Abstract
Embryos of the annual killifish Austrofundulus limnaeus acquire extreme tolerance of anoxia during embryonic development. These embryos can survive environmental and cellular conditions that would likely result in death in the majority of vertebrate cells, despite experiencing a massive loss of ATP. It is highly likely that the initial response to anoxia must quickly alter cellular physiology in order to reprogram cell signaling and metabolic pathways to support anaerobiosis. Covalent protein modifications are a mechanism that can quickly act to effect large-scale changes in protein structure and function and have been suggested by others to play a key role in mammalian ischemia tolerance. Using western blot analysis, we explored patterns of protein ubiquitylation and SUMOylation in embryos of A. limnaeus exposed to anoxia and anoxic preconditioning. Surprisingly, we report stage-specific protein ubiquitylation patterns that suggest different mechanisms for altering protein turnover in dormant and actively developing embryos that both survive long-term anoxia. Anoxic preconditioning does not appear to alter levels of ubiquitin conjugates in a unique manner. Global SUMOylation of proteins does not change in response to anoxia, but there are stage-specific changes in SUMOylation of specific protein bands. Contrary to other systems, global changes in protein SUMOylation may not be required to support long-term tolerance of anoxia in embryos of A. limnaeus. These data lead us to conclude that embryos of A. limnaeus respond to anoxia in a unique manner compared to other vertebrate models of anoxia tolerance and may provide novel mechanisms for engineering vertebrate tissues to survive long-term anoxia.
Keywords: Diapause, metabolic dormancy, metabolism, anoxia tolerance, preconditioning
Introduction
Embryos of the annual killifish Austrofundulus limnaeus exhibit the greatest tolerance of anoxia of any vertebrate and can survive for months in the complete absence of oxygen at 25°C (Podrabsky et al. 2012b; Podrabsky et al. 2007). The mechanisms that regulate the cellular physiology supporting this extreme tolerance of anoxia are currently unknown. Transitions into anoxia will quickly compromise cellular energetic status, and thus at least some of the mechanisms that support tolerance of anoxia must be fast and efficient. Post-translational modification of proteins is a fast and relatively energy-efficient way to quickly and reversibly regulate protein function. The enzymatic addition of small proteins such as ubiquitin and the small ubiquitin-like modifiers (SUMO) is one general mechanism that can drastically alter protein function. Recent evidence suggests that global changes in patterns of ubiquitylation and SUMOylation may be associated with induction of endogenous protective mechanisms in response to ischemia or oxygen and glucose deprivation in mammals(Lee and Hallenbeck 2013; Meller 2009). In this study, patterns of protein ubiquitylation and SUMOylation are explored in response to anoxia and anoxic preconditioning in developing and diapausing embryos of A. limnaeus.
The biology of Austrofundulus limnaeus
Austrofundulus limnaeus inhabits ephemeral ponds in northern Venezuela (Hrbek et al. 2005). This extreme environment regularly exposes the adults and embryos to extremes in oxygen, temperature, and pH (Podrabsky et al. 1998). Embryos are deposited into the muddy pond substrate and thus likely spend a large portion of development exposed to extreme hypoxia or anoxia (Podrabsky et al. 1998). Populations of A. limnaeus survive in this harsh environment through the production of stress-tolerant diapausing embryos that can survive for months in the complete absence of liquid water and oxygen (Podrabsky et al. 2001; Podrabsky et al. 2007; Podrabsky et al. 2012b). Diapause may occur at three distinct stages (Wourms 1972). Diapause I occurs early in development prior to formation of the embryonic axis. Diapause II occurs midway through development just prior to organogenesis in an embryo that has the foundations of the central nervous system, and a functional tubular heart. Diapause III occurs in a late-prehatching embryo. Extreme tolerance of anoxia is acquired during early development and peaks during diapause II (Podrabsky et al. 2007). Importantly, this extreme tolerance is retained for up to 4 days of post-diapause II development. However, by the time development is complete, extreme tolerance of anoxia is lost. Embryos with extreme tolerance of anoxia do not respond to anoxic preconditioning, while later stage embryos that have lost the extreme tolerance of anoxia can be induced to survive harmful anoxia longer if given a sublethal preconditioning bout of anoxia (Podrabsky et al. 2012b).
