Abstract
Background:
Amplatzer vascular plugs (AVPs) are devices ideally suited to close medium-to-large vascular communications. There is limited published literature regarding the utility of AVPs in congenital cardiovascular malformations (CCVMs).
Aims:
To describe the use of AVPs in different CCVMs and to evaluate their safety and efficacy.
Materials and Methods:
All patients who required an AVP for the closure of CCVM were included in this retrospective review of our catheterization laboratory data. The efficacy and safety of AVPs are reported.
Results:
A total of 39 AVPs were implanted in 31 patients. Thirteen (33%) were AVP type I and 23 (59%) were AVP type II. AVP type III were implanted in two patients and type IV in one patient. The major indications for their use included closure of pulmonary arteriovenous malformation (AVM) (n = 7), aortopulmonary collaterals (n = 7), closure of a patent Blalock-Taussig shunt (n = 5), systemic AVM (n = 5), coronary AVM (n = 4), patent ductus arteriosus (PDA) (n = 3), pulmonary artery aneurysms (n = 3), and venovenous collaterals (n = 2). Deployment of the AVP was done predominantly via the 5 – 7F Judkin's right coronary guide catheter. Overall 92% of the AVPs could be successfully deployed and resulted in occlusion of the target vessel in all cases, within 10 minutes. No procedure-related or access site complication occurred.
Conclusions:
AVPs are versatile, easy to use, and effective devices to occlude the vascular communications in a variety of settings. AVP II is especially useful in the closure of tubular structures with a high flow.
Keywords: Amplatzer vascular plug, congenital heart disease, device therapy
INTRODUCTION
A variety of congenital cardiovascular malformations (CCVM) require closure of abnormal vascular communications. These can be natural communications such as patent ductus arteriosus (PDA), atrial septal defects, ventricular septal defects, and arteriovenous malformations (AVMs), or surgically created ones, such as, shunts and fenestrations. Closure of septal defects is well established with an armamentarium of septal/duct occluders. A majority of the other CCVMs were generally closed with materials like coils and gel foams or with other septal/duct occluders, for embolization. However, coils and gel foams have a high risk of embolization especially in high flow situations and septal/duct occluders are at times difficult to deliver due to their bulkier profiles, especially across tortuous and angulated vessels.
The Amplatzer Vascular Plugs (AVPs) are devices that are excellent alternatives to other existing devices for embolization of medium-to-large vascular communications.[1] Since their introduction, there has been an exponential growth in the utilization of AVPs for the closure of various intra- and extracardiac vascular communications. With the increasing need for these devices, the AVPs have evolved to a great extent. As a result, currently four generations of AVPs, with different shapes and occlusion properties, are available. These devices have different structural features [Table 1].[1] Most of the existing literature regarding AVPs pertains to their use in peripheral vascular malformations. Their use in various CCVMs is expanding because of their lower profile, ease and control of delivery, and low risk of device embolism. Literature regarding their use in congenital heart disease (CHD) related intervention is limited to case reports and small series. Shwartz et al., previously described the use of AVP I and II in a series of patients with congenital cardiovascular disease, mostly patients with PDA.[2] We describe here the utility of AVP in patients with CCVM, highlighting the device selection criteria and technical tips during the deployment.
Table 1.
Device characteristics of various generations of the Amplatzer vascular plug family
MATERIALS AND METHODS
We retrospectively reviewed the data of all the patients with CCVM requiring a vascular occlusion procedure, with any AVP, at our center, from August 2005 to March 2013. The study was approved by the Institute's Ethics Committee. Informed, written consent for the procedure was obtained from all participants/parents. The baseline data was recorded and angiograms were reviewed for identification of the target vessel and for other procedural details.
The diameter of each vessel was measured in relation to the catheter diameter used for the angiogram and was approximated to the nearest 0.5 mm. The size and number of the AVPs implanted were recorded along with the angiographic result, following implantation. The ratio of the device-to-vessel diameter for all patients was calculated. Complications on follow-up were noted from the outpatient files.
Successful procedure was defined as a complete occlusion of the desired vessel at the time of procedure, without any residual flow across the deployed device and without any complication related to the procedure. Periprocedural complications including device deployment failure, device malposition, migration or embolization, stroke, death, bleeding or any other vascular complication related to the access site were noted.
In the summary statistics, continuous variables are reported as mean ± SD and categorical variables as proportions.
