Abstract
Objective
Metabolic syndrome (MetS) is associated with cardiovascular disease (CVD). Insulin resistance has been hypothesized as the underlying feature of MetS. Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) are widely used antihypertensives that may improve insulin sensitivity. The aim of the study is to evaluate the effect of ACEI/ARB on incident CVD events in older hypertensive patients with MetS.
Materials/Methods
We used the Cardiovascular Health Study, a prospective cohort study of individuals > 65 years of age to evaluate ACEI/ARB use and time to CVD events (including coronary and cerebrovascular events). The study included 777 subjects who had hypertension and ATP III-defined MetS, but free of CVD and diabetes at baseline. Cox regression models were used to evaluate the effect of ACEI/ARB as compared to other antihypertensives on the time to the first CVD events.
Results
ACEI/ARB use was associated with a decreased risk of CVD events (adjusted HR=0.658, 95 % C.I. [0.436-0.993]) compared to other antihypertensives. When CVD endpoints were evaluated separately, use of ACEI/ARB was associated with lower rates of angioplasty and coronary events (HR of 0.129 and 0.530 respectively, with 95 % CI [0.017-0.952] and [0.321-0.875]).
Conclusions
ACEI/ARB use was associated with a lower risk of CVD events in older hypertensive patients with MetS, primarily due to a reduction in coronary events. The potential protective effect of ACEI/ARB on CVD events in older individuals with MetS will need further confirmation from prospective studies.
Keywords: angiotensin converting enzyme inhibitor, angiotensin receptor blocker, insulin resistance
Introduction
Metabolic syndrome (MetS), a constellation of metabolic risk factors, increases the risk for diabetes and cardiovascular events [1-3], including cardiovascular disease mortality [4-6], all-cause mortality [4-6], and coronary heart disease mortality [6]. The pathogenesis of MetS is complex and incompletely understood, but obesity and insulin resistance contribute to its development [7].
The National Cholesterol Education Program's (NCEP) Adult Treatment Panel III (ATP) criteria represent the most widely used definition for MetS. MetS, as defined by the ATP III criteria, is estimated to be prevalent in 28% of US adults [8]. The prevalence of MetS increases with age, reaching peak levels in the sixth decade for men and the seventh decade for women [9].
Current pharmacologic management of MetS focuses on the specific risk factors without targeting the underlying insulin resistance [10]. Several lines of evidence suggest that the renin-angiotensin system is both a contributor and target for several risk factors associated with the metabolic syndrome [11]. Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) may improve insulin sensitivity [12-14], decrease the risk of type 2 diabetes [15], improve endothelial function [16], and reduce atherosclerosis and cardiovascular disease risk [17]. Whether ACEI and ARB improve clinical cardiovascular outcomes in hypertensive older patients with MetS is yet to be investigated. The purpose of this study is to evaluate the association between the use of ACEI/ARB and incident cardiovascular events in older adults with hypertension and MetS.
Methods
Data Source
We used data from the Cardiovascular Health Study (CHS), a community-based prospective cohort study conducted by the National Heart Lung and Blood Institute (NHLBI) in adults aged 65 and older, to evaluate risk factors for the development and progression of cardiovascular events. The purpose and design of CHS have been published previously [18]. Briefly, the CHS consisted of over 5800 participants randomly selected from Medicare eligibility lists in four U.S. communities in North Carolina, California, Pennsylvania and Maryland. Data collected included demographics, current medication use, blood pressure, medical history, lifestyle habits, anthropometric measures, fasting blood chemistry, echocardiography, electrocardiography and carotid ultrasonography. For each cardiovascular condition at baseline, data from self-report was confirmed using components of the baseline examination and a validation protocol that included review of medical records and confirmation by treating physicians [19]. University of Vermont's Central Blood Analysis Laboratory analyzed each participant's blood chemistry, which was drawn in the morning after an overnight fast. Further details on laboratory and blood sampling procedures, examinations, and quality assurance protocols have been published previously [18,20-22]. Subjects were followed with annual clinic visits and interim 6-month phone calls for a total of 11 years, followed by telephone follow-ups only from year 11 to 15. For this analysis, we used only the first 11 years of validated event data, as data for cardiovascular events after year 11 were obtained from telephone self report without validation from medical records. The CHS was approved by the University of Washington's Data Coordinating Center and the investigational review boards at all locations. Analysis of CHS data for the purpose of evaluating the association between ACEI/ARB use and incident cardiovascular events was approved by the Virginia Commonwealth University Institutional Review Board.
Inclusion and Exclusion criteria
The subjects included in the present analyses included individuals from CHS who had used any antihypertensive medication during the study. In addition, these subjects met the ATP III criteria for MetS [10]. We excluded subjects with baseline diabetes (defined as having a fasting blood glucose > 126 mg/dl or a 2-hour serum glucose ≥ 200 mg/dl upon an oral glucose tolerance test with 75 gm glucose, or use of diabetes medications). Subjects with a history of cardiovascular events, including myocardial infarction (MI), congestive heart failure (CHF), coronary heart disease, claudication, stroke, transient ischemic attack (TIA), angina and arrhythmia, were also excluded. Individuals with prevalent cardiovascular disease and diabetes were excluded because they were already at risk for cardiovascular events regardless of the presence of MetS. We then classified the subjects based on their exposure to ACEI or ARB during the study. Hence, the exposed group was composed of individuals who had used ACEI/ARB alone or combined with other anti-hypertensives, and the control group represented those who took anti-hypertensives other than ACEI/ARB.