Exposure to anoxia leads to a profound state of metabolic depression in embryos of A. limnaeus as evidenced by a complete cessation of cardiac activity (Fergusson-Kolmes and Podrabsky 2007), and a rapid decrease in heat dissipation (Podrabsky et al. 2012a). The vast majority of the anoxic cells arrest in the G1 phase of the cell cycle (Culpepper and Podrabsky 2012; Meller et al. 2012). Strikingly, ATP levels plummet by 80% during the initial few hours of anoxia, leading to a large increase in AMP (Podrabsky et al. 2012a). Anaerobic metabolism is supported by accumulation of lactate, succinate, and GABA (Podrabsky et al. 2007). Thus, cells of these embryos can survive cellular and environmental conditions that would be lethal to most vertebrate cells, despite using the same basic metabolic machinery to support anaerobiosis. Thus, there are likely novel mechanisms at play supporting extreme anoxia tolerance in this species.
Ubiquitin and SUMO protein modifications associated with oxygen deprivation
Ubiquitin, a 9 kDa protein, is as its name suggests, a ubiquitous protein modifier present in eukaryotic cells (Meller 2009; Kerscher et al. 2006; Ciechanover et al. 2000). Ubiquitin regulates several protein-protein interactions including protein translocation, signal transduction, gene transcription, and cell signaling associated with apoptosis and autophagy (Mukhopadhyay and Riezman 2007). However, ubiquitin is best known for its role in the proteasomal degradation process (Ciechanover et al. 1984; Ciechanover et al. 2000; Ciechanover 1994). The ubiquitin-proteasome system is the major cellular pathway for degradation and recycling of proteins in a cell. Regulation of protein degradation through this pathway is thought to be critical in supporting tolerance of cell stress and supporting metabolic dormancy in a number of animal models (Hand and Hardewig 1996; Carey et al. 2003).
A number of studies have demonstrated an increase in protein ubiquitylation following harmful ischemic conditions in rat brain (Liu et al. 2004; Liu et al. 2005). Preconditioning the brain with a low dose of ischemia, resulted in a reduction in protein ubiquitylation following a subsequent and normally harmful ischemic episode (Liu et al. 2005). In addition, proteasome inhibitors have been shown to be protective in models of stoke (Williams et al. 2003; Williams et al. 2004; Wojcik and Di Napoli 2004). In contrast, a preconditioning dose of ischemia has been shown to increase protein ubiquitylation in rapid ischemic tolerance models, and blocking the proteasome blocks rapid ischemic tolerance (Meller et al. 2008). Thus, the role of ubiquitylation in tolerance of oxygen deprivation is likely context dependent, and different amounts of cellular stress may alter the role of protein ubiquitylation in mediating cell survival.
A family of proteins related to ubiquitin, the “small ubiquitin-like modifier” or SUMO proteins can be added to other proteins to regulate a variety of processes including gene expression, chromatin structure, signal transduction, nuclear transport, cell signaling, plasma membrane depolarization, and maintenance of the genome (Hay 2005; Johnson 2004; Kerscher et al. 2006; Scheschonka et al. 2007; Wilson and Rosas-Acosta 2005). Transcription factors are the main targets for SUMOylation and conjugation with SUMO proteins typically results in down-regulation of gene expression (Girdwood et al. 2004). There may be up to four SUMO paralogues in vertebrates, SUMO-1 through SUMO-4 (Cimarosti et al. 2008). In mammalian systems, SUMO-4 is thought to be localized mainly in the kidneys (Bohren et al. 2004), while the other three are found in the brain (Cimarosti et al. 2008). SUMO-2 and SUMO-3 share 96% homology and no difference in function has yet been identified (Hay 2005). SUMO-1 shares only 45% homology with SUMO-2 and SUMO-3 and has distinct immunoreactivity.
The activity and presence of SUMO-1 and SUMO-2/3 differ in response to cellular stress events. Under non-stressful conditions in mammalian cells, there is very little free SUMO-1 and a large pool of free SUMO-2/3 (Saitoh and Hinchey 2000). Cellular stress such as production of reactive oxygen species, osmotic shock, or heat shock, causes an increase in SUMO-2/3 conjugation to proteins, suggesting that SUMO-2/3 may play a role in regulating protein activity in response to cellular stress (Saitoh and Hinchey 2000).
