Abstract
Aims
Intensive glycaemic control increases the incidence of hypoglycaemia. We sought to define the effects of hypoglycaemia on aldosterone, a hormone involved in cardiovascular injury and baroreflex impairment.
Methods
To contrast the effects of hypoglycaemia and euglycaemia on aldosterone and plasma renin activity, in Study 1, we assessed hormone levels in 13 subjects who participated in euglycaemic (5.0 mmol/l) and hypoglycaemic (2.8 mmol/l) hyperinsulinaemic clamp protocols in random order. To determine the relationship between aldosterone and the depth of hypoglycaemia, in Study 2, we assessed hormone levels in an additional 13 subjects who participated in a 3-h stepped hypoglycaemic hyperinsulinaemic clamp protocol; blood glucose was reduced in 0.55 mmol/l steps from 5.0 to 2.2 mmol/l. Subjects were healthy and consumed controlled sodium diets.
Results
In Study 1, aldosterone increased approximately 2.5-fold during hypoglycaemic hyperinsulinaemia, P < 0.001, but did not rise with euglycaemic hyperinsulinaemia. Plasma renin activity increased during both hyperinsulinaemic clamps; however, the increase was greater during hypoglycaemia (Δ = 1.5 ± 0.2 ng ml−1 h−1) vs. euglycaemia (Δ = 0.5 ± 0.1 ng ml−1 h−1), P < 0.005. In Study 2, aldosterone increased significantly at glucose levels of 2.8 mmol/l; this increase was amplified with glucose of 2.2 mmol/l. Aldosterone increases paralleled those of ACTH.
Conclusions
Hypoglycaemia increases aldosterone in a dose-dependent fashion. This increase is likely attributable to activation of the renin-angiotensin-aldosterone system and increases in ACTH. Because aldosterone activation of the mineralocorticoid receptor is implicated in the pathophysiology of cardiovascular injury, including vascular dysfunction, inflammation, baroreflex impairment and cardiac arrhythmias, these findings may be of relevance in individuals who experience hypoglycaemia.
Keywords: aldosterone, autonomic nervous system, hypoglycaemia
Introduction
Recent large-scale, prospective studies, in the outpatient (1) and the intensive care setting (2), demonstrate an increased risk of mortality in patients with hyperglycaemia randomized to highly intensive glycaemic control. The mortality increase was not attributed directly to hypoglycaemia; however, the substantial increase in the incidence of hypoglycaemia with intensive glycaemic control raises the concern that factors associated with insulin-induced hypoglycaemia may have adverse clinical effects and could potentially be implicated in the pathophysiology of the increased mortality.
Aldosterone is an important factor in the pathophysiology of inflammation, vascular injury, cardiac arrhythmias, hypertension, stroke, cardiac disease and renal disease (3,4). In addition, elevated aldosterone levels are an independent predictor of major in-hospital adverse events and mortality in patients with a recent myocardial infarction (5,6). Further, large, multi-centre randomized trials demonstrate that blockade of the mineralocorticoid receptor reduces cardiovascular morbidity and mortality in patients with chronic heart failure (4) and in patients with an acute myocardial infarction complicated by heart failure (7).
Aldosterone is primarily regulated by the renin–angiotensin–aldosterone system which is activated by hypovolaemia, hypotension and low dietary sodium intake. The effects of insulin-induced hypoglycaemia on the renin-angiotensin-aldosterone system are not well defined. Specifically, plasma renin activity is increased by an insulin infusion under euglycaemic conditions (8,9), but it is not known if it is further increased by an insulin infusion in the presence of hypoglycaemia. Similarly, the relative effects on aldosterone of an insulin infusion in the presence of euglycaemia or. hypoglycaemia are not established. To date, the only studies examining the effects on aldosterone of insulin-induced hypoglycaemia used bolus administration of insulin that results in variable insulin levels and hypoglycaemia of varying depth and duration (9-15).
