Abstract
Objective
To determine whether intraductal papillary mucinous neoplasms of the pancreas (IPMNs) have a different genetic background compared with ductal adenocarcinoma (PDAC).
Summary Background Data
The biologic and clinical behavior of IPMNs and IPMN-associated adenocarcinomas is different from PDAC in having a less aggressive tumor growth and significantly improved survival. Up to date, the molecular mechanisms underlying the clinical behavior of IPMNs are incompletely understood.
Methods
128 cystic pancreatic lesions were prospectively identified during the course of 2 years. From the corresponding surgical specimens, 57 IPMNs were separated and subdivided by histologic criteria into those with low-grade dysplasia, moderate dysplasia, high-grade dysplasia, and invasive cancer. Twenty specimens were suitable for DNA isolation and subsequent performance of array CGH.
Results
While none of the IPMNs with low-grade dysplasia displayed detectable chromosomal aberrations, IPMNs with moderate and high-grade dysplasia showed frequent copy number alterations. Commonly lost regions were located on chromosome 5q, 6q, 10q, 11q, 13q, 18q, and 22q. The incidence of loss of chromosome 5q, 6q, and 11q was significantly higher in IPMNs with high-grade dysplasia or invasion compared with PDAC. Ten of 13 IPMNs with moderate dysplasia or malignancy had loss of part or all of chromosome 6q, with a minimal deleted region between linear positions 78.0 and 130.0.
Conclusions
This study is the first to use array CGH to characterize IPMNs. Recurrent cytogenetic alterations were identified and were different than those described in PDAC. Array CGH may help distinguish between these 2 entities and give insight into the differences in their biology and prognosis.
Keywords: intraductal papillary mucinous neoplasms, comparative genomic hybridization, genetic characterization, chromosomal aberrations, KRAS
Intraductal papillary mucinous neoplasms (IPMNs) are a well described entity of cystic pancreatic tumors.1,2 Compared with ductal adenocarcinoma of the pancreas, IPMNs are characterized in having a less aggressive biologic and clinical behavior, with survival rates of more than 60% even for invasive carcinoma associated with IPMN after surgical removal.1 In pancreatic surgery referral centers, IPMNs are being increasingly diagnosed, and today they account for approximately 10 to 20% of all resected pancreatectomy specimens.2–7 IPMNs are tumors characterized by mucin production, cystic dilation of the pancreatic ducts, and intraductal growth,6 and have significant malignant potential. Similar to pancreatic ductal adenocarcinoma (PDAC), most patients with IPMNs present between the sixth and eighth decade of life, with the head of the pancreas being the predominant disease location.8,9 Although the majority of patients are symptomatic at presentation, with signs and symptoms including abdominal pain, weight loss, jaundice, diabetes, and diarrhea, many others are incidentally discovered.3,4,10,11 Histologically, IPMNs may demonstrate a spectrum of cytologic atypia ranging from mucinous epithelium with minimal cytologic atypia to frank invasive adenocarcinoma7 not infrequently in different areas of the same surgical specimen.12–14 Beside the histologic grade of atypia in the disease, IPMNs are also subclassified into main-duct IPMNs and branch-duct IPMNs based on imaging studies and pathologic examination.15,16 Branch-duct IPMNs are associated with a lower frequency of aggressive histologic features and better prognosis compared with main-duct IPMNs.16–19 In contrast to the relatively well understood molecular process in the development of conventional PDAC,20,21 only little is known about the genetic background of ductal epithelial neoplasia in IPMNs.22,23
Since the clinical behavior of IPMNs are different from PDAC, it is possible that IPMNs harbor a unique set of genetic aberrations, perhaps diverse from those more frequently involved in PDAC.24 On the molecular level, several gene mutations such as KRAS or TP53 alterations have been described, playing a role in the development of IPMNs. KRAS mutations are found in approximately 40–60% of IPMNs,14,22,25,26 compared with nearly 100% in PDAC,21 and TP53 mutations have been seen with an incidence of 8% in IPMNs27 compared with 75% in PDAC.20 In addition, there is some evidence that the Wnt-signaling pathway might be involved in the development of a proportion of IPMNs because consequent altered expression of downstream related proteins like β-catenin or E-cadherin previously has been observed.28 Finally, PIK3CA mutations have also been found in approximately 10% of IPMNs.29
However, there have been only a few studies published concerning chromosomal changes in IPMN specimens. In 1997, Fujii et al used PCR-based microsatellite analysis to detect loss of heterozygosity in 13 IPMN specimens on chromosome arms 1p, 3p, 6q, 8p, 9p, 17p, 18q, and 22q.24 More recently, Soldini et al22 investigated by interphase cytogenetics from a series of 12 IPMNs with different foci encompassing borderline lesions, intraductal (CIS) and invasive carcinoma, and concluded that monosomies, as defined by fluorescence in situ hybridization (FISH) analysis, are frequent in both IPMNs and mucinous hyperplasia of pancreatic ducts adjacent to IPMNs. To screen the whole genome for copy number changes, microarray-based comparative genomic hybridization (array CGH) has become a powerful technique.30 In recent years, studies have used array CGH to detect chromosomal aberrations in a large number of solid tumors, such as breast cancer, colon carcinoma, or PDAC specimens.31–33 Array CGH can detect recurrent genetic imbalances and global changes in IPMNs that may contribute to the progression of normal epithelium to premalignant and then invasive cancer.
In the present study, we used array CGH to analyze IPMNs, including all subtypes from low-grade dysplasia to IPMN-associated adenocarcinoma. To our knowledge, this is the first study to use this relatively novel technique to gain insights into the molecular background of IPMNs. Beside detection of a number of recurrent chromosomal alterations, we were able to identify significant differences between malignant IPMN and PDAC. This knowledge may give us insight not only into the molecular biology of IPMNs, but also it might help to clinically distinguish between benign IPMN, malignant IPMN, and PDAC.
MATERIALS AND METHODS
Tissue Samples
128 cystic lesions of the pancreas were prospectively identified over the course of 2 years. Subsequently, tissue samples from surgical specimens were collected and stored fresh frozen at the pancreatic tumor bank of the Massachusetts General Hospital, Boston, Massachusetts. From these specimens, 57 IPMN samples were evaluated by histology and 20 were suitable for DNA isolation. The patient clinical details can be found summarized in Table 1 and in detail in Supplemental Table 1 (see Table, Supplemental Digital Content 1, http://links.lww.com/A790). Additionally, one of the IPMN tumor samples derived from a third-passage xenograft tumor grown subcutaneously in immunodeficient nude mice. This xenograft tumor line will be described in detail in a separate manuscript, which is currently in submission. For all patients, written consent was obtained for the collection and use of the tumor DNA, and was approved by our Institutional Review Board. All tumors were diagnosed at the time of collection as IPMN,13 and subclassified macroscopically as main duct (n = 2), branch duct (n = 2), or mixed type IPMNs (n = 16). For tumors of mixed type, which component (main duct or branch duct) was used for DNA extraction is known from gross descriptions of the resection specimen, and this is indicated in Supplemental Table 1 (see Table, Supplemental Digital Content 1, http://links.lww.com/A790). In addition to the described 20 samples deriving from 20 different patients, we analyzed DNA from a second anatomic location for patient #10 (specimen #10a with moderate dysplasia and #10b with high-grade dysplasia). This additional sample (#10b) was not included into the statistical analysis of the data described in the paper, but the results were included in the Suppl. Table 1.
