Abstract
Objective:
To study the impact of red cell distribution width (RDW) on postoperative recovery after correction of Tetralogy of Fallot (TOF).
Background:
Increased RDW indicates dysregulated erythropoiesis and predicts survival in critical illnesses that include idiopathic pulmonary artery hypertension and chronic heart failure. Myocardial injury and oxidative stress induced by cardiopulmonary bypass potentially contribute to prolonged recovery in post TOF repair patients.
Materials and Methods:
Retrospective analysis of data on 94 consecutive children with TOF undergoing corrective repair (January 2010-March 2011) was done. RDW was higher for the study population when compared to acyanotic patients with ventricular septal defect (17.7 ± 3.7 vs. 16.2 ± 4.2; P < 0.001). The mean RDW obtained from 100 separate age-, sex-, and weight-matched TOF patients (17.8) was chosen as a cut-off. Of 93 survivors (median age: 12 (4-204) months, weight: 8.6 (3.2-70) kg), 29 patients with higher RDW (> 17.8) had a longer ICU stay (155.6 ± 71.3 vs. 122.4 ± 61.3 hours, P = 0.02), hospital stay (18.6 ± 10.5 days vs. 13.4 ± 6.5 days, P = 0.01), ventilation time (57.9 ± 41.6 vs. 38.3 ± 30.8 hours, P = 0.01), and more surgical site infection (24.1% vs. 6.2%, P = 0.01). On multivariate analysis only elevated RDW (other variables included age, weight, hemoglobin, hematocrit, and surgical support times) showed a significant association with hospital stay.
Conclusions:
Elevated RDW appears to be associated with prolonged recovery after TOF repair, the precise underlying mechanisms are worth investigating.
Keywords: Cyanosis, congenital heart defect, iron deficiency, open-heart surgery
INTRODUCTION
A proportion of patients with Tetralogy of Fallot (TOF) develop restrictive physiology and prolonged Intensive Care Unit (ICU) recovery after surgical repair.[1] No clinical or laboratory parameter has been found to consistently predict the recovery after TOF surgery. However, it has been postulated that patients with TOF are vulnerable to oxidant damage and myocardial injury potentiated by the release of redox iron from free hemoglobin, produced by hemolysis during cardiopulmonary bypass (CPB).[2] Red cell distribution width (RDW) is a marker for dysregulated erythropoiesis and is elevated in microcytic hypochromic anemia, macrocytic anemia, and myelodysplasias.[3] RDW is a robust predictor of all-cause mortality and blood stream infection in critically ill patients.[4,5] It is also a prognostic marker in chronic heart failure, probably reflecting inflammatory stress and reduced iron mobilization.[6,7,8] RDW has emerged as an independent predictor of death and a strong prognostic biomarker in patients with idiopathic pulmonary artery hypertension.[9,10] Recognizing that TOF is associated with hypoxia for a relatively prolonged period of time, with resultant deregulation of erythropoiesis, we hypothesized that elevated RDW may be associated with postoperative outcomes in patients after TOF repair. In this study, we sought to examine the impact of elevated RDW on the outcomes after TOF repair, as measured by the recovery parameters in 94 consecutive children undergoing TOF repair at our institution.
MATERIALS AND METHODS
Study design
The data on all pediatric heart surgery in our institution is now collected prospectively as a part of the International Quality Improvement Collaborative for Congenital Heart Disease (IQIC) database. This study was based on a retrospective analysis of the data on TOF patients from the above database. The IQIC was conceived at the Global Forum for Humanitarian Medicine in Cardiology and Cardiac Surgery in Geneva, in 2007, and was officially launched in Boston, in 2008. Its mission is to provide benchmarking data for congenital heart surgery, to guide quality improvement efforts and reduce mortality in developing countries, and is currently collecting data from 24 sites across the globe. We routinely obtain a complete blood count for all patients, preoperatively.
Study period
January 2010 to March 2011.
Setting
Tertiary Pediatric Cardiac Care Center in South India.