To define the effects of insulin and hypoglycaemia on plasma renin activity and aldosterone, we performed hyperinsulinaemic hypoglycaemic clamps to carefully control the insulin level and depth and duration of hypoglycaemia in healthy individuals. We contrasted the effects of hypoglycaemic and euglycaemic hyperinsulinaemic clamps on plasma renin activity and aldosterone in healthy subjects on controlled sodium diets. We determined the time course of increases in aldosterone relative to the onset of hypoglycaemia and the effect of prolonged hypoglycaemia on aldosterone levels. In addition, to determine the effect of increasing depths of hypoglycaemia on aldosterone levels, we examined the dose–response relationship between glucose levels and aldosterone using a stepped hypoglycaemic hyperinsulinaemic clamp.
Patients and methods
Subjects were recruited through advertisements in local newspapers and from electronic postings. All subjects underwent a medical history and physical examination at screening. Subjects with abnormal blood or urine chemistries, body mass index (BMI) above 29.9 kg/m2, reported use of steroids within the past year, reported use of oestrogen or progesterone within the past 4 months, post-menopausal status or current medical problems were excluded. Subjects discontinued use of prescription and non-prescription medications 2 weeks prior to the studies, and the use of acetaminophen, alcohol or caffeine was prohibited for 48 h prior to admission. Written informed consent was obtained for all subjects. The Institutional Review Boards for Brigham and Women’s Hospital and Beth Israel Deaconess Medical Center approved the study protocols.
To contrast the effects of hypoglycaemia and euglycaemia on plasma renin activity and aldosterone in subjects receiving a continuous insulin infusion, in Study 1, we assessed aldosterone levels in 13 healthy men and women who participated in euglycaemic (5.0 mmol/l) hyperinsulinaemic and hypoglycaemic (2.8 mmol/l) hyperinsulinaemic clamp protocols, separated in time by 4 weeks and in random order (16). To determine the relationship between aldosterone and the depth of hypoglycaemia, in Study 2, we assessed aldosterone levels in an additional 13 healthy women who had participated in a stepped hypoglycaemic hyperinsulinaemic clamp protocol; blood glucose was decreased from 5.0 to 2.2 mmol/l by 0.55 mmol/l every 30 min (17).
All subjects consumed a controlled, isocaloric diet for a minimum of 3 days prior to studies. In Study 1, subjects consumed a moderate sodium diet (125 mmol per day). In Study 2, subjects consumed a low sodium diet (10 mmol per day), which increases baseline activity of the renin-angiotensin-aldosterone system. Subjects were admitted to the Brigham and Women’s Hospital General Clinical Research Center and underwent hyperinsulinaemic clamp studies in the morning after fasting overnight in the supine position. During the clamp studies, subjects received a primed continuous intravenous infusion of insulin (80 mU/m2 body surface area/min); Novolin R (Novo Nordisk, Princeton, NJ, USA) was infused for 135 min for Study 1 and Humulin R (Eli Lilly and Co., Indianapolis, IN, USA) was infused for 180 min for Study 2. Blood was withdrawn through an indwelling intravenous catheter placed in a retrograde fashion near the wrist of a hand resting in a warm box (66–70° C) and assayed for serum glucose levels every 5 min and, for insulin, ACTH, epinephrine and aldosterone every 15–30 min. Blood for plasma renin activity was obtained at the beginning and end of the Study 1 clamps. Serum was analysed for glucose (Beckman Glucose Analyzer II; Beckman, Brea, CA, USA), insulin (Access High Sensitive Insulin Immunoassay; Beckman Coulter) and aldosterone (Coat-A-Count Aldosterone RIA; Siemens, Los Angeles, CA, USA). Plasma was assayed for ACTH (DiaSorin ACTH IRMA; DiaSorin, Stillwater, MN, USA) and epinephrine (2 CAT RIA kit; Immuno Biological Laboratories, Minneapolis, MN, USA). Plasma renin activity was determined by the in vitro generation of angiotensin I (DiaSorin PRA RIA; DiaSorin). Urine was collected for 24 h for measurement of sodium and creatinine (COBAS Integra 400; Roche Diagnostics, Indianapolis, IN, USA).