TABLE 1.
Clinical Characteristics of 20 Patients with IPMN
| Feature | Patients |
|---|---|
| Gender | |
| Male, n (%) | 14 (70) |
| Female, n (%) | 6 (30) |
| Age; Median age, yr (range) | 63 (46–83) |
| Location of the tumor | |
| Head of the gland, n (%) | 15 (75) |
| Body and/or tail of the gland, n (%) | 5 (25) |
| Involvement of pancreatic ducts | |
| Main duct IPMNs, n (%) | 2 (10) |
| Branch duct IPMNs, n (%) | 2 (10) |
| Mixed type IPMNs, n (%) | 16 (80) |
| Histologic grade | |
| IPMN with low-grade dysplasia, n (%) | 7 (35) |
| IPMN with moderate dysplasia, n (%) | 5 (25) |
| IPMN with high-grade dysplasia or invasion, n (%) | 8 (40) |
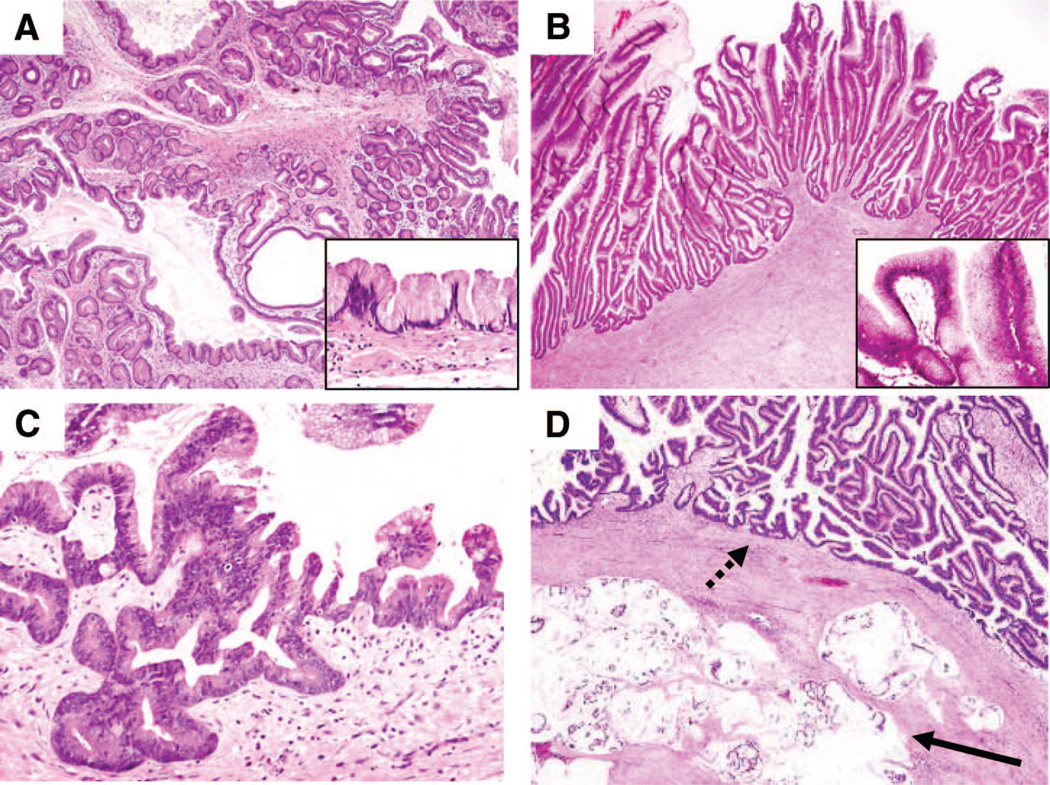
The quality of tumor specimens for DNA extraction was confirmed by Hematoxylin & Eosin staining, and only specimens which showed more than 50% tumor cells were included in this study. From a total number of 57 primary IPMN specimens, 20 samples could be identified that possessed >50% IPMN tumor cellularity. The single xenograft IPMN tumor sample showed a cellularity of more than 95% with negligible surrounding stromal tissue. IPMN samples without an invasive component were subclassified into 3 different histologic grades in accordance with the current classification system9: IPMN with low-grade dysplasia (n = 7, previously called adenoma), IPMN with moderate dysplasia (n = 5, previously called borderline tumors), and IPMN with high-grade dysplasia (n = 4, previously called intraductal papillary mucinous carcinoma, noninvasive, see representative images in Fig. 1). For statistical analysis, the remaining 4 samples with invasion were combined with those with high-grade dysplasia (n = 8). Furthermore, based on the epithelial morphology of an intraductal component and immunohistochemical profiles using mucin stains for MUC1, MUC2, MUC5AC, and MUC6, each case was categorized as gastric, intestinal, oncocytic or pancreatobiliary subtype, or unclassifiable, and invasive tumors were subclassified into colloid carcinoma and ductal carcinoma.8,9,34,35 These subclassifications were made in a blinded fashion by 3 gastrointestinal pathologists (MM-K, VD, and GYL), and microscopic images from representative examples of each subtype are shown in Supplemental Figures 1 to 3 (see Figure, Supplemental Digital Content 2, http://links.lww.com/A792). The classifications are indicated for each tumor only for the tissue sample used for array CGH analysis (there are other possible components within the same tumor).
Figure 1.
WHO Histologic Grading of IPMNs Used For Array CGH. Morphologic features of IPMNs often vary within different areas of the same pathologic specimen. Thus, each subject was classified based on a section from the tissue sample used for the array CGH analysis according to their histologic grade using current nomenclature. Hematoxylin & Eosin staining of (A) IPMN with low-grade dysplasia, (B) IPMN with moderate dysplasia, (C) IPMN with high-grade dysplasia, and (D) IPMN with moderate dysplasia (dashed arrow) with associated invasive adenocarcinoma of colloid type (solid arrow). Images shown using 4× objectives; insets show higher-power images using a 20× objective.