RESULTS
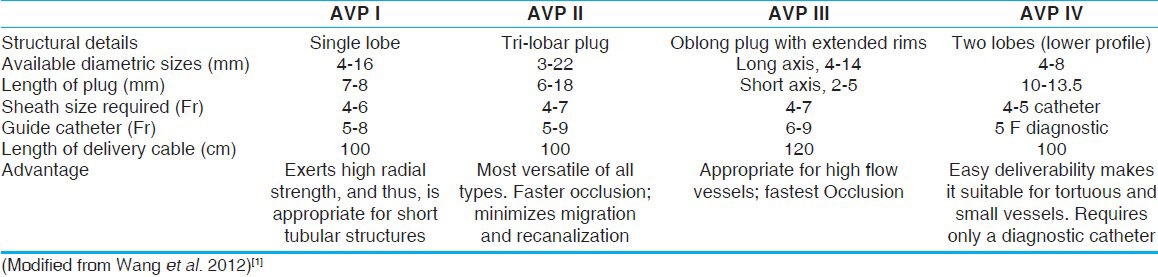
A total of 31 patients underwent a procedure requiring AVP implantation for CCVM, including the three cases reported previously.[3,4,5] The median age of the patients was 12 years, with a range from 1-60 years. Fifteen patients (48%) were male. The procedure was done under local anesthesia in 23 (74%) patients, while eight (26%) patients required general anesthesia. A total of 39 AVPs were implanted in 31 patients, with 23 (59%) of them being AVP type II and 13 (33%) AVP type I. AVP type III were implanted in two patients and type IV in one patient. Features of various plugs and a representative case highlighting their use are shown in Figure 1.
Figure 1.
Family of Amplatzer vascular plugs. Upper panel shows the in-vitro configuration of the family of Amplatzer vascular plugs comprising of type I to type IV. Lower panel shows their configuration after deployment in various vascular structures: (a) AVP type I across a modified Blalock-Taussig shunt, (b) AVP II across a large aortopulmonary collateral, (c) AVP III across an APC, and (d) AVP IV across an APC (aortopulmonary collateral)
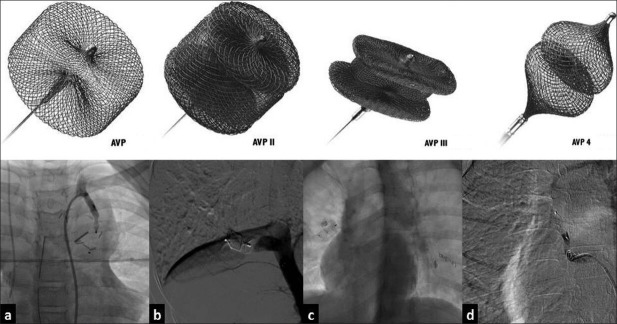
Various indications for the use of AVP are shown in Table 2. The AVPs were mostly used for the closure of extracardiac shunts [Figure 2]. AVMs in pulmonary or systemic circulation and aortopulmonary collaterals were the most common indications. Intracardiac shunts that required an AVP for their closure included coronary AVM and pulmonary valve closure in a patient with a Glenn shunt [Figure 3] and baffle leak, following a Fontan operation [Figure 4]. Two AVPs were used in four patients and three AVPs were used in two patients with pulmonary AVM/aneurysm. No patients received two AVPs for the closure of the same vessel.
Table 2.
Indications for use of an Amplatzer vascular plug
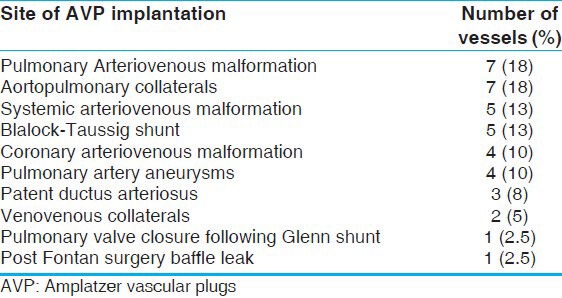
Figure 2a.
Ideal indications for use of an AVP. An angiogram showing a proximal coronary AV fistula from the right coronary artery to right ventricle
Figure 2b.
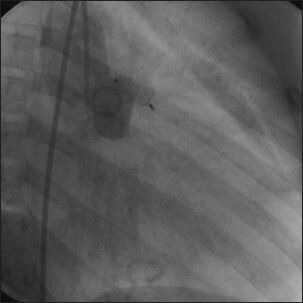
Successful deployment of AVP I across the fistula with complete occlusion of flow
Figure 2c.