Inclusion criteria
All the patients who underwent TOF repair during the above-mentioned period in the pediatric age group (< 18 years), irrespective of the anatomical variations, type of surgery, and iron supplementation status, were included.
Exclusion criteria
All patients with proven hematological abnormalities (e.g., thalassemia) and those with syndromes known to associate with hematological problems (e.g., Downs syndrome) were excluded. Those patients whose hospital stays were prolonged due to non-medical issues were excluded.
Red cell distribution width
RDW is a coefficient of variation, measuring the variation in red blood cell (RBC) volume within the RBC population and is an indicator of the extent of dysregulated erythropoiesis. RDW is calculated as a percentage of the standard deviation of RBC volume with respect to the mean RBC volume.
RDW = Standard deviation of RBC volume ÷ mean RBC volume × 100
The RDW data was obtained from the Electronic Medical Records Department of our institution, with permission. The RDW was analyzed using CELL-DYN 3700 hematology analyzer (Abbott Laboratories, Abbott Park, Illinois, USA) as a part of routine preoperative blood sampling. All the other variables were obtained from the IQIC database.
Intensive care unit protocols
In the ICU, after surgery, all patients were electively ventilated overnight. Patients with stable hemodynamics were extubated the next day, with tapering of the inotropic supports. Stable patients without inotropic supports were shifted to an intermediate ICU and observed for a period of 24 hours, after which they were transferred to the ward. The criteria for discharging from the ward were, healed surgical wound and absence of ventricular dysfunction and blood stream sepsis.
Outcome measures
The duration of ICU stay, hospital stay, mechanical ventilation, surgical site infection, and blood-borne sepsis were used as the main outcome measures for this study. The durations of outcome variables were measured in accordance with institutional ICU and postoperative policies, and applied to all patients without any bias.
Data analysis
In view of the paucity of standard normative data for RDW in the pediatric population, we first compared the mean RDW obtained from the study group with that from a group of 100 acyanotic patients with ventricular septal defects (VSD), from the database (17.7 ± 3.7 vs. 16.2 ± 4.2; P < 0.001). We then obtained the mean RDW from a separate group of 100 age-, sex-, and weight-matched patients with TOF. The mean RDW value of 17.8 obtained from this group was applied to the study cohort to form two groups; group 1 (RDW > 17.8) and group 2 (RDW ≤ 17.8). The various outcome measures were compared between the two groups. A two-tailed Student's ‘t’ test was used for all inter-group comparisons. A P < 0.05 was considered to be significant. To determine whether RDW independently impacted the outcome parameters, we performed a multivariate analysis. The variables used for the multivariate logistic regression model included, age, weight, hemoglobin, hematocrit, cardiopulmonary bypass time, and aortic cross-clamp time. The analysis was done using the SPSS 15 version.
RESULTS
A total of 94 patients were included in the study (demographic details in Table 1). The median age was 12 months (range 4-204) with 54 (57.4%) male patients. The median body weight was 8.6 kg (range 3.2-70). The mean RDW for the study group was higher when compared to the 100 patients with VSD, who underwent surgical repair in the same period (17.7 ± 3.7 vs. 16.2 ± 4.2; P < 0.001).
Table 1.
A comparison of basic characteristics between the two groups
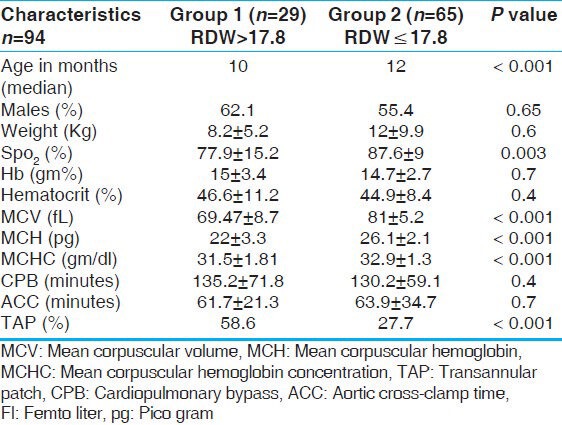
Thirty-five patients (37.2%) from the study group underwent TOF repair with a transannular patch (TAP) and 12 (12.7%) with a conduit. A single surgeon operated on 91 (96.8%) patients. One patient, a nine-month-old, 6.3 kg, female child (RDW = 17) died on the fourth postoperative day, from persistent renal failure.