Endpoints
The primary endpoint was defined as the occurrence of any first cardiovascular event, including incident MI, silent MI documented by electrocardiogram, stroke, TIA, angioplasty, coronary artery bypass graft (CABG) procedures, angina, claudication, or death due to coronary heart disease during the 11 years of follow-up. The algorithms for identifying claudication [23], MI [24], stroke [21] and deaths due to coronary disease [24] have been reported previously. Secondary outcomes for this report included investigation of each of the following incident events separately: MI, silent MI, angina, CABG, angioplasty, claudication, stroke, TIA, as well as any coronary events and any cerebrovascular events. Coronary events included MI, CABG, angioplasty, angina, silent MI and deaths due to coronary disease. Cerebrovascular events included stroke and TIA.
Statistical analyses
A Cox hazards model with time dependent covariates was used to analyze the risk of developing cardiovascular events in users of ACEI/ARB compared to non-users, adjusting for potential confounders and possible significant interactions. Important risk factors for cardiovascular events were defined a priori and were evaluated for inclusion in the multivariate model. These risk factors included age, cigarette use, family history of premature coronary heart disease, gender, alcohol use, exercise intensity as assessed by an instrument adapted from the Health Interview Survey [25], aspirin use, body mass index (BMI), LDL and HDL cholesterol levels, triglycerides, race, and income level. These were included as covariates in the multivariate model if their univariate P-values were <0.25, or as a confounder if its inclusion in the multivariate model changed the hazard ratio (HR) estimate by more than 20%. We also included systolic blood pressure and total number of antihypertensive medications used as time-dependent covariates in the preliminary models because blood pressure control itself is a risk factor for cardiovascular events. Development of diabetes and heart failure during follow-up were also included as time-dependent covariates to control for confounding by indications, as ACEI and ARB are commonly used in patients with heart failure and diabetes. Clinically plausible interactions were evaluated, including interactions between ACEI/ARB and age, ACEI/ARB and gender, and ACEI/ARB and race.
Preliminary multivariate models were compared using -2 log likelihood tests before a final multivariate model was constructed. The final model included the following covariates: development of diabetes, CHF, systolic blood pressure, age, gender (male vs. female), smoking status (current, former, never smoker), race (African American vs. other), triglycerides level, and LDL level. Subjects were censored if they did not develop any cardiovascular event during the follow-up period or if they left the study before the full follow-up. We assessed the proportional hazard assumption and the goodness of fit of the multivariable model. P-values ≤ 0.05 was considered statistically significant.
Results
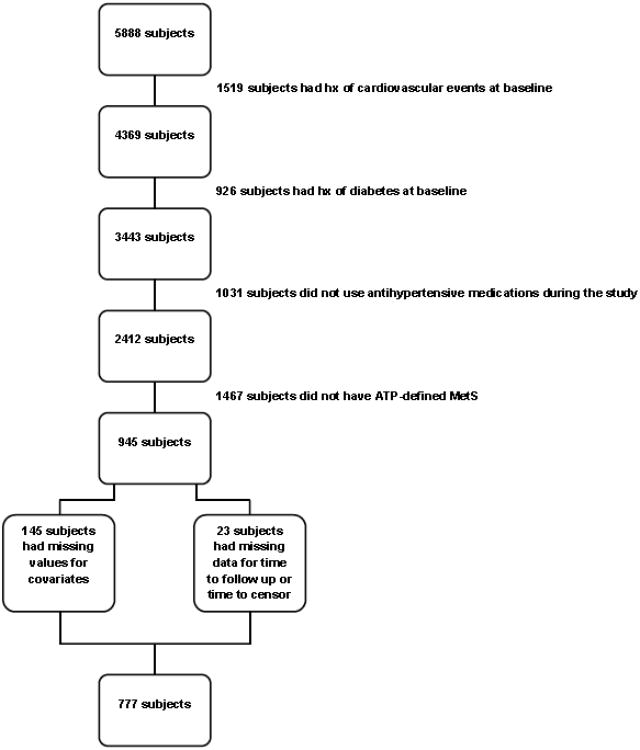
Of the original 5888 subjects enrolled in the CHS dataset, 1519 subjects had a history of cardiovascular events at baseline (including coronary heart disease, CHF, stroke or TIA). Of the remaining 4369 subjects, 3443 were free from diabetes at baseline. As we were interested in the subjects who used antihypertensive medications, we included in the analysis those who used at least one antihypertensive medication during the study (n = 2412). Out of these individuals, 945 had MetS as defined by ATP III at baseline. Twenty-three subjects had missing data for time to follow-up or time to censor, and 145 subjects had missing baseline values for covariates in the multivariable model. Our final sample size was 777 subjects as shown in figure 1. At baseline, 72 out of the 777 subjects (9.3%) were taking ACEI and none of the subjects at entry used ARB. The use of ACEI/ARB increased from baseline until year 11 where 26.1 % used ACEI and/or ARB. The average duration of use of ACEI/ARB was 1.9 years (range 0 to 12 years).