A body of evidence is accumulating that implicates SUMOylation of proteins with a protective phenotype in models of ischemic preconditioning (Lee et al. 2007; Scheschonka et al. 2007; Yang et al. 2008b; Ja Lee et al. 2009; Datwyler et al. 2011; Wang et al. 2012; Lee and Hallenbeck 2013). For example, an increase in protein SUMO-1-ylation in the brains of Thirteen-lined ground squirrels has been reported during the torpor phase of hibernation, suggesting that SUMO-1-ylation may be a mechanism for neuroprotection during periods of otherwise lethally low levels of oxygen and glucose (Lee et al. 2007). In contrast to these studies, increased SUMO-2/3 conjugation has been reported following harmful brain ischemia, using both focal and global ischemia stroke models (Yang et al. 2008a, b). Increased SUMO-2/3 conjugation has been reported following harmful ischemia that was reduced when cells were preconditioned with brief ischemia prior to the harmful ischemic insult (Loftus et al. 2009). Interestingly, Loftus et al. also reported that the SUMO-2/3-ylation was strongest during the harmful ischemic challenge when ATP levels were depleted, rather than in the recovery phase following ischemia. It is therefore unclear whether protein SUMO-2/3-ylation is protective or simply a consequence of a harmful cellular event, i.e. an “insult marker.”
The aim of this study is to explore the possible role of protein ubiquitylation and SUMOylation in regulating the survival of embryos of A. limnaeus exposed to anoxia and anoxic preconditioning. We hypothesized that ubiquitin conjugation (presumably for targeting proteins to the proteasome) following anoxic treatment would not change in embryos with extreme tolerance of anoxia, but would increase in embryos that have lost this trait. Additionally, we hypothesized that anoxic preconditioning would dampen an increase in ubiquitin conjugates in embryos more sensitive to anoxia but known to respond to anoxic preconditioning. With respect to SUMOylation, we hypothesized that levels of SUMO-1 and SUMO-2/3 protein conjugates would increase in response to anoxia in embryos with extreme tolerance of anoxia and that anoxic preconditioning would lead to a significant increase in SUMOylation. Surprisingly, we report a complex stage-specific pattern of ubiquitin conjugation associated with exposure to anoxia that suggests different regulatory mechanisms in dormant versus actively developing embryos. In addition, we find few changes in levels of protein SUMOylation associated with exposure to anoxia or anoxic preconditioning. These data lead us to conclude that embryos of A. limnaeus respond to anoxia in a unique manner compared to other vertebrate models of anoxia tolerance and may provide novel mechanisms for engineering vertebrate tissues to survive long-term anoxia.
Materials and Methods
Husbandry and collection of embryos
All procedures involving animals were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health under a protocol approved by the PSU Institutional Animal Care and Use Committee (approval number psu12.03.22.2). Adult Austrofundulus limnaeus were cared for as described previously (Podrabsky 1999). Spawning pairs of fish were housed in 9.5 l glass aquaria with 21 tanks connected to a common sump with a total system volume of 190 l. Water temperature ranged from 26-28°C. Ten percent of the system water was changed twice daily. Fish were fed twice daily during the work-week and once daily on the weekends with frozen bloodworms (chironomid larvae, Hikari) or chopped live earthworms. Embryos were collected through natural spawning activity twice weekly as described previously (Podrabsky 1999). Embryos were incubated at 25°C in the dark in constant temperature incubators (Sheldon Manufacturing, Cornelius, OR) until experiments were initiated. Embryos were incubated in 100 × 15 mm plastic Petri dishes at a density of 50 embryos per dish. Embryo incubation medium was composed of dilute salts (1 ppt salinity) that mimic the natural waters inhabited by A. limnaeus (Podrabsky 1999). For the first 4 d of development methylene blue was added to the medium to reduce bacterial and fungal growth. At 4 days post-fertilization (dpf) embryos were subjected to a dilute hypochlorite bleaching protocol to reduce microbial growth (Podrabsky 1999). Following the bleaching protocol embryos were incubated in embryo medium containing 10 μg/l gentamycin. For post-diapause II embryos, diapause II was broken experimentally by exposing embryos for 48 hr to a long day photoperiod (14 hr light, 10 hr dark) at 30°C after which they were returned to incubation at 25°C in the dark. A large percentage of the embryos break diapause II within 2-3 d of this treatment. Embryos were inspected daily following the treatment and sorted into synchronously developing groups of post-diapause II embryos.