Using a cutoff value of > 17.8, 29 patients (30.8%) had higher RDW in the study group (group 1). The indices of iron sufficiency were significantly lower among the group 1 patients. They had a low mean corpuscular volume (MCV: 69.4 ± 8.7 vs. 81 ± 5.2 fl; P < 0.001), mean corpuscular hemoglobin (MCH: 22 ± 3.3 vs. 26.1 ± 2.1 pgm; P < 0.001), and mean corpuscular hemoglobin concentration (MCHC: 31.5 ± 1.8 vs. 32.9 ± 1.3 gm/dl; P < 0.001) compared to group 2 [Table 1]. Among the 29 patients with elevated RDW, 17 (58.6%) had hypochromia (based on MCH for age[11]), of whom 15 (51.7%) had associated microcytosis of RBC (based on MCV for age[11]) suggesting iron deficiency, while only three (4.6%) in group 2 had microcytosis, with only two among them having hypochromia, the difference being significant (P < 0.001) [Table 1].
Of the seven patients (24.1%) who had surgical site infection in group 1, two had mediastinitis and the remaining five had superficial wound infection, while in group 2, four (6.2%) had superficial wound infection (P < 0.001), and none from either group had associated blood stream sepsis. The only microbe isolated in these patients was methicillin resistant staphylococcus aureus (MRSA) from the pus in both the groups. Culture-proven blood stream sepsis (n = 7 in total) was not associated with elevated RDW.
Group 1 patients had a longer ICU stay (155.6 ± 71.3 hours vs. 122.4 ± 61.3 hours, P = 0.02), hospital stay (18.6 ± 10.5 days vs. 13.4 ± 6.5 days, P = 0.01), ventilation time (57.9 ± 41.6 hours vs. 38.3 ± 30.8 hours, P = 0.01), and more frequent surgical site infection (24.1% vs. 6.2%, P = 0.01) when compared to group 2 [Table 2]. The groups were comparable with respect to weight, hemoglobin concentration, hematocrit, and surgical variables like cardiopulmonary bypass time and cross-clamp time. Group 1 patients were more hypoxic (SPO2: 77.9 ± 15.2% vs. 87.6 ± 9%; P value 0.003) and were marginally younger (median age: 10 months vs. 12 months; P < 0.001). However, TAP was required more frequently in group 1 (58.6% vs. 27.7%; P = 0.004) although ventilation time, ICU stay, and hospital stay did not differ between TAP and non-TAP patients in the whole cohort. No other specific anatomic variables were analyzed in the study.
Table 2.
Comparison of outcome measures between the two groups
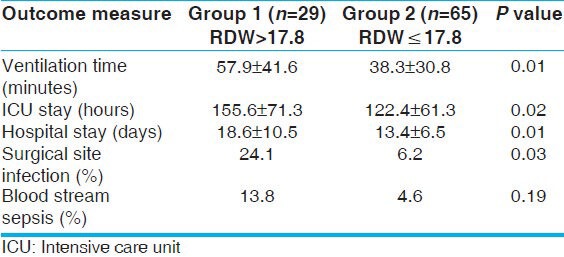
Multivariate analysis showed a strong independent association of elevated RDW with hospital stay (Odds ratio for hospital stay exceeding 14 days of 11.4; 95% confidence limits of 2.3-55.3; P < 0.001). There was no independent association of RDW with the other outcome variables.