Figure 1. Flow chart showing final sample size after applying inclusion/exclusion criteria.
ATP = Adult Treatment Panel; MetS = metabolic syndrome
Baseline characteristics of subjects were compared between those who took ACEI and/or ARB at baseline and the control group (Table 1). At baseline, there were no statistically significant differences between the 2 groups regarding their age, gender, smoking habits, triglycerides, HDL, LDL levels, BMI, total number of blood pressure medications used, fasting glucose or systolic blood pressure. However, the ACEI/ARB group contained a higher percentage of African Americans (21.0% in the exposed group vs. 11.1% in the control group, P = 0.0065). Rates of use of most antihypertensive medications (thiazide diuretics, potassium sparing diuretics, vasodilators and alpha blockers) were similar between the 2 groups. However, ACEI and/or ARB users were significantly less likely to use beta blockers (5.6% vs. 18.7%, P = 0.0051) but more likely to use loop diuretics (11.1% vs. 4.4%, P = 0.0129) or calcium channel blockers (18.1% vs. 8.7%, P = 0.0096) compared to the control group.
Table 1. Baseline comparison between subjects exposed to ACEI/ARB and the control group.
| Covariate | ACEI/ARB users (N=72) | Control group (N=705) | P -value |
|---|---|---|---|
| Male | 30 (42.0%) | 230 (33.0%) | 0.1214 |
| Smoking | |||
| Never | 29 (40.0%) | 360 (51.0%) | |
| Former | 34 (47.0%) | 262 (37.0%) | 0.1937 |
| Current | 9 (13.0%) | 83 (12.0%) | |
| Race | |||
| White | 56 (78.0%) | 626 (88.8%) | |
| Black | 15 (21.0%) | 78 (11.1%) | 0.0065 |
| Other | 1 (1.0%) | 1 (0.1%) | |
| Triglycerides (mg/dl) | 160.2 (±61.5) | 165.4 (±63.9) | 0.5109 |
| HDL (mg/dl) | 47.6 (±11.5) | 49 (±12.6) | 0.3785 |
| LDL (mg/dl) | 130.0 (±34.9) | 136.2 (±35.0) | 0.1502 |
| Age (years) | 71.8 (±4.4) | 72.5 (±5.0) | 0.2968 |
| BMI (kg/m2)a | 28.7 (±3.8) | 28.6 (±3.9) | 0.9278 |
| Number of antihypertensives | 1.9 (±0.9) | 1.7 (±0.8) | 0.2074 |
| Systolic blood pressure (mmHg) | 143.8 (±21.4) | 140.0 (±20.1) | 0.2120 |
| Fasting glucose (mg/dl) | 105.3 (±7.5) | 104.4 (±8.9) | 0.3737 |
| Frequencies of antihypertensive use at baseline | |||
| Beta blockers | 4 (5.6%) | 132 (18.7%) | 0.0051 |
| Thiazides | 7 (9.7%) | 118 (16.7%) | 0.1228 |
| Loop diuretics | 8 (11.1%) | 31 (4.4%) | 0.0129 |
| K sparing diuretic | 0 (0.0%) | 10 (1.4%) | 0.3091 |
| Calcium channel blocker | 13 (18.1%) | 61 (8.7%) | 0.0096 |
| Vasodilators | 10 (13.9%) | 66 (9.4%) | 0.2180 |
| Alpha blockers | 0 (0.0%) | 28 (4.0%) | 0.0850 |
| Angiotensin receptor blocker | 0 (0.0%) | 0 (0.0%) | |
Data are given as mean (SD) for continuous variables and as numbers (percent %) for categorical variables
BMI calculated as weight in kilograms divided by the square of height in meters
The percentage of subjects with uncontrolled blood pressure over the 11 years of follow up was compared between the ACEI/ARB and control group (Table 2). Over the follow-up period, blood pressure control was not significantly different between those who used ACEI/ARB and those who did not use any of these 2 classes of drugs except for year 3. In year 3, a higher percentage of subjects had uncontrolled blood pressure in the control group. To account for any possible difference in the control of blood pressure between the ACEI/ARB and control groups, systolic blood pressure was included in the model as time dependent variables. We used systolic blood pressure and not other measures of blood pressure in our analysis because systolic blood pressure has been more strongly associated with coronary heart disease than diastolic blood pressure. In addition, elevated systolic blood pressure is common among older individuals, which is our population under study [26,27].