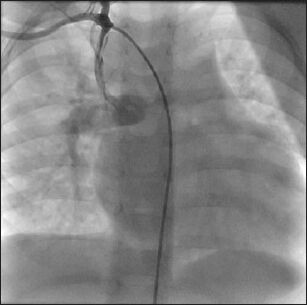
An angiogram shows a patent right modified Blalock-Taussig shunt filling the bilateral pulmonary arteries
Figure 2d.
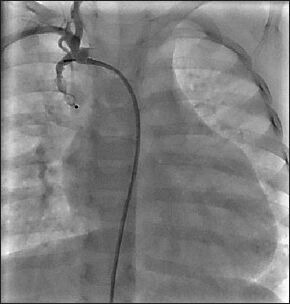
An angiogram showing successful deployment of AVP type II in the BT shunt to close it, prior to definitive surgery in a patient with Tetrology of Fallot's
Figure 2e.
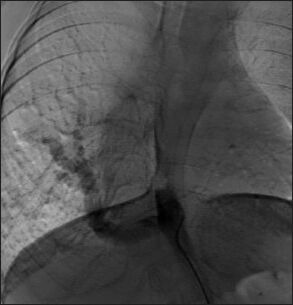
This angiogram shows a large aortopulmonary collateral arising from the abdominal aorta and supplying the right lung in a patient with Tetralogy of Fallot
Figure 2f.
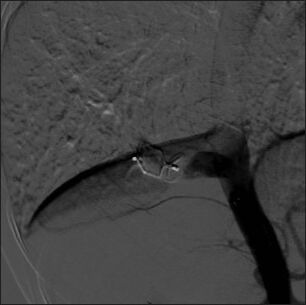
No flow across the collateral after successful deployment of AVP type II
Figure 2g.
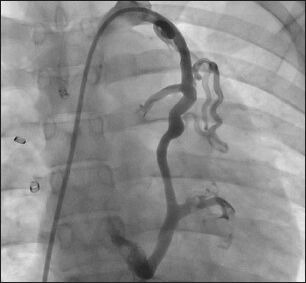
An angiogram in a patient with a history of Fontan surgery presenting with desaturation, showing a large venovenous collateral arising from the left innominate vein and draining into the left inferior pulmonary vein
Figure 2h.
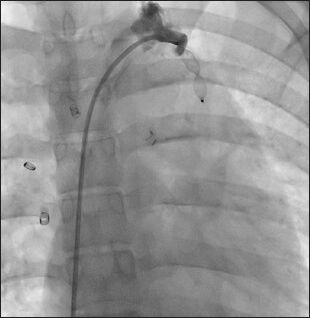
AVP type II was implanted in the venovenous collateral causing complete closure of the communication and significant improvement in the systemic saturation
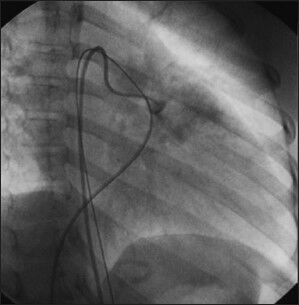
Figure 3a.
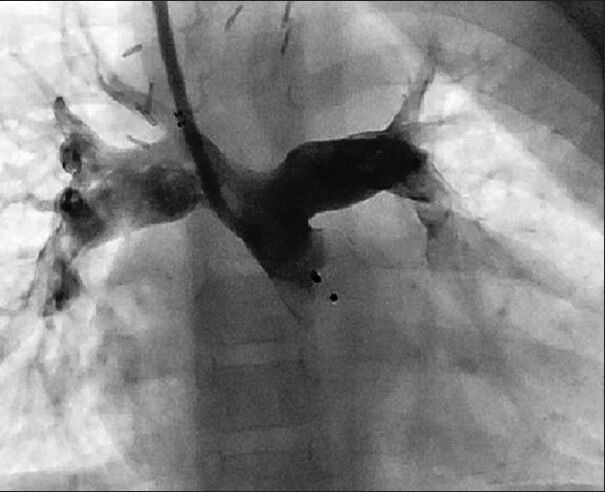
Unusual indications for AVP use. An angiogram with a Judkin's right catheter in the main pulmonary artery of a patient with a prior history of Glenn surgery. As the pulmonary valve was not ligated during surgery, the antegrade flow caused a significant reversal of flow in the superior vena cava. AVP type I was implanted successfully at the pulmonary valve, with complete closure of the antegrade flow
Figure 3b.