Embryonic stages chosen for study
Three different embryonic stages were chosen for study (Table 1) to represent dormant (diapause II) and active (4 days post-diapause II, dpd) embryos with extreme tolerance of anoxia, and active embryos (12 dpd) with reduced tolerance of anoxia that respond to anoxic preconditioning (Meller et al. 2012). Three independent sample sets (spawning events) were prepared for each of the developmental stages. A matched normoxic control sample was prepared for each set of anoxic samples at t = 0 for the anoxic exposures.
Table 1.
Three developmental stages of A. limnaeus were exposed to long-term anoxia for one-half of the time to reach 50% mortality.
| dpf1 | dpd2 | Stage | Anoxia LT503 | 0.5LT504 | Precond5 |
|---|---|---|---|---|---|
| 24 | 0 | Diapause II | 42 | 21 | no |
| 4 | Early organogenesis | 65 | 32 | no | |
| 12 | Late organogenesis | 6 | 3 | yes |
days post-fertilization
days post-diapause II
Lethal time to 50% mortality in days at 25°C (Podrabsky et al. 2007; Podrabsky et al. 2012b)
days of long-term exposure to anoxia used in this study
response of embryos to anoxic preconditioning
Exposure to anoxia
Embryos were exposed to anoxia in a Bactron III anaerobic chamber (Sheldon Manufacturing, Cornelius, OR) that uses positive pressure to maintain an atmosphere of 5% H2, 5% CO2, and 90% N2 gas. Residual oxygen was removed from the chamber through the formation of water from hydrogen and oxygen via the action of a palladium catalyst. Anoxic embryo medium was created by bubbling embryo medium containing 10 μg/l gentamycin sulfate with N2 gas for 20-30 min and then allowing the medium to equilibrate inside the anaerobic chamber for 24 hr. Two different protocols were used to expose embryos to anoxia (Figure 1). First, all three stages were exposed to a single bout of long-term anoxia equal to one half of the lethal time to 50% mortality (LT50) for each embryonic stage followed by 24 hr of aerobic recovery from anoxia. In addition, embryos at 12 dpd were exposed to an anoxic preconditioning regime prior to a bout of long-term anoxia (Figure 1).
Figure 1. Protocol for exposing embryos to anoxia and anoxic preconditioning.
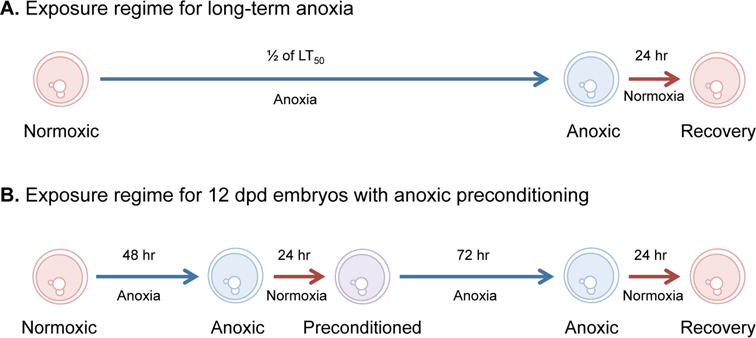
(A) Long-term anoxia was defined as one half of the lethal time to 50% mortality (LT50) for each embryonic stage investigated. Times of exposure for each developmental stage are presented in Table 1. (B) Anoxic preconditioning in embryos at 12 days post-diapause II (dpd) was defined as exposure to 48 hr of anoxia followed by 24 hr of aerobic recovery. Embryos at this stage were exposed to 72 hr of anoxia with and without anoxic preconditioning.
Separation of embryonic cells from yolk
Embryos (50-100) were dissociated into cell suspensions mechanically by using a flat-bottom glass pestle to mash the embryos through a 500 μm nylon mesh (24 mm Netwell™, Corning Product #3480) into a 50 mm plastic Petri dish containing 2 ml of phosphate buffered saline (PBS) and protease inhibitor (Roche Complete Mini, #11836153001). Following dissociation, cell suspensions were kept on ice at all times. Cell suspensions were transferred to a 1.7 ml microcentrifuge tube and pelleted by centrifugation at 300 × g for 5 min. The cells were rinsed and pelleted two times in PBS with protease inhibitor to remove yolk proteins. After the final rinse all the supernatant was removed and the cell pellet was snap-frozen in liquid nitrogen. Cell pellets were stored at -80°C until the day of analysis.