Table 2. Prevalence of uncontrolled blood pressure (> 140/90mmHg) in subjects exposed to ACEI/ARB and the control group over 11 years of follow-up.
| Covariate | ACEI/ARB users (%) | Control group (%) | P -value |
|---|---|---|---|
| Baseline | 56.94 | 62.37 | 0.3719 |
| Year 1 | 95.56 | 92.91 | 0.5057 |
| Year 2 | 88.64 | 92.41 | 0.3820 |
| Year 3 | 38.16 | 55.84 | 0.0038 |
| Year 4 | 47.47 | 56.50 | 0.0975 |
| Year 5 | 54.87 | 58.93 | 0.4289 |
| Year 6 | 49.55 | 59.64 | 0.0516 |
| Year 7 | 51.24 | 60.16 | 0.0739 |
| Year 8 | 53.15 | 61.15 | 0.0881 |
| Year 9 | 56.58 | 64.47 | 0.0817 |
| Year 10 | 50.00 | 56.09 | 0.1800 |
| Year 11 | 58.24 | 61.79 | 0.4265 |
Other important characteristics were also compared during the follow up years between the users of ACEI/ARB and non-users. There were minimal statistically significant differences regarding the percentage of subjects who developed CHF or diabetes over the 11 years of follow up between the ACEI/ARB group and the control group. However, to account for any possible differences, development of CHF and diabetes were assessed for inclusion in the multivariate model as time dependent variables (see Statistical Analyses).
The results of the univariate analyses in determining the risk of incident cardiovascular events are shown in Table 3.
Table 3. Univariate analyses of potential risk factors, including exposure to ACEI/ARB, for incident cardiovascular events.
| Variable | Hazard Ratio (HR) | 95% HR | P-value | |
|---|---|---|---|---|
| Confidence | Limits | |||
| Age (years) | 1.052 | 1.027 | 1.077 | <.0001 |
| Gender (male vs. female) | 2.039 | 1.593 | 2.611 | <.0001 |
| Smoking (former vs. never) | 1.363 | 1.041 | 1.785 | 0.0242 |
| Smoking (current vs. never) | 2.149 | 1.509 | 3.062 | <.0001 |
| Race (black vs. non-black) | 0.621 | 0.379 | 1.019 | 0.0594 |
| Number of alcohol beverages/week | 1.017 | 1.000 | 1.033 | 0.0460 |
| Aspirin use (user vs. non-user) | 1.174 | 0.906 | 1.521 | 0.2246 |
| Exercise intensity level (absent, low, moderate, high) | 1.124 | 0.958 | 1.319 | 0.1506 |
| BMI (kg/m2) | 0.989 | 0.957 | 1.021 | 0.4817 |
| Income level a | 1.030 | 0.965 | 1.098 | 0.3769 |
| Family history of myocardial infarction | 1.013 | 0.778 | 1.319 | 0.9230 |
| Triglycerides (mg/dl) | 1.002 | 1.000 | 1.004 | 0.0400 |
| HDL (mg/dl) | 0.986 | 0.976 | 0.997 | 0.0122 |
| LDL (mg/dl) | 1.001 | 0.998 | 1.005 | 0.4481 |
| Use of ACEI/ARB | 0.782 | 0.523 | 1.168 | 0.2292 |
| Systolic blood pressure (mmHg) | 1.008 | 1.002 | 1.014 | 0.0064 |
| Development of diabetes | 1.030 | 0.454 | 2.335 | 0.9439 |
| Development of CHF | 7.122 | 5.094 | 9.958 | <.0001 |
| Number of antihypertensives | 0.922 | 0.820 | 1.038 | 0.1785 |
Income level is divided into 8 categories: under $5,000, ($5,000-$7,999), ($8,000-$11,999), ($12,000-$15,999), ($16,000-$24,999), ($25,000-$34,999), ($35,000-$49,999), (> $50,000)
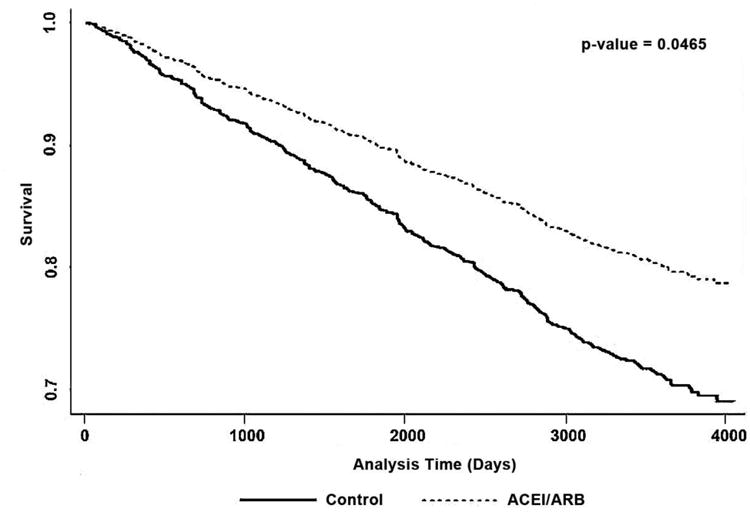
The final model included exposure to ACEI/ARB, development of diabetes, CHF, systolic blood pressure, age, gender (male vs female), smoking status (current, former, never smoker), race (African-American vs other), triglycerides level, and LDL level. We tested for interactions between the use of ACEI/ARB and race, age, and gender; however, none of these interactions showed any statistically significant effects. The final multivariable model is presented in Table 4. In this final model, after adjusting for confounding variables, the use of ACEI or ARB was associated with a reduction in the risk of incident cardiovascular events (HR=0.658, 95 % CI [0.436-0.993], P = 0.0462, Figure 2).