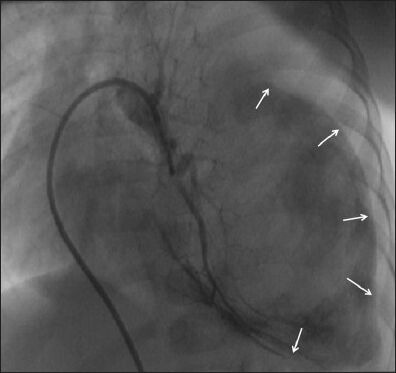
A massive pseudoaneurysm arising from the left pulmonary artery occupying almost the left hemithorax. (Reproduced with permission from reference 5)
Figure 3c.
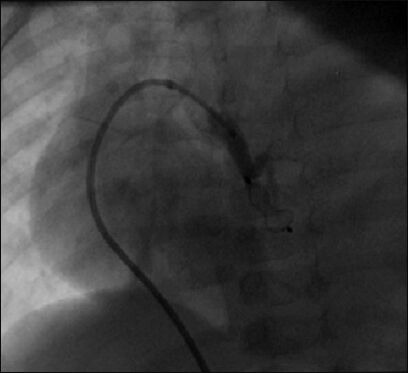
AVP type I was implanted with the help of the mother and child technique (a 5F multipurpose guide catheter advanced through a 7F Judkin's right guide catheter) in the neck of a pseudoaneurysm, causing the collapse of the pseudoaneurysm. (Reproduced with permission from reference 5)
Figure 4a.
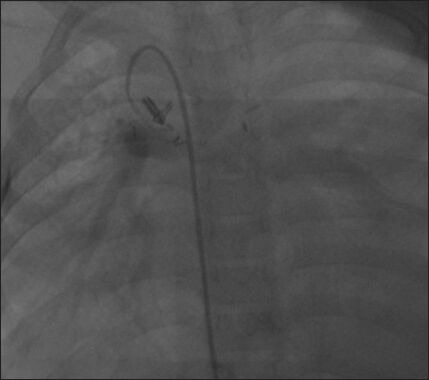
Successful use of AVP deployment through a Jugular approach. An angiogram shows a patent right modified Blalock.Taussig shunt in a patient who underwent Glen surgery. The BT shunt caused overflow in the pulmonary circulation, requiring its closure. Deployment of a duct occluder could not be done due to difficulty in advancing a catheter through the arterial side. (Reproduced with permission from reference 4)
Figure 4b.
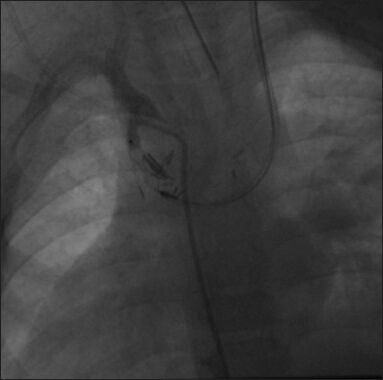
AVP type I was deployed successfully in the right BT shunt with the help of a JR guide catheter advanced through the left Glen pathway. (Reproduced with permission from reference 4)
Figure 4c.
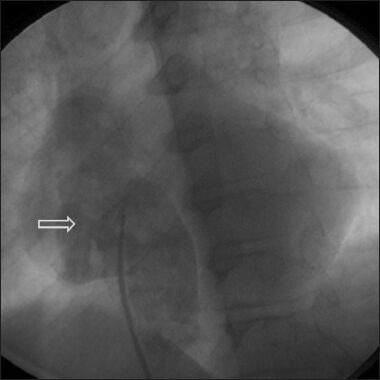
An angiogram showing a baffle leak following a lateral tunnel Fontan, leading to significant desaturation (Reproduced with permission from reference 3)
Figure 4d.
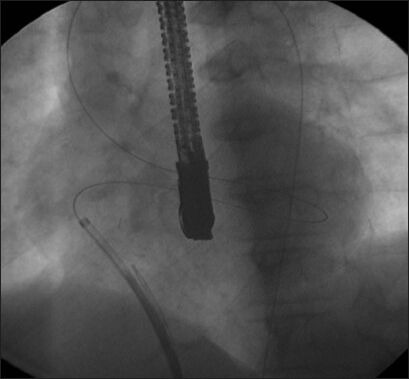
Initially, a venoarterial loop was created, but the duct occluder sheath could not be advanced due to difficult angulation (Reproduced with permission from reference 3)[3]
Figure 4e.