Array CGH
Genomic DNA was extracted from approximately 200 mg of fresh frozen tumor tissue using the Puregene DNA Purification Kit (Qiagen Inc., Valencia, California) according to standard procedures. A pooled normal male DNA (Promega, Madison, Wisconsin) was used as reference, independent of the patient’s gender. Array CGH was performed using Agilent Technologies 244k oligonucleotide arrays according to the recommended protocol (Agilent Technologies, Santa Clara, California).36 Briefly, one microgram of genomic tumor DNA, or male reference DNA, was digested at 37°C for 2 hours using AluI and RsaI. Digested DNA was labeled by random priming (BioPrime Array CGH Labeling Module, Invitrogen, Carlsbad, California) using Cy3-dUTP or Cy5-dUTP (GE Healthcare, Piscataway, NJ). After an incubation of 2 hours at 37°C, labeled DNAs were purified using the Millipore Microcon YM-30 purification kit (Millipore, Billerica, Massachusetts) according to the manufacturer’s instructions. Labeled tumor and reference DNA were combined together with Human Cot-1 DNA (Invitrogen, Carlsbad, California) using the Agilent Oligo aCGH Hybridization Kit (Agilent Technologies, Santa Clara, California). Subsequently, the DNA was denatured for 3 minutes at 95°C, prehybridized for 30 minutes at 37°C and hybridized for 35 to 40 hours at 65°C. After hybridization, slides were washed with Agilent Oligo aCGH Wash Buffer 1 and Buffer 2, at room temperature for 5 minutes and at 37°C for one minute respectively. These 2 washes were followed by a third wash in stabilization and drying solution.
Washed slides were scanned using the Agilent G2565 Microarray Scanner (Agilent Technologies, Santa Clara, California). Data was extracted from the microarray TIFF images (.tif) using Agilent’s Feature Extraction Software v9.1 and analysis was performed using Agilent’s CGH Analytics software. Before analyzing pathologic chromosomal aberrations, known copy number variants (CNVs) were identified and excluded from further statistical analysis.37 Copy number alterations are considered significant if the log2 ratio is ±2 standard deviations from the mean intensity of the entire experiment.37
Fluorescence in Situ Hybridization
Chromosome 6q copy number was evaluated in paraffin tumor sections using a 6q BAC probe (RP11-411H20) labeled in SpectrumOrange (Vysis/Abbott Molecular, Des Plaines, Illinois) and a 6p copy number control BAC (RP11-710L16) labeled in SpectrumGreen. Chromosome 18q copy number was evaluated using an 18q BAC probe (RP11-729G3) labeled in SpectrumOrange and a 6p copy number control BAC (RP11-14P14) labeled in SpectrumGreen. Slide pretreatment, hybridization, and washes should be performed in each laboratory according to standard laboratory paraffin FISH procedures. DAPI in antifade solution (0.3 µg DAPI in Vectashield Mounting Medium, Vector Laboratories, Burlingame, California) was used as mounting media and counter-stain. Images were acquired with a fluorescence microscope (Olympus BX61, Olympus America, Center Valley, PA) equipped with a mercury-arc lamp and fitted with combination with filters for multiple probe acquisition (SpectrumOrange, SpectrumGreen, and DAPI) and Z-stacking capability.
KRAS Mutation Analysis at Codon 12 and 13
The same tumor DNA samples used for array CGH were used to amplify the KRAS locus on exon 2 by means of polymerase chain reaction (PCR). For the amplification step the following primers were used: 5′-CTTAAGCGTCGATGGAGGAG-3′ and 5′-AGAATGGTCCTGCACCAGTAA-3′. PCR amplification products were purified using the Wizard SV Gel and PCR Clean-Up system (Promega, Madison, Wisconsin) and were sequenced bi-directionally with appropriate forward and reverse primers.
Statistical Analysis
Statistical analysis was performed with the SPSS software (SPSS Standard version 8.0; SPSS, Chicago, Illinois). Genetic alterations were compared between various groups by the two-tailed unpaired Student t test or Fisher exact test where appropriate. P-values of 0.05 or less were considered as significant.
RESULTS
Array CGH Analysis
Array CGH analysis revealed chromosomal copy number alterations in 13 out of 20 samples. The 7 specimens that did not display chromosomal aberrations were all in the group of IPMNs with low-grade dysplasia (7/7). In contrast, all IPMNs with moderate dysplasia, high-grade dysplasia, or invasion possessed at least 3 copy number changes (summarized in Table 2 and in detail in Supplemental Table 1 [see Table, Supplemental Digital Content 1, http://links.lww.com/A790]). IPMNs with moderate dysplasia had an average number of 10.0 (range 3–19) copy number aberrations. The increased numbers of chromosomal copy number changes in IPMNs with moderate dysplasia compared with IPMNs with low-grade dysplasia (no aberrations) was highly significant (P = 0.002). Furthermore, IPMNs with high-grade dysplasia or invasion, including 4 IPMNs with high-grade dysplasia and 4 with invasive carcinoma, showed more aberrations (average of 16.25 per case, range 8–28) compared with IPMNs with moderate dysplasia. However, this increase of chromosomal changes correlated to disease progression was not statistically significant (P = 0.142). IPMNs with moderate dysplasia showed an average of 3.6 gains (range 1–7) and 6.4 losses (range 2–14), and IPMNs with high-grade dysplasia or invasion an average of 7.1 gains (range 1–16) and 10.3 losses (range 6–14). Summarized, loss of chromosomal material was more frequent (average 8.82) compared with gains (average 5.55) and to high-level amplifications (only seen in 2 out of the 20 specimens). Overall, in both IPMNs with moderate dysplasia and IPMNs with high-grade dysplasia or invasion, the most frequent lost regions were located on chromosome 2 (38.5%), 4q (46.2%), 5q (53.8%), 6q (76.9%), 8p (46.2%), 10q (61.5%), 11q (61.5%), 13q (53.8%), 15q (46.2%), 18q (53.8%), and 22q (53.8%).
TABLE 2.
Frequent Chromosomal Aberrations by Array CGH
| Chromosomal Aberration |
Low-Grade IPMNs (n = 7) |
Moderate Grade IPMNs (n = 5) |
High Grade Dysplasia or Invasion (n = 8) |
% in Non-Low Grade (total) (n = 13) |
|---|---|---|---|---|
| Loss of 2 | 0 | 1 | 4 | 38.5 |
| Loss of 4q | 0 | 1 | 5 | 46.2 |
| Loss of 5q | 0 | 2 | 5 | 53.8 |
| Loss of 6q | 0 | 4 | 6 | 76.9 |
| Gain of 7 | 0 | 4 | 4 | 61.5 |
| Loss of 8p | 0 | 2 | 4 | 46.2 |
| Loss of 10q | 0 | 3 | 5 | 61.5 |
| Loss of 11q | 0 | 3 | 5 | 61.5 |
| Loss of 13q | 0 | 3 | 4 | 53.8 |
| Loss of 15q | 0 | 2 | 4 | 46.2 |
| Loss of 18q | 0 | 2 | 5 | 53.8 |
| Gain of 19q | 0 | 0 | 4 | 30.8 |
| Loss of 22q | 0 | 4 | 3 | 53.8 |
Overview of frequent chromosomal aberrations (prevalence of ≥50% within each group).
