity ratio on MR angiography for hemodynamic impact of intracranial atherosclerosis. J Stroke Cerebrovasc Dis. 2013 doi: 10.1016/j.jstrokecerebrovasdis.2013.07.036. published online ahead of print September 25, 2013. http://www.strokejournal.org/inpress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, et al. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95:20–26. doi: 10.1136/hrt.2007.138537. [DOI] [PubMed] [Google Scholar]
- 40.Ni J, Yao M, Gao S, Cui LY. Stroke risk and prognostic factors of asymptomatic middle cerebral artery atherosclerotic stenosis. J Neurol Sci. 2011;301:63–65. doi: 10.1016/j.jns.2010.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