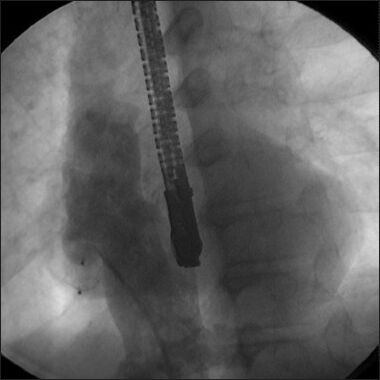
An AVP I was successfully deployed across the baffle leak through the jugular approach. (Reproduced with permission from reference 3)
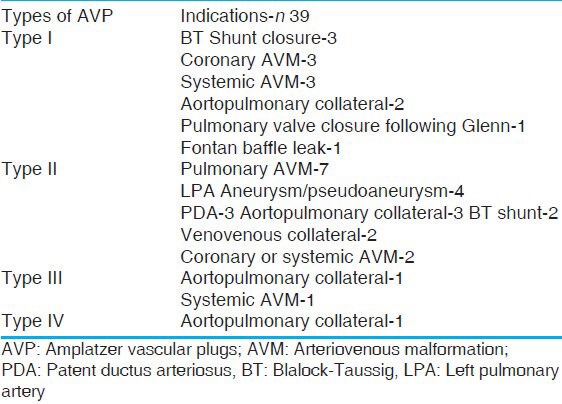
The type of AVPs used and their indications are shown in Table 3. AVP I was the most common type used before AVP II became available for clinical use. In the later part of the study period, however, AVP II was the most common type used, due to better occlusive properties and availability in a wide range of diameters. Type III is preferred for medium-sized high flow tubular structures, where faster occlusion is desired. We have used type III in two patients only. A vast majority of the aortopulmonary collaterals are closed with coils and gel foams in our institute and AVP is reserved for large collaterals [Figure 2e and 2f]. In one patient with a ventricular septal defect and pulmonary atresia, a large aortopulmonary collateral was occluded in the operating room as a hybrid procedure. Three patients underwent occlusion of the patent Blalock-Taussig shunt in the immediate postoperative period after failed surgical ligation during a surgical repair, while in two patients occlusion of the BT shunt was performed just prior to the corrective surgery.
Table 3.
Types of AVP used and their indications
Procedural details
The device size was selected based on the most restrictive diameter along the length of the vascular channel to be closed. We oversized the AVP by 30-50%, relative to the size of the native vessel. Thus, for a vessel size of 5 mm, a plug of 8 mm and for a vessel of size 4 mm a plug of 6 mm was chosen. We used AVP sizes ranging from 6 mm to 22 mm. The choice of the device size is also dependent on the overall available length of the vessel, as an oversized AVP tends to lengthen significantly. For instance, for tubular structures with a uniform diameter, an AVP that is large enough to ensure device stability, but not so large that the device elongates significantly to protrude into nearby structures, should be chosen. The mean device-to-vessel ratio was 1.42.
The device was placed through the femoral venous or femoral artery access as needed. In three patients, a jugular approach was used [Figure 4]. In a patient after a superior cava-pulmonary connection with a residual BT shunt, a retrograde approach through the jugular vein was employed after the initial antegrade arterial approach failed [Figure 4]. The most common catheter used for plug delivery was the Judkin's right (JR) guide catheter (Cordis Corporation, Miami, FL). The other catheters included Multipurpose (MP), Amplatzer duct occluder (ADO) delivery sheath (AGA Medical Corp, Golden Valley, MN), Cook delivery sheaths (Cook, Bloomington, USA), Amplatz left (AL), and Amplatz right (AR) guide catheters. In most instances, a Judkin's right coronary, Picard or Conard catheter was used to hook the vessel and an exchange length Terumo wire (Terumo Medical Corporation, Somerset, NJ, USA) or exchange length ordinary guidewire was advanced, over which a guide catheter of choice was taken across the intended site of closure. Occasional instances in which advancement of a guiding catheter was technically difficult, a catheter-in-catheter (mother and child) technique was used [Figure 3b and 3c]. For instance, for advancing a 6F or 7F guide catheter, another 4F or 5F diagnostic catheter of sufficient length was kept inside the guide catheter and the guide catheter was advanced over the diagnostic catheter. An appropriate AVP of the desired diameter was advanced and delivered with ease in most of the cases, including cases in which other duct/septal occluders failed. The technique for delivering the AVP is similar to all other Amplatzer devices except that traction on the assembly is minimized and the nitinol mesh is allowed to assume its natural configuration. Recapturing and redeployments are relatively easy as compared to duct and septal occluders due to its lower profile. If the device is oversized, or too much pull is applied during delivery, then the AVP tends to elongate, which may lead to obstruction of the nearby structures and also make the AVPs less occlusive. In a majority of the circumstances, a Tohue Bosht can be attached and a good quality angiogram maybe obtained with the same guide catheter, as the thickness of the delivery cable of the AVP is lesser. In some instances we used additional access and diagnostic catheters to demonstrate total occlusion prior to the release of AVP. An adequately oversized device that had deformed and produced a near complete occlusion of flow suggests a tight fit and negligible chance of embolization, even in very high flow situations.