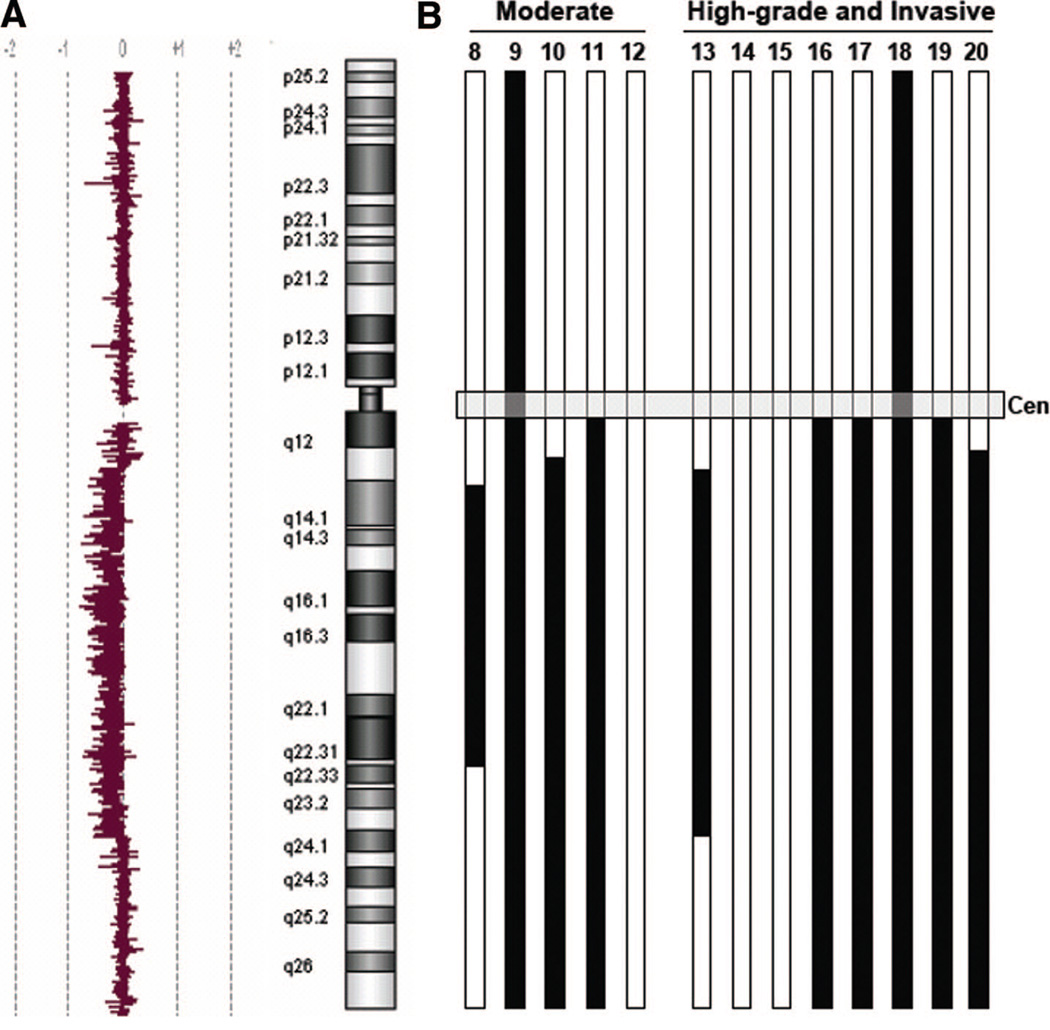
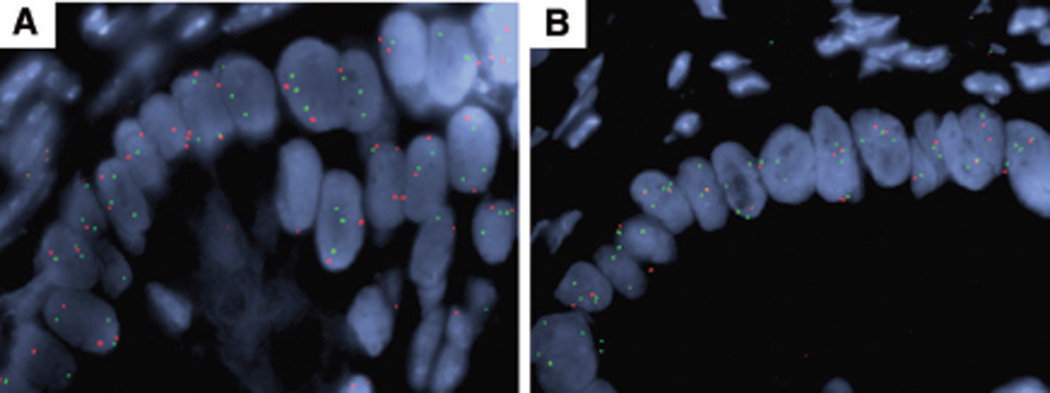
The most frequently observed chromosomal large-scale aberration was loss of chromosome 6q with 4/5 IPMNs, with moderate dysplasia and 6/8 IPMN samples with high-grade dysplasia or invasion showing this abnormality. Some of the samples displayed loss of the entire chromosome 6, some loss of just the long arm of chromosome 6, and others only loss of parts of the long arm. The minimal deleted region could be determined by aligning 6q loss patterns for all cases and is defined as a region located between 6q linear positions 78.0 and 130.0 (Fig. 2), a region of 52 mega-basepairs that contains approximately 180 known genes. There are no well-described tumor suppressor genes located in this area, even though 6q loss has been observed in a number of other tumor types such as breast cancer, neuroblastoma, malignant melanoma, osteosarcoma, ovarian, or prostate cancer.38–42 FISH analysis using a 6q probe within the minimally-deleted region was used to confirm copy number loss in tumor specimens in situ (Fig. 3A).
Figure 2.
Array CGH Data for Chromosome 6q. Summary of all array CGH results concerning copy number changes on chromosome 6. (A); A representative array CGH chromosome 6 tracing from a tumor with partial loss of 6q, shown as the log2 ratio of tumor/normal fluorescence. (B); Black bars indicate extent of loss of chromosomal material on chromosome 6. 4/5 IPMNs with moderate dysplasia and 6/8 IPMNs with high-grade dysplasia or invasion showed loss of parts or loss of the whole long arm of chromosome 6. The numbers on the top of each bar8–20 refer to the patients described in detail in Supplemental Table 1 [see Table, Supplemental Digital Content 1, http://links.lww.com/A790]. Patients 1 through 7 had low-grade IPMNs with no genetic alterations detected by array CGH.
Figure 3.
FISH Verification of Array CGH Findings. FISH analysis of paraffin sections of a representative tumor with relative 6q loss (A) and 18q loss (B). 6q loss is seen as fewer copies of the red 6q probe relative to the green 6p probe in tumor epithelium. 18q loss is seen as fewer copies of the red 18q probe relative to the green 6p probe in tumor epithelium. There is significant polysomy for both chromosomes.