Preparation of embryo lysates
Embryo lysates were prepared by lysing the cells in a buffer (100 mM NaCl, 20 mM Tris-HCl, pH 7.6, 1 mM EDTA, pH 8.0, 1% NP-40) containing protease inhibitors (0.99 μg/ml aprotinin, 0.3 μg/ml leupeptin, 1.5 μg/ml pepstatin and 15 μg/ml PMSF and a phosphatase inhibitor cocktail (15 μg/ml, Sigma P2850) used as prescribed by the manufacturer. Lysates were sonicated at 15% amplitude for 10 to 15 seconds or until homogenized (Branson Digital Sonifier, S-450D). Total protein was determined using a Bradford Protein Assay kit according to the manufacturer's instructions (Bio-rad #500-0205). Absorbance was quantified using a Molecular Devices Spectramax plate reader.
Immunoblot analysis of SUMO-1, SUMO-2/3 and ubiquitylated proteins
Lanes were loaded with equal amounts of protein (10 μg for ubiquitin blots and 25 μg for SUMO-1 and SUMO-2/3 blots). Lysates were heated for 5 min in a 100°C water bath, loaded on a 10% SDS-polyacrylamide gel, and subjected to electrophoresis (115mV for ∼2 hours). Proteins were transferred onto PVDF membranes (30V, overnight). Membranes were blocked with 5% nonfat dried milk in Tris-buffered saline with 0.1% Tween (TBST solution) for 30 min at room temperature on an orbital shaker. After blocking, the membranes were incubated on a rotator with primary antibody in 5% nonfat dried milk/TBST solution in a sealed plastic bag for 2 hr at room temperature. The SUMO-1 (diluted 1:1000) and SUMO-2/3 (diluted 1:2000) primary antibodies (both rabbit polyclonal) were provided by R. Meller (Loftus et al. 2009). The ubiquitin primary antibody (diluted 1:5000) was obtained commercially (Santa Cruz #8017, mouse monoclonal). Following incubation in primary antibody, the membranes were washed four times in TBST for 5-10 min at room temperature on an orbital shaker. For secondary antibody incubation, membranes were incubated as described above with HRP-conjugated secondary antibodies diluted in 5% nonfat dried milk/TBST solution. An anti-rabbit IgG secondary antibody diluted 1:2000 was used for both SUMO-1 and SUMO-2/3 detection (Cell Signaling #7074), while ubiquitin was detected using an anti-mouse IgG secondary antibody (Cell Signaling #7076).
Antigen binding was determined by chemiluminescence (Visualizer™ Western Blot Detection Kit, Millipore #64-201). Chemiluminescence was detected and quantified using a digital image station (Kodak image station 2000RT) and analyzed with 1D 3.6 software. Protein levels (intensity) were normalized across blots using a common sample loaded onto each gel, and all protein levels are expressed relative to this common sample. Thus the data presented represent relative levels of protein modifications across an equal amount of protein for each developmental stage.
Data presentation and statistics
Graphs were prepared and statistical analyses were performed using GraphPad Prism 5.0 software. Statistical significance was set at p < 0.05 for all comparisons. When appropriate Student Newman-Keuls post-hoc comparison test was used to compare individual means. Percentage data were transformed using either a log or square root transformation as appropriate prior to statistical analysis.