Table 4. Multivariate model for the risk of incident cardiovascular events in ACEI/ARB users vs. non-users.
| Parameter | Hazard Ratio (HR) | 95% HR | P-value | |
|---|---|---|---|---|
| Confidence | Limits | |||
| Use of ACEI/ARB | 0.658 | 0.436 | 0.993 | 0.0462 |
| Development of CHF | 7.566 | 5.312 | 10.775 | <.0001 |
| Systolic blood pressure (mmHg) | 1.007 | 1.001 | 1.013 | 0.0151 |
| Development of diabetes | 1.419 | 0.618 | 3.256 | 0.4093 |
| Age (years) | 1.035 | 1.010 | 1.061 | 0.0065 |
| Gender (male vs. female) | 2.140 | 1.643 | 2.788 | <.0001 |
| Former smoker vs. never | 1.218 | 0.919 | 1.615 | 0.1707 |
| Current smoker vs. never | 2.142 | 1.486 | 3.088 | <.0001 |
| Race (black vs. non-black) | 0.808 | 0.488 | 1.339 | 0.4090 |
| Triglycerides (mg/dl) | 1.003 | 1.001 | 1.005 | 0.0050 |
| LDL (mg/dl) | 1.004 | 1.001 | 1.008 | 0.0153 |
Figure 2. Cox regression survival plot comparing the survival rate (free from incident cardiovascular events) in subjects exposed to ACEI/ARB as compared to the control group.
We also assessed the effect of ACEI/ARB separately on coronary events (incident MI, silent MI, coronary heart disease death, CABG, angioplasty, or angina) and cerebrovascular events (incident stroke and/or TIA). The use of ACEI/ARB had a significant protective effect against the development of coronary events (HR=0.530, 95 % CI [0.321-0.875], P = 0.0130). In particular, when angioplasties were evaluated alone, the ACEI/ARB exposure decreased risk of first angioplasty (HR=0.129, 95% CI [0.017-0.952], P = 0.0446). However, there were no effects on cerebrovascular events (HR=1.173, 95% CI [0.621-2.217], P = 0.6228). These data suggest that the effects of ACEI/ARB may differ between coronary and non-coronary cardiovascular events.
Additionally, we also evaluated the effect of ACEI/ARB on all-cause mortality. The use of ACEI/ARB did not have a significant effect on all-cause mortality in both univariate (HR =1.068, 95% C.I. [0.713-1.600], P = 0.7494) and multivariate models (HR = 1.078, 95% C.I. [0.714-1.629], P = 0.7198).
Sensitivity analyses
We performed sensitivity analyses by including waist circumference and insulin sensitivity in the multivariate model because they are closely related to the metabolic syndrome. Waist circumference did not have a significant association with incident cardiovascular events in univariate (HR=1.010, 95% CI [0.998-1.022]) or multivariate (HR=1.007, 95 % C.I. [0.995-1.020]) models. Inclusion of waist circumference in the multivariate Cox model shows that the hazard ratio for incident cardiovascular events was 0.656 in ACEI/ARB users vs. control (95% C.I. [0.435-0.991], P = 0.0452), which is similar to the estimates without the inclusion of waist circumference. Similarly, insulin sensitivity (by homeostasis model assessment [HOMA]) did not have a significant association with incident cardiovascular events in both univariate (HR=0.953, 95% CI [0.902-1.008]) or multivariate (HR=0.953, 95 % C.I. [0.899-1.010]) analyses. Inclusion of HOMA in the multivariate Cox regression model shows that the hazard ratio for incident cardiovascular events in ACEI/ARB users was 0.660 as compared to the control group (95% CI [0.437-0.996], P = 0.0476), which is similar to the estimate obtained without the inclusion of HOMA as a covariate. Because waist circumference and HOMA were not independently associated with risk of incident cardiovascular events, and their inclusion did not result in a change in the original estimates, the final model did not include waist circumference or HOMA.
To validate the conclusions obtained from the multivariable model, we also tested the effect of using ACEI/ARB on incident cardiovascular events after further adjusting for the concurrent use of other antihypertensives by including them as time dependent variables in the models (Table 5). These models also allowed us to evaluate the effect of each of the following classes of anti-hypertensives: beta blockers, alpha blockers, calcium channel blockers, diuretics and vasodilators on the outcomes. Concurrent use of these other anti-hypertensive classes did not change the magnitude of association between ACEI/ARB and the incidence of cardiovascular events. We found that the use of ACEI/ARB, independent of concurrent use of other antihypertensive class of drugs, was associated with a significant protective effect against the development of cardiovascular events (HR=0.644, 95 % CI [0.426-0.976], P = 0.0379).