In contrast to loss of 6q, which was seen in the majority of IPMN specimens (except IPMNs with low-grade dysplasia), loss of chromosome 4q was seen in 62.5% of IPMNs but only in one case (20%) of IPMNs with moderate dysplasia. Furthermore, loss of 18q was seen in 40% of moderate dysplasia but in 62.5% of IPMNs with high-grade dysplasia or invasion. FISH analysis using an 18q probe was used to confirm copy number loss in tumor specimens in situ (Fig. 3B). Gain of 19q was seen in 50% of IPMNs with high-grade dysplasia or invasion but in none of the IPMNs with moderate dysplasia. Other chromosomal aberrations occurred in approximately the same proportion in IPMNs, with moderate dysplasia and IPMNs with high-grade dysplasia or invasion (Table 2). We examined 2 distinct regions from IPMN specimen (#10), one region containing moderate dysplasia (#10a) and a second with high-grade dysplasia (#10b), revealed predominantly the same chromosomal aberrations, indicating many chromosomal abnormalities are present already at the moderate dysplasia stage.
The most common chromosomal gains were located on chromosome 7 and 19q with a total incidence of 61.5% and 30.8% respectively. Other gains in the copy number of chromosomes affected different regions and chromosomal arms without noticeable high frequencies. Only 2 distinct high-level amplifications were seen in total, one on 11q13.2 (case #16; high-grade dysplasia, intestinal subtype) involving the Cyclin D1 gene (CCND1) and 12p13.2 (case #11; moderate dysplasia, intestinal subtype) involving the Cyclin D2 gene (CCND2).
Overall, there was no significant difference in chromosomal aberrations between the subtypes based on the epithelial morphology. While the majority of cases, as noted, were of mixed branch duct and main duct type, we know based on gross specimen descriptions of the 20 cases analyzed by array CGH that DNA for 9 were derived from side branch portions and 11 from the main duct portion. The side branch specimens were associated with 6q loss in 2/9 cases versus 8/11 main duct specimens (P = 0.04). While such genetic findings are potentially interesting, they may be more linked to grade than anatomic location. 5/9 branch duct specimens were of the low-grade type, and harbored no genetic alterations. Only 2/11 main duct specimens were low-grade.
Comparing the array CGH results to PDAC,31–33,43,44 we found loss of 5q, 6q, and 11q to be significant more frequent in IPMNs with high-grade dysplasia or invasion (P = 0.022, P = 0.026, and P = 0.00039). Especially the incidence of loss of 11q was extensively higher in IPMNs (50%) compared with PDAC where this aberration was rarely reported (5.5%).
KRAS status
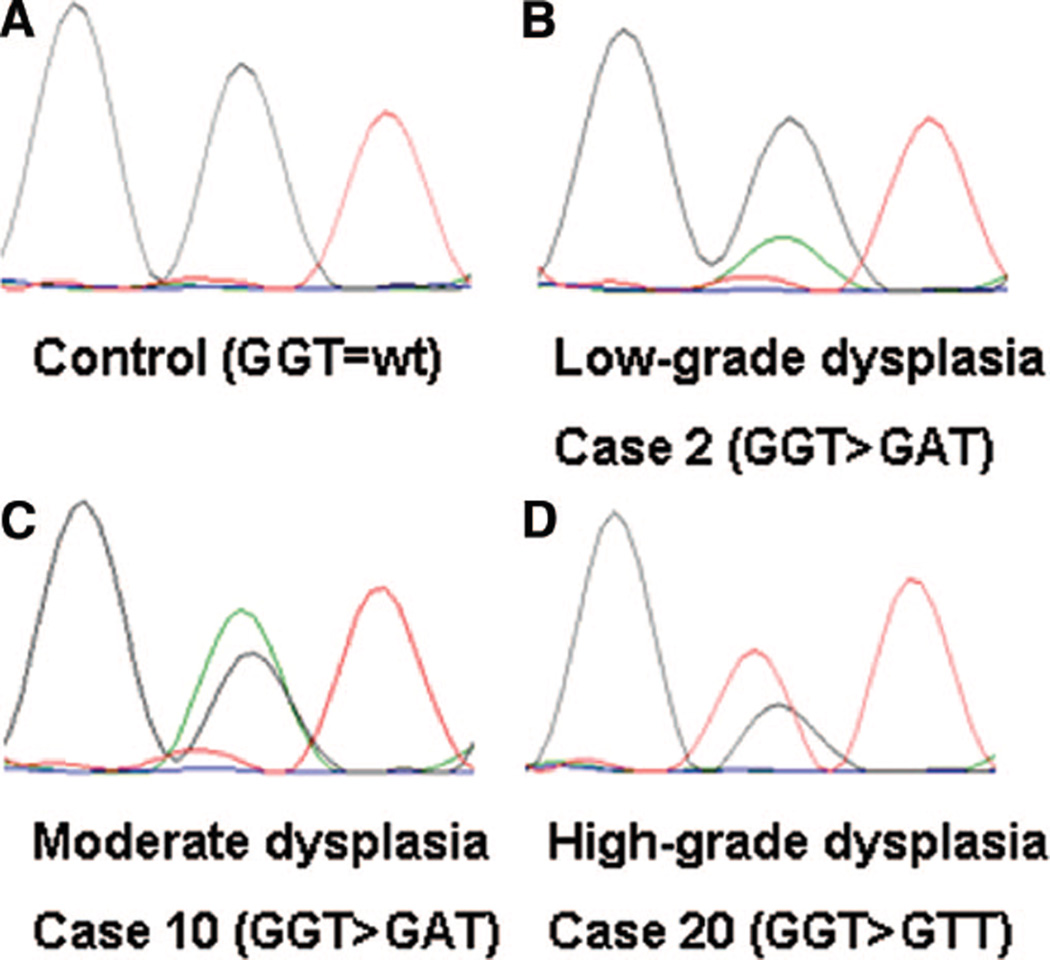
Eight out of 20 IPMN specimens (40%) displayed KRAS mutations at codon 12 of exon 2. No KRAS mutations at codon 13 could be detected. Concerning the different histologic grades, 4 out of 7 IPMNs with low-grade dysplasia showed KRAS mutations at codon 12 (see Table, Supplemental Digital Content 1, http://links.lww.com/A790), 2 out of 5 IPMNs with moderate dysplasia and 2 out of 8 IPMNs with high-grade dysplasia or invasion (Fig. 4). There was no statistically significant difference between these subgroups (P > 0.05). However, KRAS mutations occurred more frequent (7/10) in IPMNs with gastric morphologic subtype compared with IPMNs of intestinal, oncocytic, or unclassifiable type (1/10) (P = 0.0198).
Figure 4.
Sequencing for KRAS Mutation at Codon 12. Sequence chromatograms of KRAS codon 12. (A); Codon 12 of a control sample consisting of DNA deriving from normal pancreas. (B); Example for a KRAS mutation at codon 12 in an IPMN specimen with low-grade dysplasia showing the sequence change GGT > GAT. (C); and (D); show KRAS mutations in IPMN with moderate dysplasia, high-grade dysplasia and invasion respectively.