Technical success
Successful deployment of the device and complete flow occlusion across the vessel could be achieved in 36 (92%) of the vessels that were closed. In a majority of cases, angiographic evidence of residual shunting reduced significantly in the laboratory itself, despite full heparinization. After 10 minutes of deployment, a significant residual flow was observed across only two AVPs. In one patient in whom AVP type IV was deployed in an aortopulmonary collateral, there was persistence of flow across it, which was later on completely occluded with a giant coil deployed proximally [Figure 1d]. Another patient had a very high flow coronary AVM in which an AVP II was implanted. However, because of the persistent flow as well as lack of stability of the device in the desired position, the procedure was abandoned and the patient was subjected to a surgical repair of the AVM. Another case of unsuccessful plug deployment was a case of large PDA with pulmonary arterial hypertension. Pulmonary hypertension was reversible, and the patient was considered operable on the basis of cardiac catheterization data and oxygen reversibility testing. After deployment of the AVP type II, of 22 mm, across the duct, there was a progressive tenting of the distal lobe of the plug and ultimately the device was pushed into the pulmonary arteries. However, as the plug was not detached from the delivery cable, it could be easily retrieved. The PDA was then closed using a 20–18 mm duct occluder device (Cocoon Duct Occluder, Vascular Innovations Inc., Thailand).
Complications
None of the patients had any serious complications in the periprocedural period. As previously described an additional coil was required to close the residual flow in the aorto-pulmonary collateral closed with AVP IV, and in a patient with PDA and pulmonary hypertension an AVP II prolapsed. No access site complication had occurred in any of these patients, neither was there any periprocedural requirement of blood transfusion. In three patients, an imaging evaluation confirmed the absence of a residual shunt after one week to three months from the initial deployment of the plug. No patient reported any complication related to the AVP on follow up.
DISCUSSION
There has been a tremendous advancement in transcatheter closure of various abnormal vascular communications using coils, gel foams, septal, and ductal occluders.[6] A major addition was the introduction of AVP type I in 2004 followed by a series of AVP type II, III, and the latest AVP type IV. The AVPs are self-expanding devices made of nitinol wire mesh. The AVPs have features of both closure and embolization devices, making them particularly versatile for closure of vessels not ideally treated by other existing devices. The major advantages of AVP over other closure devices like ADO and the Ampltzer septal occluder (ASO) include a lower profile and the ability to deploy the device through the usual guiding and diagnostic catheters. This makes AVP ideally suited for deployment across tortuous vessels or a tortuous approach route, wherein, advancing the ADO/ASO sheath could be problematic. The same size of AVP is generally delivered through a lower size sheath as compared to the ADO or ASO, and is a distinct advantage in smaller children. In some of our cases also, the delivery of an ADO/ASO failed, but the AVP was successfully deployed [Figure 4]. The use of AVP for closure of different CCVMs is increasing. The clinical conditions in which these vascular plugs are utilized include intracardiac and extracardiac shunts, abnormal arterioarterial, arteriovenous, venovenous malformations, paravalvular leaks, tumor embolization and portosystemic shunts.[7,8,9,10,11,12,13,14,15,16,17,18,19]
A detailed understanding of the different features of AVPs helps in the right selection of the device [Table 1]. AVP I has a single lobe of a single layer nitinol mesh and has been replaced by a three-lobed AVP II, with better occlusive properties and has six occlusive planes. The multilayered, multisegmented design of AVP II reduces the time to occlusion, provides full cross-sectional vessel coverage, and minimizes the migration and recanalization potential. Our study emphasizes the importance of AVP type II, which can be utilized in a majority of conditions requiring vascular occlusion, because of its versatile nature. AVP I is still useful in vessels with short landing zones. AVP III and IV have two lobes that are made for specific indications. AVP III has the fastest occlusive properties and is ideally suited for medium-sized, high-flow tubular structures. AVP IV has the lowest profile and in fact can be delivered through a 5F diagnostic catheter [Table 1]. However, the use of AVP IV is limited in our institute. AVP type IV is available till 8 mm only and can be utilized in a vessel up to a size of 5 mm, but we generally prefer to use coils, as they are a cheaper alternative.