Statistical analysis
The hormonal response to hypoglycaemia as compared with euglycaemia was analysed using repeated measures analysis of variance. The main effects were treatment (hypoglycaemia and euglycaemia) and time (Study 1) and time (Study 2). Treatment differences in response by time were assessed as interactions between treatment and time. Measures that were not repeated were analysed using the Student two-tailed t-test. A P-value less than 0.05 was considered statistically significant. Data are expressed as the mean ± standard error of the mean (SEM).
Results
Study 1
Thirteen healthy subjects (27 ± 2 years; 5 males) completed both the euglycaemic (5.0 mmol/l) and hypoglycaemic (2.8 mmol/l) hyperinsulinaemic clamp studies. Subjects had an average BMI of 23.9 ± 0.5 kg/m2, resting heart rate of 63 ± 2 bpm, systolic blood pressure of 105 ± 3 mmHg and diastolic blood pressure of 68 ± 2 mmHg at baseline.
Glucose levels were maintained at a target level of 2.8 mmol/l (median 2.82 mmol/l, range 2.56–2.93 mmol/l) during the final 105 min of the hypoglycaemic clamp and 5.0 mmol/l (median 4.96 mmol/l, range 4.69–5.06 mmol/l) during the euglycaemic clamp. Insulin levels were similar during the hypoglycaemic (751 ± 54 pmol/l) and euglycaemic (712 ± 22 pmol/l) clamps. Baseline levels of plasma renin activity and aldosterone were similar at the start of the hypoglycaemic and euglycaemic clamp studies (Fig. 1b and c) and 24-h urinary sodium levels were similar between studies averaging 129 ± 12 mmol/24 h.
Figure 1.
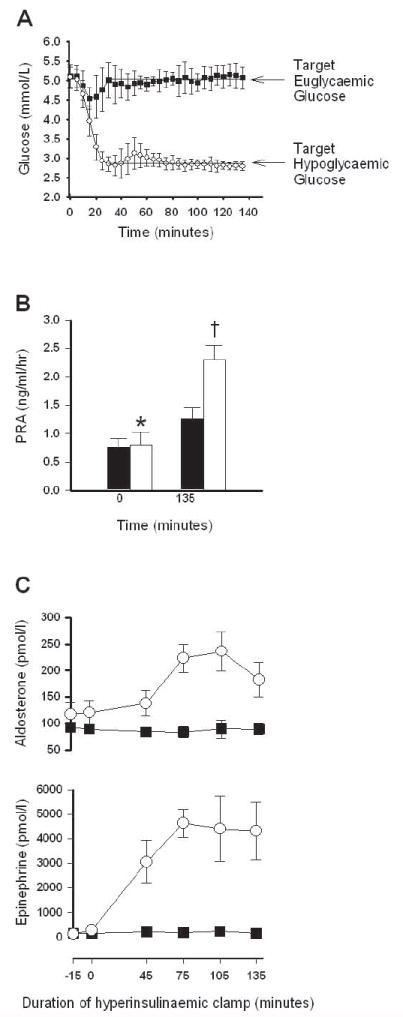
(a) Serum glucose levels during euglycaemic hyperinsulinaemic clamp studies (closed squares) and during hypoglycaemic hyperinsulinaemic clamp studies (open circles). Arrow indicates target glucose of 5.0 mmol/l for the euglycaemia protocol and 2.8 mmol/l for the hypoglycaemia protocol. (b) Plasma renin activity (PRA) levels at baseline (T = 0 min) and end (T = 135 min) of the euglycaemic (black bars) and hypoglycaemic (open bars) hyperinsulinaemic clamp studies. *P < 0.05 vs. baseline euglycaemia; †P < 0.001 vs. all other groups; n = 8 per group. (c) Aldosterone (n = 12) and epinephrine (n = 5) levels during the hypoglycaemic hyperinsulinaemic clamp (open circles) and euglycaemic hyperinsulinaemic clamp (black squares), P < 0.001 for aldosterone and P < 0.0001 for epinephrine by repeated measures ANOVA.