Results
Developmental patterns of normoxic protein ubiquitylation
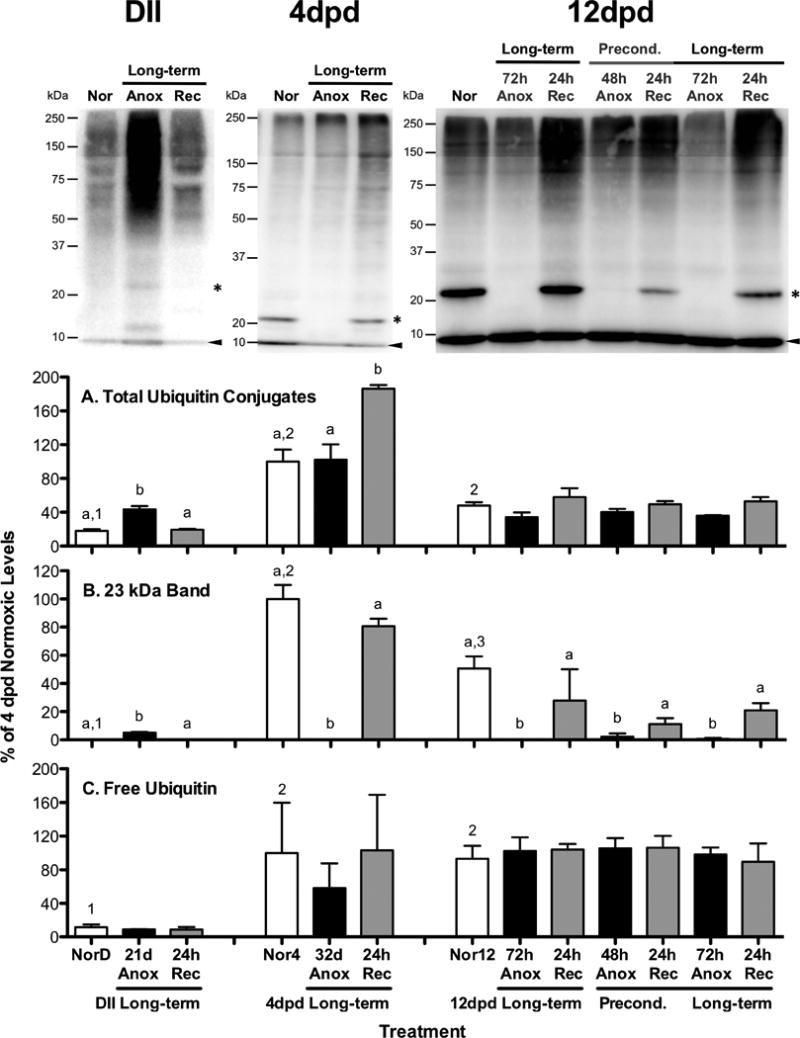
Normoxic diapause II embryos have significantly less total ubiquitin-conjugated proteins than both 4 and 12 dpd embryos (Fig 2A; ANOVA, Student Newman-Keuls, p < 0.01). A ubiquitylated protein band around 23 kDa is absent in normoxic diapause II embryos but is present in normoxic post-diapause II embryos (Fig 2B; ANOVA, Student Newman-Keuls, p < 0.01). In addition, free ubiquitin levels are extremely low in diapause II embryos compared to post-diapause II embryos (Fig 2C; ANOVA, Student Newman-Keuls, p < 0.03).
Figure 2. Ubiquitin conjugate levels in A. limnaeus embryos exposed to anoxia.
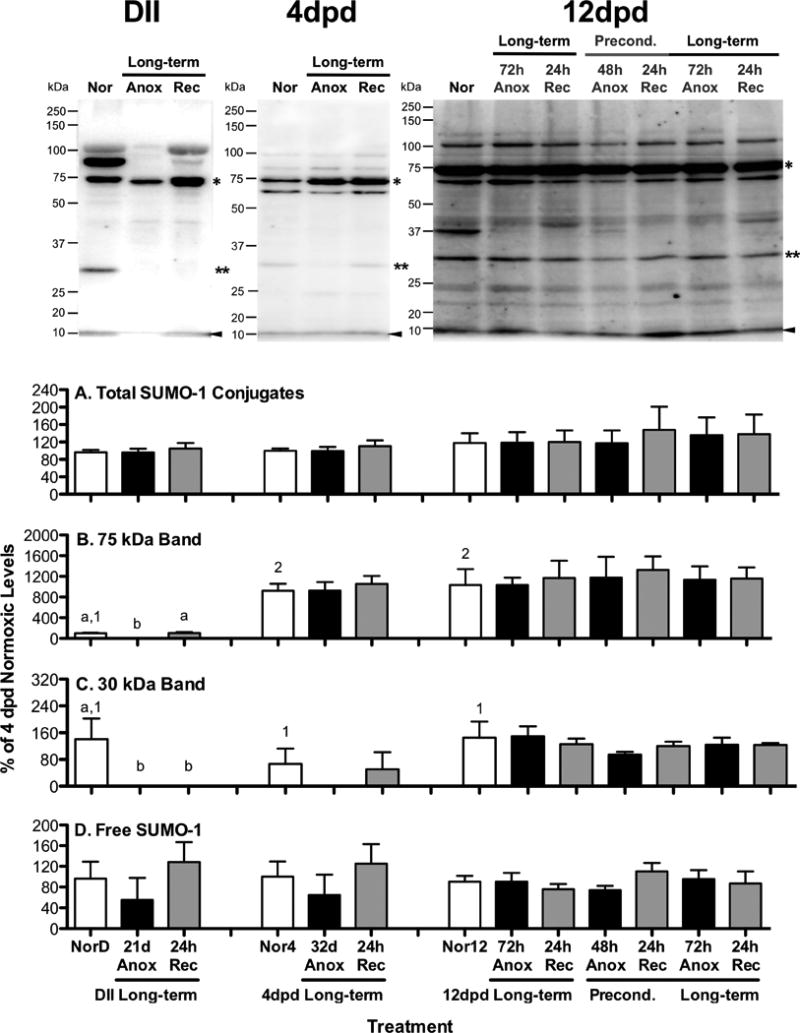
Representative blots for each embryonic stage investigated (DII = diapause II, dpd = days post-diapause II) are presented at the top of the figure. Bar charts represent quantification of ubiquitin conjugated proteins. Total ubiquitin conjugates were quantified (A) between 20 and 250 kDa. The 23 kDa band quantified (B) is denoted with an asterisk in the gels, while the free ubiquitin band (C) is marked with an arrowhead in the gels. Each lane of each gel was loaded with 10 μg total protein. All quantifications were normalized between blots to a control sample loaded onto each gel and expressed as a percentage of the average levels in 4 dpd normoxic embryos. Bars represent means ± S.E.M. (n=3). Different letters above bars denote means that are statistically different (Student Newman-Keuls, p < 0.05, see text for details) between treatments within each developmental stage. Different numbers above normoxic bars indicate means that are statistically different between different developmental stages under normoxic conditions (Student Newman-Keuls, p < 0.05, see text for details). NorD = normoxic diapause II embryos; Nor4 = normoxic embryos for 4 dpd embryos; Nor12 = normoxic 12 dpd embryos. Times for exposure to long-term anoxia are equal to one half of the LT50 for anoxia in days as detailed in Table 1.
Ubiquitylation of proteins in response to long-term anoxia