DISCUSSION
We identified RDW to be significantly elevated in children undergoing surgery for TOF. As expected, elevated RDW correlated with the other hematological indices of iron deficiency. However, elevated RDW was not associated with elevated hematocrit or hemoglobin. Those with elevated RDW were younger and more hypoxic. The group of children with elevated RDW had longer duration of mechanical ventilation, ICU stay, hospital stay, and more frequent surgical site infections. On multivariate analysis, however, elevated RDW was significantly associated with a prolonged hospital stay alone.
These results suggest that RDW may be a potentially useful biomarker for delayed recovery after TOF correction. However, because the present study was not designed to identify potential mechanisms, we can only speculate on a possible hypothesis [Figure 1].
Figure 1.
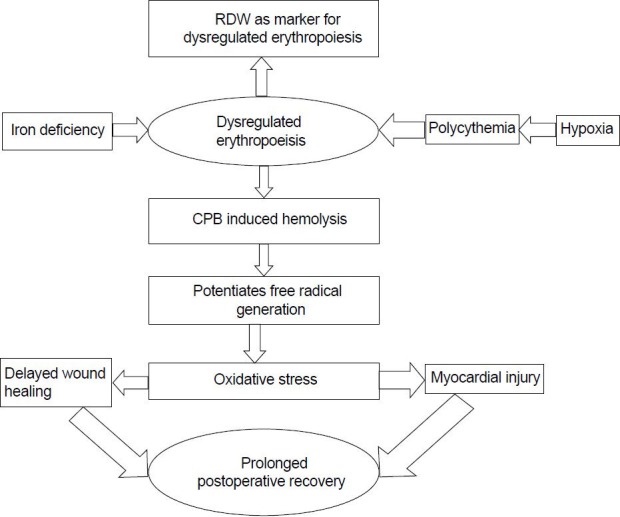
Proposed mechanism for correlation of elevated RDW and prolonged recovery. RDW: Red cell distribution width, CPB: Cardio pulmonary bypass
Dysregulated erythropoiesis in cyanotic patients may allow a greater release of free iron by excessive hemolysis during CPB.[12] Acute restrictive physiology after TOF repair has been shown to be associated with oxidative stress, due to an increased iron concentration released by excessive hemolysis of the abnormal red blood cells.[13,14,15]
The usual reasons for elevated RDW in the general population include microcytic anemias, macrocytic anemias, and myelodysplasias.[3] Dysregulated erythropoiesis is a known hematological event in cyanotic heart disease, secondary to chronic hypoxia-induced RBC proliferation. This can be accentuated by superimposed iron deficiency, to which cyanotic patients are particularly vulnerable. The prevalence of iron deficiency is well documented in congenital cyanotic heart diseases.[16,17,18]
The association of oxidative stress and impaired wound healing is well documented.[19] A higher incidence of delayed surgical site healing is noted in patients with elevated RDW, with the majority having documented the absence of microbial infection, probably implying alternative non-infective mechanisms for the same. Delayed wound healing definitely cotributes delayed recovery and prolonged hospital stay.
The results of this study underscore the need to aggressively treat iron deficiency in patients with TOF. It reinforces the traditional teaching that recommends prophylactic iron administration to all infants and children with cyanotic heart disease until corrective surgery is undertaken.
Study limitations
The study was designed as a retrospective analysis of prospectively collected data. As no standard age-related values for RDW were available in the pediatric population, the cut off was arbitrarily taken from the measurement in other TOF patients as described. We did not explore the reasons for elevated RDW in the study group including micronutrient deficiencies. The impact of elevated RDW on older children could not be analyzed separately because there were relatively few older patients with elevated RDW. The reasons for prolonged hospital stay could not be ascertained. A variable number of patients were on iron supplementation and this could not be identified.