Plasma renin activity levels were elevated at the end of both the euglycaemic and hypoglycaemic hyperinsulinaemic clamps (Fig. 1b). However, the plasma renin activity increase over baseline was three times greater during hypoglycaemia (Δ = 1.5 ± 0.2 ng ml−1 h−1) as compared with euglycaemia (Δ=0.5 ± 0.1 ng ml−1 h−1), P < 0.005. There was a significant increase in aldosterone levels during the hypoglycaemic clamp as compared with the euglycaemic clamp (Fig. 1c). Aldosterone levels remained elevated throughout the hypoglycaemic clamp but did not change during the euglycaemic clamp. The counterregulatory hormone epinephrine also increased during hypoglycaemia but not euglycaemia.
Study 2
Thirteen healthy pre-menopausal women (40 ± 2 years) completed the stepped hypoglycaemic hyperinsulinaemic clamp protocol. Subjects had an average BMI of 24.4 ± 1.2 kg/m2, resting heart rate of 71 ± 6 bpm, systolic blood pressure of 120 ± 3 mmHg and diastolic blood pressure of 77 ± 1.9 mmHg.
During the insulin infusion, insulin levels averaged 975 ± 57 pmol/l. Glucose levels were decreased from a baseline of 5.0 to 2.2 mmol/l in decrements of 0.55 mmol/l (Fig. 2a). Baseline levels of plasma renin activity and aldosterone were 2.0 ± 0.4 ng ml−1 h−1 and 417 ± 57 pmol/l, respectively, consistent with subjects consuming a low sodium diet; 24-h urinary sodium was 22 ± 3 mmol/24 h. There were no significant increases in aldosterone levels between target glucoses of 5.0 and 3.3 mmol/l. Further decreases in glucose to 2.8 and 2.2 mmol/l led to glucose-level-dependent increases in aldosterone, with aldosterone levels reaching 1314 ± 69 pmol/l at the end of the hypoglycaemic clamp (Fig. 2). ACTH rose with hypoglycaemia (Fig. 2).
Figure 2.
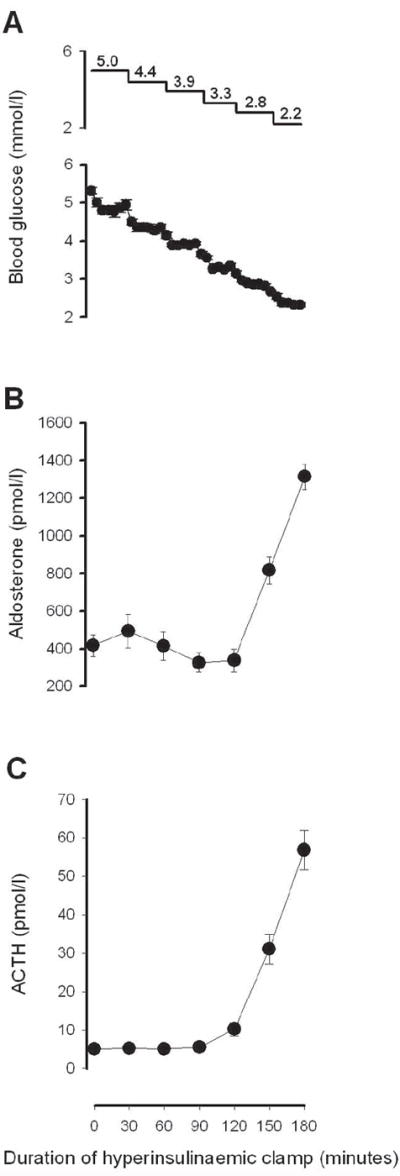
(A) Serum glucose, (B) aldosterone and (C) ACTH levels during the 180-min stepped, hypoglycaemic, hyperinsulinaemic clamp study, n = 13; P < 0.001 for each hormone by repeated measures ANOVA. ACTH values were reported previously (17). Upper panel in (a) indicates target blood glucose levels.