Total ubiquitin-conjugated proteins increased significantly from normoxic values following long-term anoxic treatment in diapause II and 4 dpd embryos (Fig 2A). For diapause II embryos, ubiquitin-conjugates accumulated during the 21 d anoxic exposure and were cleared after 24 hr of aerobic recovery (Fig 2A; ANOVA, Student Newman-Keuls, p < 0.001). In contrast, embryos at 4 dpd did not accumulate ubiquitin conjugated proteins during 32 d of anoxia, but rather during the 24 hr of aerobic recovery from anoxia (Fig 2A; ANOVA, Student Newman-Keuls, p < 0.01). In the 12 dpd embryos, ubiquitin conjugates actually decrease by nearly 30% during anoxia (ANOVA, p = 0.047) compared to normoxic samples, although post-hoc tests were unable to determine which means are actually different (Fig 2A). Anoxic preconditioning appears to have no unique effect on levels of total ubiquitin conjugates compared to exposure to direct long-term anoxia (compare levels in long-term treated versus preconditioned embryos in Fig 2A). Free ubiquitin levels are not affected by exposure to anoxia in any of the developmental stages investigated (Fig 2C).
Levels of a ubiquitylated 23kDa protein with interesting expression patterns were quantified (Fig 2B). In the diapause II samples the band was below the level of detection in the normoxic embryos but was visible after 21 d of anoxia (Fig 2B; ANOVA, Student Newman-Keuls p < 0.001). Upon 24 h of aerobic recovery this band once again disappeared. In 4 and 12 dpd embryos the opposite pattern of expression was observed and the band present in normoxic embryos dropped below detection levels after 32 d of anoxia in 4 dpd embryos (Fig 2B; ANOVA, Student Newman-Keuls, p < 0.001) and after 72 h of anoxia in 12 dpd embryos (Fig 2B; ANOVA, Student Newman-Keuls, p < 0.01). Recovery from anoxia for 24 h was sufficient to return levels of ubiquitylation of this protein to normoxic levels in both post-diapause II developmental stages. Anoxic preconditioning doesn't appear to statistically alter the levels of ubiquitylation of the 23 kDa protein band.