The present series in one of the largest report of AVP in CCVMs. Previously, Shwartz et al., described the utilization of 50 AVP type I or II in patients with congenital cardiovascular diseases.[2] However, the majority (38%) of their patients had AVP implanted in a PDA. The other common indications described in their series were venous and arterial collaterals, BT shunts, pulmonary and peripheral AVM's, and Fontan fenestration.[2] Another large multicenter study across 11 centers of US reported the use of AVP I in 52 children, for similar indications.[20] In the present report, we describe some unusual indications for the use of AVP, such as, for left pulmonary artery aneurysm and for pulmonary valve closure [Figure 3a], not described previously. Technical success in our study can be achieved in 92% of the patients as compared to 100% in a previous study.[2] This is mainly because of the definition of success used. Using our definition, success in the earlier series would have been 87% only, as 13% of the patients had significant residual flow and 96% patients had occlusion documented after one week of procedure. It should be noted that the intracardiac use of AVP is not approved in some countries.
The use of AVPs for closing the PDA is evolving.[2,20] We have used AVP type II for closing a PDA in only three (8%) patients. In one patient with a hypertensive PDA, an AVP II prolapsed, while the same was subsequently closed by the usual duct occluder, perhaps suggesting less sturdiness in the vascular plugs. Recently, AVP II was successfully used to close all types of PDAs in infants.[21] AVPs may emerge as more cost-effective devices for PDAs as compared to the ADO, especially in tubular PDA, which is not suitable to be closed by the duct occluder.[2]
No instances of device migration, embolization or significant device lengthening causing obstruction of the nearby structures were encountered. Only one patient had significant residual flow requiring additional coil placement. The procedural protocol at our center mandated a check angiogram done after 10 minutes of deployment of the AVP. This suggests a closure time of less than 10 minutes for the AVP to close the vessel by thrombus formation. We did not encounter any specific complication related to the procedure involving AVP implantation. The recanalization rate could not be determined as we did not perform an angiogram in the later period after AVP implantation; neither did any patient present with symptoms suggestive of recanalization. In all the three patients who had an imaging evaluation following AVP implantation, there was no evidence of recanalization. Recanalization with AVP is extremely rare, with only five reports of recanalization among more than 1200 AVP placements.[1] Reconfiguration of plugs due to nitinol memory is an interesting phenomenon and may happen in up to 50% of AVPs, but is rarely clinically important.[22] We did not specifically look for reconfiguration-related problems.
CONCLUSION
The Amplatzer series of vascular plugs are very useful in the closure of a wide spectrum of abnormal vascular communications associated with congenital heart disease, with high technical success and low complication rates. Ease of use, lower profile, different designs, and controlled deliverability of AVPs makes them an ideal choice for occluding various extracardiac congenital cardiovascular communications.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Wang W, Li H, Tam MD, Zhou D, Wang DX, Spain J. The amplatzer vascular plug: A review of the device and its clinical applications. Cardiovasc Intervent Radiol. 2012;35:725–40. doi: 10.1007/s00270-012-0387-z. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz M, Glatz AC, Rome JJ, Gillespie MJ. The Amplatzer vascular plug and Amplatzer vascular plug II for vascular occlusion procedures in 50 patients with congenital cardiovascular disease. Catheter Cardiovasc Interv. 2010;76:411–7. doi: 10.1002/ccd.22370. [DOI] [PubMed] [Google Scholar]
- 3.Ramakrishnan S, Kothari SS, Singh N, Singh S. Baffle leak following lateral tunnel Fontan--treated with a vascular plug. Indian Heart J. 2009;61:285–7. [PubMed] [Google Scholar]