DISCUSSION
This study has shown that with array CGH, all IPMNs with at least moderate dysplasia exhibited chromosomal copy number imbalances, while none of the IPMNs with low-grade dysplasia showed copy number changes. Our current understanding of the natural history of low-grade IPMNs is incomplete, and it is not entirely clear if all low-grade IPMNs have the potential to progress to malignancy.45 It has been previously shown that the malignant progression of pancreatic neoplasms, such as moderate dysplasia lesions/borderline tumors, is associated with successive genomic alterations, which may generate cell clones with growth advantages over other cancerous cells.22 This cumulative process of genomic alterations is compatible with the increasing complexity of array CGH patterns found in malignant versus borderline IPMNs.24 With our study, we were able to confirm the accumulation of chromosomal abnormalities from low-grade dysplasia through moderate dysplasia to high-grade dysplasia and invasive carcinoma. In one case, however, the examination of 2 different locations of one surgical IPMN specimen (#10a and #10b) reflecting the progression from moderate to severe dysplasia, revealed the same chromosomal aberrations. Nonetheless, it appears that comparable to other tumor progression models, IPMNs show an accumulation of chromosomal copy number changes reflecting the progression from low-grade dysplasia to frank invasive carcinoma. Array CGH revealed that the most common gains of chromosomal material seen in IPMNs were gains of chromosome 7 and 19q that were seen in 50% of IPMNs with high-grade dysplasia or invasion. Distinct high-level amplifications were rare and seen on 11q13.2 (including CCND1) and 12p13.2 (including CCND2). No amplifications of receptor tyrosine kinases (eg, EGFR) were observed. The most frequent copy number loss that we found in IPMNs, that was also the most common aberration overall, was loss of the long arm of chromosome 6. Because it was seen in 4 out of 5 moderately dysplastic tumors, we suggest that this event occurs at the relatively early stage during the course of tumor progression, and is likely important for progression from low-grade dysplasia. This agrees with data published by Soldini et al,22 who have shown similar findings on IPMN specimens using 6q FISH. Although 6q loss is a common aberration in a number of human tumors, a tumor suppressor in that region has not been convincingly identified.38 Based on alignment of 6q losses in our dataset, a minimally deleted region is defined within 6q linear position 78.0 –130.0 (Fig. 2). This region contains approximately 180 genes. While a number of genes could have potential roles in tumorigenesis, none are well-studied tumor suppressors. Cancer gene resequencing projects of colon and breast tumors have identified mutations in a number of genes in this region, some with possible roles in tumorigenesis including PHIP, KIAA1117, NCB5OR, EPHA7, MANEA, GRIK2, APG5L, MICAL1, DDO, REV3L, KPNA5, C6orf204 and LAMA2.46 Resequencing these genes in IPMNs and PDAC may be warranted.
Loss of 18q was only seen in 2 out of 5 IPMNs with moderate dysplasia but in 5 out of 8 IPMNs with high-grade dysplasia or invasion. This might indicate that loss of 18q, possibly driven by loss of the SMAD4 tumor suppressor gene, plays an important role in the progression from IPMNs with moderate dysplasia to IPMNs with high-grade dysplasia and finally frank invasive carcinoma. Again, our data is consistent with data published by Soldini et al,22 which have suggested that synchronous loss of chromosome 6, 17, and 18 appears to be associated with carcinoma in situ or invasive cancer. Our findings are also in agreement with the study previously published by Fujii et al,24 who have used polymerase chain reaction (PCR-)-based microsatellite analysis to detect loss of heterozygosity in 13 IPMN specimens on chromosome arms 1p, 3p, 6q, 8p, 9p, 17p, 18q, and 22q. They found frequent loss of heterozygosity (LOH) at several chromosomal loci, including 6q (54%), 8p (31%), 9p (62%), 17p (38%), and 18q (38%). Abe et al47 found LOH of 10q to be among the most common finding in a cohort of 33 sporadic IPMNS, 25 of which had associated invasive carcinoma. Our array CGH data also suggests that beside loss of chromosome 18q, loss of chromosome 2 and 4q as well as gain of 19q might play a role in the progression of IPMNs with moderate dysplasia to IPMNs with high-grade dysplasia and invasive carcinoma (see Table 2).
Genomic Alterations in IPMN Versus PDAC
In PDAC, frequent aberrations have been previously reported and include gains of chromosome arms 8q, 11q, 12p, 17q, and 20q, and losses of 9p, 15q, and 18q.33,43,48,49 Comparing our data to previously published array CGH and cytogenetics data on PDAC reveals significant differences, but also similarities concerning frequent chromosomal copy number changes (Table 3). In contrast to previously published data on PDAC,31–33,43,44 we detected loss of 5q, 6q, and 11q significant more frequently in IPMNs (P = 0.022, P = 0.026, and P = 0.00039). This difference in frequency might indicate that IPMNs have distinct genetic alterations compared with conventional PDAC, a finding which is supported by several previous studies.16,23,24
TABLE 3.
Chromosomal Aberrations of IPMNs in Comparison to PDAC
| Chromosomal Aberration |
% IPMNs With High Grade Dysplasia or Invasion (n = 8) |
% in PDAC* | P Values† |
|---|---|---|---|
| Loss of 2 | 50.0 | 24.3 | 0.202 |
| Loss of 4q | 62.5 | 32.2 | 0.121 |
| Loss of 5q | 62.5 | 18.9 | 0.0224 |
| Loss of 6q | 75.0 | 33.6 | 0.0264 |
| Gain of 7 | 50.0 | 43.3 | 0.727 |
| Loss of 8p | 50.0 | 42.2 | 0.721 |
| Loss of 10q | 62.5 | 25.0 | 0.0878 |
| Loss of 11q | 50.0 | 5.5 | 0.00039 |
| Loss of 13q | 50.0 | 27.0 | 0.224 |
| Loss of 15q | 50.0 | 40.0 | 0.704 |
| Loss of 18q | 62.5 | 67.8 | 0.714 |
| Gain of 19q | 50.0 | 23.3 | 0.197 |
| Loss of 22q | 37.5 | 35.6 | 1.000 |
Prevalence of chromosomal aberrations in PDAC were calculated by compiling the data of 5 representative publications (Aguirre AJ,31 Schleger C,32 Mahlamaki EH,43 Solinas-Toldo S,33 and Bardi G),44 with the percentages calculated by dividing the total number of described aberrations by the total number of samples included into these 5 publications. P-values were calculated with a 2-tailed Fisher exact test.
Previously published data.
Fisher exact test; 2-tailed.