CONCLUSION
Elevated RDW is a potentially useful biomarker that appears to be associated with prolonged recovery after TOF repair. Prospective and mechanistic studies are required to understand the precise underlying mechanisms of this association.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Seamus C, Darryl S, Andrew R. Characterization of right ventricular diastolic performance after complete repair of Tetralogy of Fallot. Restrictive physiology predicts slow postoperative recovery. Circulation. 1995;91:1782–5. doi: 10.1161/01.cir.91.6.1782. [DOI] [PubMed] [Google Scholar]
- 2.Chathurvedi RR, Darryl FS, Christopher L, Redington AN. Acute right ventricular restrictive physiology after repair of Tetralogy of Fallot: Association with myocardial injury and oxidative stress. Circulation. 1999;100:1540–7. doi: 10.1161/01.cir.100.14.1540. [DOI] [PubMed] [Google Scholar]
- 3.Bessman JD, Gilmer PR, Gardner FH. Improved classification of anemias by MCV and RDW. Am J Clin Pathol. 1983;80:322–6. doi: 10.1093/ajcp/80.3.322. [DOI] [PubMed] [Google Scholar]
- 4.Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913–21. doi: 10.1097/CCM.0b013e31821b85c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F, Pan W, Pan S, Ge J, Wang S, Chen M. Red cell distribution width as a novel predictor of mortality in ICU patients. Ann Med. 2011;43:40–6. doi: 10.3109/07853890.2010.521766. [DOI] [PubMed] [Google Scholar]
- 6.Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16:230–8. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. CHARM Investigators. Red cell distribution width as a novel prognostic marker in heart failure: Data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50:40–7. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 8.Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width: A powerful prognostic marker in heart failure. Eur J Heart Fail. 2010;12:415. doi: 10.1093/eurjhf/hfq018. [DOI] [PubMed] [Google Scholar]
- 9.Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol. 2009;104:868–72. doi: 10.1016/j.amjcard.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Christopher JR, John W, Luke SH, Simon RG, Martin RW. Red cell distribution width outperforms other potential circulating biomarkers in predicting survival in idiopathic pulmonary arterial hypertension. Heart. 2011;97:1054–60. doi: 10.1136/hrt.2011.224857. [DOI] [PubMed] [Google Scholar]
- 11.Kleigman, Behrman, Jenson, Stanton . 18th ed. Vol. 2. Philadelphia: Saunders, Elsevier Inc; 2011. Nelsons Text Book of Pediatrics; p. 2944. [Google Scholar]
- 12.Everse J, Hsia N. Toxicities of native and modified hemoglobins. Free Radic Biol Med. 1977;22:1075–99. doi: 10.1016/s0891-5849(96)00499-6. [DOI] [PubMed] [Google Scholar]
- 13.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease. An overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 14.Li RK, Mickle DA, Weisel RD, Tumiati LC, Jackowski G, Wu TW, et al. Effect of oxygen tension on the antioxidant enzyme activities of Tetralogy of Fallot ventricular myocytes. J Mol Cell Cardiol. 1989;21:567–75. doi: 10.1016/0022-2828(89)90822-5. [DOI] [PubMed] [Google Scholar]
- 15.Allen BS, Rahman S, Ilbawi MN, Kronon M, Bolling KS, Halldorsson AO, et al. Detrimental effects of cardiopulmonary bypass in cyanotic infants; preventing the reoxygenation injury. Ann Thorac Surg. 1997;64:1381–8. doi: 10.1016/S0003-4975(97)00905-3. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Quintana E, Rodríguez-González F. Iron deficiency anemia detection from hematology parameters in adult congenital heart disease patients. Congenit Heart Dis. 2013;8:117–23. doi: 10.1111/j.1747-0803.2012.00708.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaemmerer H, Fratz S, Braun SL, Koelling K, Eicken A, Brodherr-Heberlein S, et al. Erythrocyte indexes, iron metabolism, and hyperhomocysteinemia in adults with cyanotic congenital cardiac disease. Am J Cardiol. 2004;94:825–8. doi: 10.1016/j.amjcard.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Onur CB, Sipahi T, Tavil B, Karademir S, Yoney A. Diagnosing iron deficiency in cyanotic heart disease. Indian J Pediatr. 2003;70:29–31. doi: 10.1007/BF02722740. [DOI] [PubMed] [Google Scholar]
- 19.Matthias S, Sabine W. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008;58:165–71. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]