Table 5. Effect of different antihypertensives on incident cardiovascular events.
| Parameter | Hazard Ratio (HR) | 95% HR | P-value | |
|---|---|---|---|---|
| Confidence | limits | |||
| Use of ACEI/ARB | 0.644 | 0.426 | 0.976 | 0.0379 |
| Use of beta blockers | 0.864 | 0.609 | 1.226 | 0.4130 |
| Use of CCB | 0.920 | 0.653 | 1.296 | 0.6323 |
| Use of vasodilators | 0.854 | 0.428 | 1.704 | 0.6548 |
| Use of diuretics | 0.965 | 0.737 | 1.263 | 0.7968 |
| Use of alpha blockers | 0.881 | 0.359 | 2.158 | 0.7811 |
| Development of CHF | 7.547 | 5.279 | 10.791 | <.0001 |
| SBP | 1.008 | 1.002 | 1.014 | 0.0103 |
| Development of diabetes | 1.429 | 0.623 | 3.282 | 0.3994 |
| Age | 1.034 | 1.009 | 1.06 | 0.0087 |
| Gender (male vs.female) | 2.142 | 1.642 | 2.795 | <.0001 |
| Former smoker vs. never | 1.209 | 0.911 | 1.604 | 0.1886 |
| Current smoker vs. never | 2.124 | 1.473 | 3.062 | <.0001 |
| Race (black vs. other) | 0.826 | 0.497 | 1.373 | 0.4610 |
| Triglycerides | 1.003 | 1.001 | 1.005 | 0.0046 |
| LDL | 1.004 | 1.001 | 1.008 | 0.0185 |
Discussion
MetS is highly prevalent in older individuals [9] and has been associated with future cardiovascular events [1-3]. ACEIs and ARBs may have beneficial effects on insulin sensitivity [12-14], the major underlying pathophysiologic feature of MetS. Few studies reported the effect of ACEI/ARB in patients with MetS [28-30]. These studies were short-term, and the effects of ACEI/ARB on the clinical cardiovascular endpoints were not assessed. Therefore, we sought to determine whether ACEI/ARB inhibition prevents cardiovascular events in hypertensive older patients with MetS, after excluding those with diabetes and any history of cardiovascular disease at baseline.
In this study, we observed a lower risk of incident cardiovascular events among older hypertensive individuals with MetS who used ACEI/ARB (adjusted HR=0.658, 95% CI [0.436-0.993], P = 0.0462]). Our findings complement those of large randomized controlled trials such as HOPE and EUROPA [17,31], which supported the use of ACEI for secondary prevention of cardiovascular events. HOPE showed a significant reduction in the rate of death, MI, stroke, revascularization, cardiac arrest, and heart failure with the use of an ACEI (ramipril) in patients at high risk for cardiovascular events (age > 55 years old with preexisting coronary disease or equivalent, with at least one other risk factor such as smoking, hypertension, or dyslipidemia). Similarly, EUROPA showed that an ACEI (perindopril) reduced the primary endpoint of cardiovascular death, MI or cardiac arrest in a population with stable coronary heart disease. Both EUROPA and HOPE evaluated a population at higher risk of cardiovascular events (individuals with preexisting coronary heart disease) than our current evaluation (individuals with hypertension and MetS but without diabetes or preexisting coronary disease). To our knowledge, the current study is the first to evaluate this lower-risk population.
The Cox regression survival curves in Figure 2 for individuals taking ACEI/ARB and those using other anti-hypertensives start to separate at one to two years after the start of the follow-up period, and the difference in incident cardiovascular events (survival curves) between the 2 groups increases with time. Although survival benefits seen within such a relatively short time frame may seem like a chance finding, our results are congruent with the findings of HOPE, where Kaplan-Meier curves for the ramipril and placebo groups started to separate between the first and second years of follow up, and continued to diverge during the 4.5 years of follow-up. In the HOPE Study Extension (HOPE-TOO), cardiovascular benefits of ramipril were maintained during an additional 2.6 years of post-trial follow-up, regardless of baseline risk or other concomitant treatments [32]. This suggests that treatment with ramipril in HOPE may have prolonged beneficial effects. The survival curves in Figure 2 corroborate findings in HOPE-TOO, and suggest that exposure to ACEI/ARBs may have extended benefits. However, this will need to be further evaluated in future studies.
When coronary and cerebrovascular events were evaluated separately, we found a significant reduction in risk of coronary events (MI, silent MI, coronary heart disease death, CABG, angioplasty, or angina) with the use of ACEI/ARB (adjusted HR=0.530, 95% CI [0.321-0.875], P = 0.013]). However, the risk of developing cerebrovascular events was not different between users and non-users of ACEI/ARB. This suggests that ACE/ARB's effects might be different between coronary and non-coronary cardiovascular events.