Discussion
Our physiologic studies, contrasting the effects of hypoglycaemic and euglycaemic hyperinsulinaemic clamps under controlled dietary sodium intake in healthy subjects, clearly demonstrated that hypoglycaemia increases plasma rennin activity and aldosterone independently from hyperinsulinaemia. The rise in aldosterone was dependent on the depth of hypoglycaemia and was sustained at least for the duration of hypoglycaemia. As aldosterone mediates adverse cardiovascular effects (3, 4-7), these findings in healthy subjects may have implications for the pathophysiology underlying the mortality and morbidity associated with hypoglycaemia (1,2).
Hyperinsulinaemia in the presence of hypoglycaemia resulted in an increase in plasma renin activity that was three times higher than during euglycaemic hyperinsulinaemia. Increases in plasma renin activity under hyperinsulinaemic euglycaemic conditions have been attributed to activation of the sympathetic nervous system by insulin, insulin-mediated vasodilation and/or insulin-induced reduction in sodium chloride delivery to the macula densa (8,18). The further increase in plasma renin activity with hypoglycaemia is likely mediated through two mechanisms involving the sympathetic nervous system. Hypoglycaemia is a powerful activator of central sympathetic nervous system outflow, resulting in increased renal sympathetic nervous activity that leads to activation of β1-adrenoreceptors on juxtaglomerular cells that stimulates renin secretion (19). In addition, hypoglycaemia increases circulating epinephrine levels. This rise in epinephrine, which is a central feature of the counterregulatory response to hypoglycaemia, also activates renal β1-adrenoreceptors.
Two likely mechanisms for the increase in aldosterone with hypoglycaemia are activation of the renin-angiotensin-aldosterone system and activation of the hypothalamic-pituitary-adrenal axis with ACTH stimulating aldosterone production (10,11,15).
Acutely ill patients in the intensive care unit commonly have an activated rennin-angiotensin-aldosterone system as a result of hypotension and/or hypovolaemia. In Study 2, we used a low sodium diet to activate the renin-angiotensi-aldosterone system in the healthy subjects. Under these conditions, hypoglycaemia led to marked elevations in serum aldosterone. Acute infusions of angiotensin II or aldosterone in humans lead to attenuation of baroreflex sensitivity in healthy subjects, while angiotensin type 1 (AT1) receptor antagonism and angiotensin-converting enzyme inhibition restores attenuated baroreceptor sensitivity (20-22). Levels of aldosterone similar to those observed during hypoglycaemia have been associated with decreases in baroreflex sensitivity in healthy subjects receiving aldosterone infusions (23). The baroreflex mediates cardiovascular circulatory homeostasis and impaired baroreflex sensitivity is a predictor of mortality in individuals who experienced a recent myocardial infarction, possibly attributable to increased arrhythmias (24,25). We previously showed that baroreflex sensitivity is attenuated in healthy subjects more than 12 h after exposure to two 90-min periods of hypoglycaemia (2.8 mmol/l) (16). The mechanism whereby baroreflex function is attenuated after antecedent hypoglycaemia is not known. The present data raise the possibility that activation of the renin-angiotensin-aldosterone system may be a factor.