KRAS Status
KRAS mutations in IPMNs have previously been described with frequencies ranging from 40 to 81%.14,25,26 Z’graggen et al14 described KRAS mutations at codon 12 in at least one of the microdissected lesions from 13 of 16 IPMNs, with a stepwise increase in the frequencies from papillary hyperplasia (low-grade dysplasia/adenoma), through borderline (moderate grade dysplasia), and to carcinoma in situ (high-grade dysplasia) and invasive carcinoma. In our dataset, activating KRAS point mutations occurred already in 4 out of 7 IPMNs with low-grade dysplasia (66.6%) without significant difference (P = 0.356) compared with the remaining higher-grade IPMNs. The data suggest that activating KRAS mutations already occur in an early stage of the neoplastic transformation in IPMNs, and precede the development of genomic instability. This is in accordance with the current literature,31 which reports the occurrence of activating mutations in KRAS as an early event in neoplastic epithelial transformation, for example, in PDAC of the pancreas. Indeed, KRAS mutations seem to be the first known genetic alterations and are detected in approximately 30% of early neoplastic lesions (ie, PanIN-1) with the frequency rising nearly to 100% in PDAC.50 Unlike PDAC where KRAS mutations occur in 90–100%,21,51 many IPMNs did not harbor an active KRAS mutation (60% in our study). The observation that IPMNs of the gastric morphologic subtype are associated with a higher prevalence of KRAS mutation (7/10 in this study), suggests that gastric type IPMNs may be more similar to the better studied Pancreatic intraepithelial neoplasia (PanIN) and PDACs that frequently harbor KRAS mutations. Immunoprofiles of gastric subtype IPMNs show staining for MUC5AC and MUC6, which is the same as PanIN, supporting the possible similarity. Since KRAS mutation is present in only 40% of our cohort overall, IPMNs likely have mutations in other genes besides KRAS that stimulate mitogenic (eg, MAPK) signal transduction pathways.25
Clinical Importance
While radiologic and pathologic features of IPMN are increasingly well understood, the diagnosis of IPMN can be difficult. This is especially true of the histologic diagnosis on fine needle aspiration cytology. On one end of the dysplastic spectrum, low-grade IPMNs can appear similar to normal gastric epithelium acquired during the needle pass, similar to PanIN lesions, or to reactive epithelial processes. On the high-grade end, there is overlap with conventional PDAC. Our data indicates that copy number assays such as FISH could play an important role in distinguishing these possibilities. As listed in Table 2, the most common alterations in IPMNs with moderate dysplasia, high-grade dysplasia or invasive carcinoma include 6q loss (76.9%), 7q gain (61.5%), 10q loss (61.5%), and 11q loss (61.5%). By analyzing the case by case array CGH results in Supplementary Table 1 (see Table, Supplemental Digital Content 1, http://links.lww.com/A790), a panel of FISH probes to these 4 chromosomes would detect at least 1 alteration in all 13 moderate and IPMNs with high-grade dysplasia or invasion (sensitivity 100%), and 0/7 of the low-grade IPMNs (specificity 100%). A FISH study to analyze these loci in larger numbers is underway, and should yield more realistic sensitivity and specificity calculations of such a panel. Such a panel could be effective at both diagnosing a neoplastic lesion that may require an operative approach (IPMN with high-grade dysplasia, invasive cancer or that with moderate dysplasia) from low-grade lesions for which observation will suffice.
Chromosomal alterations can significantly improve our ability to diagnose a neoplastic process from endoscopically obtained cyst fluid. Since the number of intact neoplastic cells in cyst fluid can be rare, cell-free DNA could be analyzed by nonarray CGH and non-FISH techniques, such as PCR-based 6q loss of heterozygosity analysis. Analysis of 5q, 6q, and 11q loss by FISH could be an effective panel in differentiating IPMN lesions from PDAC, as these are most specific to IPMNs (Table 3). Whether invasive carcinoma associated with IPMN (especially associated with 5q, 6q, or 11q) would behave differently than PDACs remains an important open question.
In conclusion, recurrent chromosomal copy number changes are observed in all moderately dysplastic IPMNs and IPMNs with high-grade dysplasia or invasion. Low-grade IPMNs lack genomic instability but are driven commonly by KRAS mutation. The aberrations found in IPMNs to a certain degree showed different patterns compared with conventional PDAC, which might explain the distinct clinical behavior of this entity of pancreatic tumors. Furthermore, the detected, overrepresented, or underrepresented chromosomal regions will be important for diagnostic applications and may contain candidate regions for potential oncogenes and tumor suppressor genes, which might be involved in the multistep tumorigenesis of IPMNs.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Julie M. Batten and Anhthu T. Nguyen for technical assistance.
Grant Support: This work was supported by funding from the Karin Grunebaum Cancer Research Foundation and the German Research Foundation (DFG).
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239(5):678–685. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234(3):313–321. doi: 10.1097/00000658-200109000-00005. discussion 321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conlon KC. Intraductal papillary mucinous tumors of the pancreas. J Clin Oncol. 2005;23(20):4518–4523. doi: 10.1200/JCO.2005.22.517. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-del Castillo C, Targarona J, Thayer SP, et al. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138(4):427–423. doi: 10.1001/archsurg.138.4.427. discussion 433–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarr MG, Murr M, Smyrk TC, et al. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg. 2003;7(3):417–428. doi: 10.1016/s1091-255x(02)00163-4. [DOI] [PubMed] [Google Scholar]
- 6.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239(6):788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189(5):632–636. doi: 10.1016/j.amjsurg.2005.01.020. discussion 637. [DOI] [PubMed] [Google Scholar]
- 8.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28(7):839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hruben RH, Pitman MB, Klimstra DS. Intraductal Neoplasms. In: Hruban RH, Pitman MB, Klimstra DS, editors. AFIP ATLAS OF TUMOR PATHOLOGY, Series 4: Tumors of the pancreas. Series 4 ed. Washington, DC: Washington, DC: American Registry of Pathology; 2007. pp. 75–110. 2007. [Google Scholar]
- 10.Adsay NV, Conlon KC, Zee SY, et al. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94(1):62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- 11.D’Angelica M, Brennan MF, Suriawinata AA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239(3):400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloppel G, Solcia E, Longnecker DS, et al. World Health Organization International Histological Typing of Tumors of the Exocrine Pancreas. Berlin: Springer-Verlag; 1996. [Google Scholar]
- 13.Longnecker DS, Adler G, Hruban RH, Kloppel G. Intraductal papillary-mucinous neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumors. Lyon: IARC Press; 2000. [Google Scholar]