One possible reason that ACEI/ARB do not have beneficial effects on cerebrovascular accidents in this study is that MetS according to the ATP-III definition may not be a strong predictor of stroke and/or TIA risk [33-35]. This may explain the lack of effect in our study consisting only of individuals with MetS based on the ATP-III definition. Another reason may be that regardless of the presence of MetS, ACEI/ARB may not be protective against the risk of cerebrovascular events, as was previously reported [36]. Sub-analysis by race in the LIFE study, for example, showed that African-American participants treated with losartan were actually at higher risk for stroke events than African-American individuals who received atenolol [36]. The analysis of several double-blinded randomized controlled trials have also suggested that the use of ACEI might not be protective against stroke and may be associated with greater risk for stroke [37].
Although it is encouraging that ACEI/ARB may be protective against cardiovascular events, and especially coronary events, in this cohort of older individuals with hypertension and MetS, we should interpret our findings in light of the strengths and weaknesses of the study. The strengths of our study include the long duration of follow-up, reliable recording of cardiovascular events, prospective documentation of cardiovascular risk factors and medication assessments, which occurred at baseline and at annual follow-ups. Study limitations include the relatively small sample size after applying the inclusion and exclusion criteria on the total number of participants. In addition, residual confounding may be present. For example, there may be unmeasured systematic differences between patients prescribed ACEI/ARB and those who were not. It is possible that the healthcare providers selected ACEI or ARB for patients who were at increased risk of developing cardiovascular disease, such as patients with risk of developing diabetes and heart failure. Although we have adjusted for the development of diabetes and heart failure in our final model by including them as time-dependent variables to control for confounding by indication bias, it is still possible that participants who received ACEI/ARB may have received a different level of care as compared to participants who did not. In addition, the proportion of subjects taking ACEI/ARBs is small, which reflects the practice pattern at the time of observation. Despite the smaller sample, use of ACEI/ARBs was significantly associated with a lower incidence of cardiovascular events, even after adjusting for other risk factors. Another limitation is that some patients may have changed their lifestyle (smoking habits, exercise intensity, number of alcohol beverages consumed per week) during the study and this could not be accounted for in the analysis. Finally, the results of this study may not be generalizable to patients younger than 65 years as only older subjects were included in CHS.
Our results are translatable to the care of older patients with hypertension and MetS. ACEI/ARBs are the preferred treatment for blood pressure control in patients with diabetes [38]. Whether this class of drug is also first-line for hypertensive patients with MetS is unknown. To the authors' knowledge, this current report is the first to address this knowledge gap. Pending validation from prospective clinical trials, ACEI/ARBs may become the preferred treatment for hypertension management in patients with MetS.
In summary, the results of the present study show that use of ARBs or ACEIs may be associated with decreased risk of cardiovascular events, particularly coronary events, in hypertensive older subjects with MetS. Our results require validation from prospective clinical trials.
Acknowledgments
None
Funding: This work was supported in part by National Institutes of Health Grant K23HD049454 (to K.I.C) and Fulbright Scholarship (to H.H.Z.). The Cardiovascular Health Study (CHS) was conducted and supported by the NHLBI in collaboration with the CHS investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the CHS or the NHLBI.
List of abbreviations
- NCEP
National Cholesterol Education Program
- ATP
Adult Treatment Panel
- MetS
metabolic syndrome
- ACEI
angiotensin converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- CHS
Cardiovascular Health Study
- NHLBI
National Heart Lung and Blood Institute
- MI
myocardial infarction
- CHF
congestive heart failure
- TIA
transient ischemic attack
- CABG
coronary artery bypass graft
- HR
hazard ratio
- BMI
body mass index
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- LIFE
Losartan Intervention for Endpoint Reduction
- EUROPA
European Trial on Reduction of Cardiovascular Events with Perindopril in Stable Coronary Artery Disease
- HOPE
Heart Outcomes Prevention Evaluation
Footnotes
Disclosure statement: There is no relevant conflict of interest to be disclosed.