Aldosterone also increases oxidative stress, inflammation, vascular dysfunction, vascular injury, apoptosis and fibrosis, leading to vascular, cardiac and renal injury (3). Aldosterone increases interleukin-6 and other pro-inflammatory cytokines (26,27) and thus could mediate the increase in these factors during hypoglycaemia (28,29). Further, aldosterone activation of the mineralocorticoid receptor causes defective regulation of the cardiac ryanodine receptor and impaired functioning of this receptor is an important cause of the initiation of cardiac arrhythmias (30). In acutely ill patients with a myocardial infarction, high aldosterone levels are associated with an increased mortality and adverse clinical outcomes including ventricular fibrillation (5,6). Furthermore, in patients with acute myocardial infarction and heart failure, blockade of the mineralocorticoid receptor improves cardiovascular mortality and decreases the incidence of sudden death from cardiac causes (7).
Our findings in non-obese, healthy individuals need to be extended to patient populations that experience hypoglycaemia, such as critically ill patients treated with insulin and individuals with diabetes. Nevertheless, our results raise the possibility that hypoglycaemia-induced increases in aldosterone could play a role in the pathophysiology of cardiovascular disease and/or autonomic dysfunction in individuals who experience repeated hypoglycaemic episodes.
Acknowledgments
This work was supported in part by the US Public Health Service, National Institutes of Health grants RO1 DK063296, R01 AR43130 and MO1 RR002635. The authors thank Jackson Chang, Laura Colburn, Marcelo Risk, Rajesh Garg and the Brigham and Women’s Hospital GCRC staff for contributions to this research.
Footnotes
Competing interests Nothing to declare.
References
- 1.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.XXXXX. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 3.Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51:161–167. doi: 10.1161/HYPERTENSIONAHA.107.095489. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 5.Guder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, et al. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964. [DOI] [PubMed] [Google Scholar]
- 6.Beygui F, Collet JP, Benoliel JJ, Vignolles N, Dumaine R, Barthelemy O, et al. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST-elevation myocardial infarction. Circulation. 2006;114:2604–2610. doi: 10.1161/CIRCULATIONAHA.106.634626. [DOI] [PubMed] [Google Scholar]
- 7.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 8.Perlstein TS, Gerhard-Herman M, Hollenberg NK, Williams GH, Thomas A. Insulin induces renal vasodilation, increases plasma renin activity, and sensitizes the renal vasculature to angiotensin receptor blockade in healthy subjects. J Am Soc Nephrol. 2007;18:944–951. doi: 10.1681/ASN.2006091026. [DOI] [PubMed] [Google Scholar]
- 9.Rooney DP, Edgar JD, Sheridan B, Atkinson AB, Bell PM. The effects of low dose insulin infusions on the renin angiotensin and sympathetic nervous systems in normal man. Eur J Clin Invest. 1991;21:430–435. doi: 10.1111/j.1365-2362.1991.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez G, Ganguly A, Brueggemeyer CD. Acute effect of captopril on aldosterone secretory responses to endogenous or exogenous adrenocorticotropin. J Clin Endocrinol Metab. 1988;66:46–50. doi: 10.1210/jcem-66-1-46. [DOI] [PubMed] [Google Scholar]
- 11.Worck RH, Ibsen H, Frandsen E, Dige-Petersen H. AT1 receptor blockade and the sympathoadrenal response to insulin-induced hypoglycemia in humans. Am J Physiol. 1997;272:E415–E421. doi: 10.1152/ajpendo.1997.272.3.E415. [DOI] [PubMed] [Google Scholar]
- 12.Trovati M, Massucco P, Mularoni E, Cavalot F, Anfossi G, Mattiello L, et al. Insulin-induced hypoglycaemia increases plasma concentrations of angiotensin II and does not modify atrial natriuretic polypeptide secretion in man. Diabetologia. 1988;31:816–820. doi: 10.1007/BF00277483. [DOI] [PubMed] [Google Scholar]