- 14.Z’Graggen K, Rivera JA, Compton CC, et al. Prevalence of activating K-ras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg. 1997;226(4):491–498. doi: 10.1097/00000658-199710000-00010. discussion 498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furukawa T, Takahashi T, Kobari M, Matsuno S. The mucus-hypersecreting tumor of the pancreas. Development and extension visualized by three-dimensional computerized mapping. Cancer. 1992;70(6):1505–1513. doi: 10.1002/1097-0142(19920915)70:6<1505::aid-cncr2820700611>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24(10):1372–1377. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133(1):72–79. doi: 10.1053/j.gastro.2007.05.010. quiz 309–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvia R, Crippa S, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56(8):1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6(1–2):17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 20.Goggins M, Kern SE, Offerhaus JA, Hruban RH. Progress in cancer genetics: lessons from pancreatic cancer. Ann Oncol. 1999;10(Suppl 4):4–8. [PubMed] [Google Scholar]
- 21.Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 22.Soldini D, Gugger M, Burckhardt E, et al. Progressive genomic alterations in intraductal papillary mucinous tumours of the pancreas and morphologically similar lesions of the pancreatic ducts. J Pathol. 2003;199(4):453–461. doi: 10.1002/path.1301. [DOI] [PubMed] [Google Scholar]
- 23.Raut CP, Cleary KR, Staerkel GA, et al. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13(4):582–594. doi: 10.1245/ASO.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Fujii H, Inagaki M, Kasai S, et al. Genetic progression and heterogeneity in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 1997;151(5):1447–1454. [PMC free article] [PubMed] [Google Scholar]
- 25.Schonleben F, Qiu W, Bruckman KC, et al. BRAF and KRAS gene mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/IPMC) of the pancreas. Cancer Lett. 2007;249(2):242–248. doi: 10.1016/j.canlet.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wada K, Takada T, Yasuda H, et al. Does “clonal progression” relate to the development of intraductal papillary mucinous tumors of the pancreas? J Gastrointest Surg. 2004;8(3):289–296. doi: 10.1016/j.gassur.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Sessa F, Solcia E, Capella C, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425(4):357–367. doi: 10.1007/BF00189573. [DOI] [PubMed] [Google Scholar]
- 28.Chetty R, Serra S, Salahshor S, et al. Expression of Wnt-signaling pathway proteins in intraductal papillary mucinous neoplasms of the pancreas: a tissue microarray analysis. Hum Pathol. 2006;37(2):212–217. doi: 10.1016/j.humpath.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Schonleben F, Qiu W, Ciau NT, et al. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res. 2006;12(12):3851–3855. doi: 10.1158/1078-0432.CCR-06-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrett MT, Scheffer A, Ben-Dor A, et al. Comparative genomic hybridization using oligonucleotide microarrays and total genomic DNA. Proc Natl Acad Sci U S A. 2004;101(51):17765–17770. doi: 10.1073/pnas.0407979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguirre AJ, Brennan C, Bailey G, et al. High-resolution characterization of the pancreatic adenocarcinoma genome. Proc Natl Acad Sci U S A. 2004;101(24):9067–9072. doi: 10.1073/pnas.0402932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schleger C, Arens N, Zentgraf H, et al. Identification of frequent chromosomal aberrations in ductal adenocarcinoma of the pancreas by comparative genomic hybridization (CGH) J Pathol. 2000;191(1):27–32. doi: 10.1002/(SICI)1096-9896(200005)191:1<27::AID-PATH582>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Solinas-Toldo S, Wallrapp C, Muller-Pillasch F, et al. Mapping of chromosomal imbalances in pancreatic carcinoma by comparative genomic hybridization. Cancer Res. 1996;56(16):3803–3807. [PubMed] [Google Scholar]
- 34.Mino-Kenudson M, Richards C, Crippa S, et al. Intraductal papillary mucinous neoplasms of the pancreas consist of heterogenous clinicopathological groups: A single institution experience of157 resected cases. Gastroenterology. 2007;132(4):A84. [Google Scholar]
- 35.Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447(5):794–799. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 36.Albertson DG, Pinkel D. Genomic microarrays in human genetic disease and cancer. Hum Mol Genet. 2003;12(Spec No 2):R145–R152. doi: 10.1093/hmg/ddg261. [DOI] [PubMed] [Google Scholar]
- 37.Iafrate AJ, Feuk L, Rivera MN, et al. Detection of large-scale variation in the human genome. Nat Genet. 2004;36(9):949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Huang J, Zhao YL, et al. UTRN on chromosome 6q24 is mutated in multiple tumors. Oncogene. 2007;26(42):6220–6228. doi: 10.1038/sj.onc.1210432. [DOI] [PubMed] [Google Scholar]
- 39.Mertens F, Johansson B, Hoglund M, Mitelman F. Chromosomal imbalance maps of malignant solid tumors: a cytogenetic survey of 3185 neoplasms. Cancer Res. 1997;57(13):2765–2780. [PubMed] [Google Scholar]
- 40.Nathrath MH, Kuosaite V, Rosemann M, et al. Two novel tumor suppressor gene loci on chromosome 6q and 15q in human osteosarcoma identified through comparative study of allelic imbalances in mouse and man. Oncogene. 2002;21(38):5975–5980. doi: 10.1038/sj.onc.1205764. [DOI] [PubMed] [Google Scholar]
- 41.Sun M, Srikantan V, Ma L, et al. Characterization of frequently deleted 6q locus in prostate cancer. DNA Cell Biol. 2006;25(11):597–607. doi: 10.1089/dna.2006.25.597. [DOI] [PubMed] [Google Scholar]
- 42.Saito S, Saito H, Koi S, et al. Fine-scale deletion mapping of the distal long arm of chromosome 6 in 70 human ovarian cancers. Cancer Res. 1992;52(20):5815–5817. [PubMed] [Google Scholar]
- 43.Mahlamaki EH, Hoglund M, Gorunova L, et al. Comparative genomic hybridization reveals frequent gains of 20q, 8q, 11q, 12p, and 17q, and losses of 18q, 9p, and 15q in pancreatic cancer. Genes Chromosomes Cancer. 1997;20(4):383–391. doi: 10.1002/(sici)1098-2264(199712)20:4<383::aid-gcc10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 44.Bardi G, Johansson B, Pandis N, et al. Karyotypic abnormalities in tumours of the pancreas. Br J Cancer. 1993;67(5):1106–1112. doi: 10.1038/bjc.1993.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamisawa T, Fujiwara T, Tu Y, et al. Long-term follow-up of intraductal papillary adenoma of the pancreas. J Gastroenterol. 2002;37(10):868–873. doi: 10.1007/s005350200144. [DOI] [PubMed] [Google Scholar]
- 46.Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314(5797):268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 47.Abe T, Fukushima N, Brune K, et al. Genome-wide allelotypes of familial pancreatic adenocarcinomas and familial and sporadic intraductal papillary mucinous neoplasms. Clin Cancer Res. 2007;13(20):6019–6025. doi: 10.1158/1078-0432.CCR-07-0471. [DOI] [PubMed] [Google Scholar]
- 48.Fukushige S, Waldman FM, Kimura M, et al. Frequent gain of copy number on the long arm of chromosome 20 in human pancreatic adenocarcinoma. Genes Chromosomes Cancer. 1997;19(3):161–169. doi: 10.1002/(sici)1098-2264(199707)19:3<161::aid-gcc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 49.Ghadimi BM, Schrock E, Walker RL, et al. Specific chromosomal aberrations and amplification of the AIB1 nuclear receptor coactivator gene in pancreatic carcinomas. Am J Pathol. 1999;154(2):525–536. doi: 10.1016/S0002-9440(10)65298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lohr M, Kloppel G, Maisonneuve P, et al. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia. 2005;7(1):17–23. doi: 10.1593/neo.04445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hruban RH, van Mansfeld AD, Offerhaus GJ, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143(2):545–554. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.