Author contributions: H.H.Z. collected and analyzed data, and drafted the manuscript. K.I.C. designed the study, obtained funding, supervised data collection and analysis, and finalized the manuscript. S.E.H., D.P.M. and P.W.S. contributed to data interpretation and analyses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonora E, Kiechl S, Willeit J, et al. Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck study. Diabetes Care. 2003;26(4):1251–1257. doi: 10.2337/diacare.26.4.1251. [DOI] [PubMed] [Google Scholar]
- 2.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28(2):385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 3.McNeill AM, Katz R, Girman CJ, et al. Metabolic syndrome and cardiovascular disease in older people: The cardiovascular health study. J Am Geriatr Soc. 2006;54(9):1317–1324. doi: 10.1111/j.1532-5415.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 4.Hunt KJ, Resendez RG, Williams K, et al. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110(10):1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 5.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 6.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 7.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am. 2004;33(2):283–303. doi: 10.1016/j.ecl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27(10):2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 9.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163(4):427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 11.Putnam K, Shoemaker R, Yiannikouris F, et al. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302(6):H1219–H1230. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogari R, Zoppi A, Preti P, et al. Differential effects of ACE-inhibition and angiotensin II antagonism on fibrinolysis and insulin sensitivity in hypertensive postmenopausal women. Am J Hypertens. 2001;14(9 Pt 1):921–926. doi: 10.1016/s0895-7061(01)02140-9. [DOI] [PubMed] [Google Scholar]
- 13.Gans RO, Bilo HJ, Nauta JJ, et al. The effect of angiotensin-I converting enzyme inhibition on insulin action in healthy volunteers. Eur J Clin Invest. 1991;21(5):527–533. doi: 10.1111/j.1365-2362.1991.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 14.Lind L, Pollare T, Berne C, et al. Long-term metabolic effects of antihypertensive drugs. Am Heart J. 1994;128(6 Pt 1):1177–1183. doi: 10.1016/0002-8703(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 15.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369(9557):201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 16.Kishi T, Hirooka Y, Konno S, et al. Angiotensin II receptor blockers improve endothelial dysfunction associated with sympathetic hyperactivity in metabolic syndrome. J Hypertens. 2012;30(8):1646–1655. doi: 10.1097/HJH.0b013e328355860e. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 19.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 20.Cushman M, Cornell ES, Howard PR, et al. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41(2):264–270. [PubMed] [Google Scholar]
- 21.Price TR, Psaty B, O'Leary D, et al. Assessment of cerebrovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(5):504–507. doi: 10.1016/1047-2797(93)90105-d. [DOI] [PubMed] [Google Scholar]
- 22.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(4):358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 23.Newman AB, Naydeck BL, Sutton-Tyrrell K, et al. The role of comorbidity in the assessment of intermittent claudication in older adults. J Clin Epidemiol. 2001;54(3):294–300. doi: 10.1016/s0895-4356(00)00308-5. [DOI] [PubMed] [Google Scholar]
- 24.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 25.Siscovick DS, Fried L, Mittelmark M, et al. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997;145(11):977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease. The Framingham study. Am J Cardiol. 1971;27(4):335–346. doi: 10.1016/0002-9149(71)90428-0. [DOI] [PubMed] [Google Scholar]
- 27.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153(5):598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 28.Anichkov DA, Shostak NA, Schastnaya OV. Comparison of rilmenidine and lisinopril on ambulatory blood pressure and plasma lipid and glucose levels in hypertensive women with metabolic syndrome. Curr Med Res Opin. 2005;21(1):113–119. doi: 10.1185/030079904x20277. [DOI] [PubMed] [Google Scholar]
- 29.Bitkin EC, Boyraz M, Taskin N, et al. Effects of ACE Inhibitors on Insulin Resistance and Lipid Profile in Children with Metabolic Syndrome. J Clin Res Pediatr Endocrinol. 2013;5(3):164–169. doi: 10.4274/Jcrpe.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan BV, Sola S, Lauten WB, et al. Quinapril, an ACE inhibitor, reduces markers of oxidative stress in the metabolic syndrome. Diabetes Care. 2004;27(7):1712–1715. doi: 10.2337/diacare.27.7.1712. [DOI] [PubMed] [Google Scholar]
- 31.Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362(9386):782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 32.Bosch J, Lonn E, Pogue J, et al. Long-term effects of ramipril on cardiovascular events and on diabetes: results of the HOPE study extension. Circulation. 2005;112(9):1339–1346. doi: 10.1161/CIRCULATIONAHA.105.548461. [DOI] [PubMed] [Google Scholar]
- 33.Chen XY, Thomas GN, Chen YK, et al. Atherosclerotic vascular disease rather than metabolic syndrome predicts ischemic stroke in diabetic patients. Cerebrovasc Dis. 2010;30(4):374–379. doi: 10.1159/000319570. [DOI] [PubMed] [Google Scholar]
- 34.Jia Z, Wu S, Zhou Y, et al. Metabolic syndrome and its components as predictors of stroke in middle-aged and elderly Chinese people. Neurol Res. 2011;33(5):453–459. doi: 10.1179/016164111X13007856083882. [DOI] [PubMed] [Google Scholar]
- 35.Vinluan CM, Zreikat HH, Levy JR, et al. Comparison of different metabolic syndrome definitions and risks of incident cardiovascular events in the elderly. Metabolism. 2012;61(3):302–309. doi: 10.1016/j.metabol.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siragy HM. Comparing angiotensin II receptor blockers on benefits beyond blood pressure. Adv Ther. 2010;27(5):257–284. doi: 10.1007/s12325-010-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fournier A, Messerli FH, Achard JM, et al. Cerebroprotection mediated by angiotensin II: a hypothesis supported by recent randomized clinical trials. J Am Coll Cardiol. 2004;43(8):1343–1347. doi: 10.1016/j.jacc.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 38.Wu HY, Huang JW, Lin HJ, et al. Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: systematic review and bayesian network meta-analysis. BMJ. 2013;347:f6008. doi: 10.1136/bmj.f6008.:f6008. [DOI] [PMC free article] [PubMed] [Google Scholar]