- 13.Frier BM, Al Dujaili EA, Corrall RJ, Pritchard JL, Edwards CR. Autonomic neural control mechanisms and the release of adrenal steroids after hypoglycaemia in man. Horm Metab Res. 1984;16:S138–141. doi: 10.1055/s-2007-1014918. [DOI] [PubMed] [Google Scholar]
- 14.Trovati M, Massucco P, Anfossi G, Cavalot F, Mularoni E, Mattiello L, et al. Insulin influences the renin angiotensin aldosterone system in humans. Metabolism. 1989;38:501–503. doi: 10.1016/0026-0495(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 15.Hata S, Kunita H, Okamoto M. Aldosterone response to hypoglycemia: evidence of ACTH mediation. J Clin Endocrinol Metab. 1976;43:173–177. doi: 10.1210/jcem-43-1-173. [DOI] [PubMed] [Google Scholar]
- 16.Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function – implications for rigorous glycemic control. Diabetes. 2009;58:360–366. doi: 10.2337/db08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler GK, Kinsley BT, Hurwitz S, Mossey CJ, Goldenberg DL. Reduced hypothalamic-pituitary and sympathoadrenal responses to hypoglycemia in women with fibromyalgia syndrome. Am J Med. 1999;106:534–543. doi: 10.1016/s0002-9343(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 18.Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation. 1997;96:4104–4113. doi: 10.1161/01.cir.96.11.4104. [DOI] [PubMed] [Google Scholar]
- 19.DiBona GF. Neural control of the kidney: past, present, and future. Hypertension. 2003;41:621–624. doi: 10.1161/01.HYP.0000047205.52509.8A. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher J, Buch AN, Routledge HC, Chowdhary S, Coote JH, Townend JN. Acute aldosterone antagonism improves cardiac vagal control in humans. J Am Coll Cardiol. 2004;43:1270–1275. doi: 10.1016/j.jacc.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 21.Yee KM, Struthers AD. Aldosterone blunts the baroreflex response in man. Clin Sci (Lond) 1998;95:687–692. doi: 10.1042/cs0950687. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt BM, Horisberger K, Feuring M, Schultz A, Wehling M. Aldosterone blunts human baroreflex sensitivity by a nongenomic mechanism. Exp Clin Endocrinol Diabetes. 2005;113:252–256. doi: 10.1055/s-2005-837650. [DOI] [PubMed] [Google Scholar]
- 23.Monahan KD, Leuenberger UA, Ray CA. Aldosterone impairs baroreflex sensitivity in healthy adults. Am J Physiol Heart Circ Physiol. 2007;292:H190–H197. doi: 10.1152/ajpheart.00622.2006. [DOI] [PubMed] [Google Scholar]
- 24.De Ferrari GM, Sanzo A, Bertoletti A, Specchia G, Vanoli E, Schwartz PJ. Baroreflex sensitivity predicts long-term cardiovascular mortality after myocardial infarction even in patients with preserved left ventricular function. J Am Coll Cardiol. 2007;50:2285–2290. doi: 10.1016/j.jacc.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 25.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. see comments. [DOI] [PubMed] [Google Scholar]
- 26.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, et al. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension. 2006;48:1050–1057. doi: 10.1161/01.HYP.0000248135.97380.76. [DOI] [PubMed] [Google Scholar]
- 28.Dotson S, Freeman R, Failing HJ, Adler GK. Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes Care. 2008;31:1222–1223. doi: 10.2337/dc07-2243. [DOI] [PubMed] [Google Scholar]
- 29.Razavi NL, Kitabchi AE, Stentz FB, Wan JY, Larijani BA, Tehrani MM, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism. 2009;58:443–448. doi: 10.1016/j.metabol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Gomez AM, Rueda A, Sainte-Marie Y, Pereira L, Zissimopoulos S, Zhu X, et al. Mineralocorticoid modulation of cardiac ryanodine receptor activity is associated with downregulation of FK506-binding proteins. Circulation. 2009;119:2179–2187. doi: 10.1161/CIRCULATIONAHA.108.805804. [DOI] [PubMed] [Google Scholar]