- 4.Ramakrishnan S, Kothari SS. Amplatzer vascular plug closure of a Blalock-Taussig shunt through a Glenn shunt. Catheter Cardiovasc Interv. 2008;72:413–5. doi: 10.1002/ccd.21643. [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishnan S, Kothari SS, Sharma S. Massive left pulmonary artery pseudoaneurysm in a young child. JACC Cardiovasc Interv. 2010;3:362–3. doi: 10.1016/j.jcin.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Holzer R, Hijazi ZM. Interventional approach to congenital heart disease. Curr Opin Cardiol. 2004;19:84–90. doi: 10.1097/00001573-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Brown MA, Balzer D, Lasala J. Multiple coronary artery fistulae treated with a single amplatzer vascular plug: Check the back door when the front is locked. Catheter Cardiovasc Interv. 2009;73:390–4. doi: 10.1002/ccd.21860. [DOI] [PubMed] [Google Scholar]
- 8.Wiegand G, Sieverding L, Kaulitz R, Hofbeck M. Transarterial and transvenous approach for transcatheter closure of a large coronary artery fistula with the amplatzer vascular plug. Pediatr Cardiol. 2009;30:172–5. doi: 10.1007/s00246-008-9266-4. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer MH. Novel use of the amplatzer plug for closure of a patent ductus arteriosus. Catheter Cardiovasc Interv. 2005;65:577–80. doi: 10.1002/ccd.20347. [DOI] [PubMed] [Google Scholar]
- 10.Jang GY, Son CS, Lee JW. Transcatheter occlusion of a modified Blalock-Taussig shunt using the amplatzer vascular plug with the catheter-snare technique. Pediatr Cardiol. 2008;29:670–2. doi: 10.1007/s00246-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 11.Ebeid MR, Mehta I, Gaymes CH. Closure of external tunnel Fontan fenestration: A novel use of the amplatzer vascular plug. Pediatr Cardiol. 2009;30:15–9. doi: 10.1007/s00246-008-9268-2. [DOI] [PubMed] [Google Scholar]
- 12.Tuite DJ, Kessel DO, Nicholson AA, Patel JV, McPherson SJ, Shaw DR. Initial clinical experience using the amplatzer vascular plug. Cardiovasc Interv Radiol. 2007;30:650–4. doi: 10.1007/s00270-007-9044-3. [DOI] [PubMed] [Google Scholar]
- 13.Farra H, Balzer DT. Transcatheter occlusion of a large pulmonary arteriovenous malformation using the amplatzer vascular plug. Pediatr Cardiol. 2005;26:683–5. doi: 10.1007/s00246-004-0857-4. [DOI] [PubMed] [Google Scholar]
- 14.Peirone AR, Spillman A, Pedra C. Successful occlusion of multiple pulmonary arteriovenous fistulas using amplatzer vascular plugs. J Invasive Cardiol. 2006;18:E121–3. [PubMed] [Google Scholar]
- 15.Santos CS, Norte A, Ferreira I, Almeida P, Segorbe Luís A, Loureiro M, et al. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations—Embolization with Amplatzer vascular plug. Rev Port Pneumol. 2009;15:331–7. [PubMed] [Google Scholar]
- 16.Hares DL, Tometzki AJ, Martin R. Use of the amplatzer vascular occluder to occlude large venous vessels in adults and children with congenital heart disease: A case series. Catheter Cardiovasc Interv. 2007;69:33–9. doi: 10.1002/ccd.20921. [DOI] [PubMed] [Google Scholar]
- 17.Aranzulla TC, Cosgrave J, La Canna G, Maisano F, Montorfano M, Sangiorgi G, et al. Percutaneous treatment of periprosthetic mitral valve leaks: Is it just a futile exercise? Ann Thorac Surg. 2008;86:996–8. doi: 10.1016/j.athoracsur.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Kim SK, Chun HJ, Choi BG, Lee HG, Bae SH, Choi JY. Transcatheter venous embolization of a massive hepatic arteriovenous shunt complicating hepatocellular carcinoma using an Amplatzer Vascular Plug. Jpn J Radiol. 2011;29:156–60. doi: 10.1007/s11604-010-0516-z. [DOI] [PubMed] [Google Scholar]
- 19.Evans WN, Galindo A, Acherman RJ, Rothman A, Berthoty DP. Congenital portosystemic shunts and amplatzer vascular plug occlusion in newborns. Pediatr Cardiol. 2009;30:1083–8. doi: 10.1007/s00246-009-9501-7. [DOI] [PubMed] [Google Scholar]
- 20.Hill SL, Hijazi ZM, Hellenbrand WE, Cheatham JP. Evaluation of the AMPLATZER vascular plug for embolization of peripheral vascular malformations associated with congenital heart disease. Catheter Cardiovasc Interv. 2006;67:113–9. doi: 10.1002/ccd.20555. [DOI] [PubMed] [Google Scholar]
- 21.Delaney JW, Fletcher SE. Patent ductus arteriosus closure using the Amplatzer vascular plug II for all anatomic variants. Catheter Cardiovasc Interv. 2013;81:820–4. doi: 10.1002/ccd.24707. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan B, Ward C, Justo R. Reconfiguration of the Amplatzer vascular plug II 5 months after occlusion of venovenous collateral in a bidirectional cavopulmonary circulation. Catheter Cardiovasc Interv. 2010;75:857–60. doi: 10.1002/ccd.22459. [DOI] [PubMed] [Google Scholar]