SUMO-1 protein modifications associated with normoxic development
Amounts of total SUMO-1 conjugated proteins are similar in all three developmental stages under normoxic conditions (Fig 3A; ANOVA, p = 0.56). However, the specific pattern of SUMO-1 conjugates is unique in all three developmental stages. For instance, a 75 kDa protein band is low in diapause II embryos compared to both post-diapause II developmental stages (Fig 3B; ANOVA, Student Newman-Keuls, p < 0.004). Levels of free SUMO-1 were similar in all three developmental stages (Fig 3D; ANOVA, p = 0.99).
Figure 3. SUMO-1 conjugate levels in A. limnaeus embryos exposed to anoxia.
Representative blots for each embryonic stage investigated (DII = diapause II, dpd = days post-diapause II) are presented at the top of the figure. Bar charts represent quantification of SUMO-1 conjugated proteins. Total SUMO-1 conjugates (A) were quantified between 20 and 250 kDa. The 75 kDa band quantified (B) is denoted by a single asterisk in the gel images, while the 30 kDa band quantified (C) is marked by a double asterisk in the gel images. The free SUMO-1 band quantified (D) is identified by an arrowhead in the representative gel images. Each lane of each gel was loaded with 20 μg total protein. All quantifications were normalized between blots to a control sample loaded onto each gel and expressed as a percentage of the average levels in 4 dpd normoxic embryos. Bars represent means ± S.E.M. (n=3). Different letters above bars denote means that are statistically different (Student Newman-Keuls, p < 0.05, see text for details) between treatments within each developmental stage. Different numbers above normoxic bars indicate means that are statistically differrent between different developmental stages under normoxic conditions (Student Newman-Keuls, p < 0.05, see text for details). NorD = normoxic diapause II embryos; Nor4 = normoxic embryos for 4 dpd embryos; Nor12 = normoxic 12 dpd embryos. Times for exposure to long-term anoxia are equal to one half of the LT50 for anoxia in days as detailed in Table 1.
SUMO-1 protein modifications in response to anoxia
There were no significant changes in total SUMO-1 protein modifications in response to exposure to or recovery from anoxia or anoxic preconditioning in any of the developmental stages investigated (Fig 3A; ANOVA, p > 0.56). Similarly, levels of free SUMO-1 were unaffected by treatment with anoxia or anoxic preconditioning (Fig 3D; ANOVA, p > 0.34).
Protein bands at 75 and 30 kDa appear to respond to anoxic exposure uniquely in embryos with high tolerance of anoxia (Fig 3B,C). The 30 kDa band was observed in roughly equal abundance in normoxic embryos for all three stages examined (Fig 3C; ANOVA, p = 0.37). In embryos with long-term anoxia tolerance, the band disappeared completely after exposure to long-term anoxia, although due to sample variability and likely a low sample size its disappearance was only statistically significant in the diapause II samples (Fig 3B; ANOVA, Student Newman-Keuls, p < 0.001 for diapause II and p = 0.8 for 4 dpd embryos). While this band was never observed in anoxic 4 dpd embryos, it was observed after 24 hr of aerobic recovery from anoxia in 1 of the 3 4 dpd samples. In the 12 dpd embryo samples there was no significant change in the levels of this SUMO-1 conjugate associated with exposure to anoxia or anoxic preconditioning (Fig 3C; ANOVA, p = 0.77).
A SUMO-1 conjugate band in the 75 kDa range is a dominant feature in blots from all three developmental stages, with post-diapause embryos expressing significantly higher amounts during normoxic development (Fig 3B; ANOVA, Student Newman-Keuls, p < 0.01). Long-term anoxia causes a significant decline in the levels of this conjugate in diapause II embryos (Fig 3B; ANOVA, Student Newman-Keuls, p < 0.001) while post-diapause II embryo conjugate levels are unaffected by exposure to anoxia (Fig 3B; 4 and 12 dpd embryos, ANOVA, p > 0.8) or anoxic preconditioning (Fig 3B; 12 dpd embryos, p = 0.99). Additionally, there is a SUMO-1 conjugate band between 75 and 100 kDa bands that is strongly expressed during normoxia in diapause II embryos that disappears in response to anoxia (compare MW range in representative gel images, amounts were not quantified). This band does not appear to be present in high amounts in post-diapause II embryos.
SUMO-2/3 protein modifications associated with normoxic development
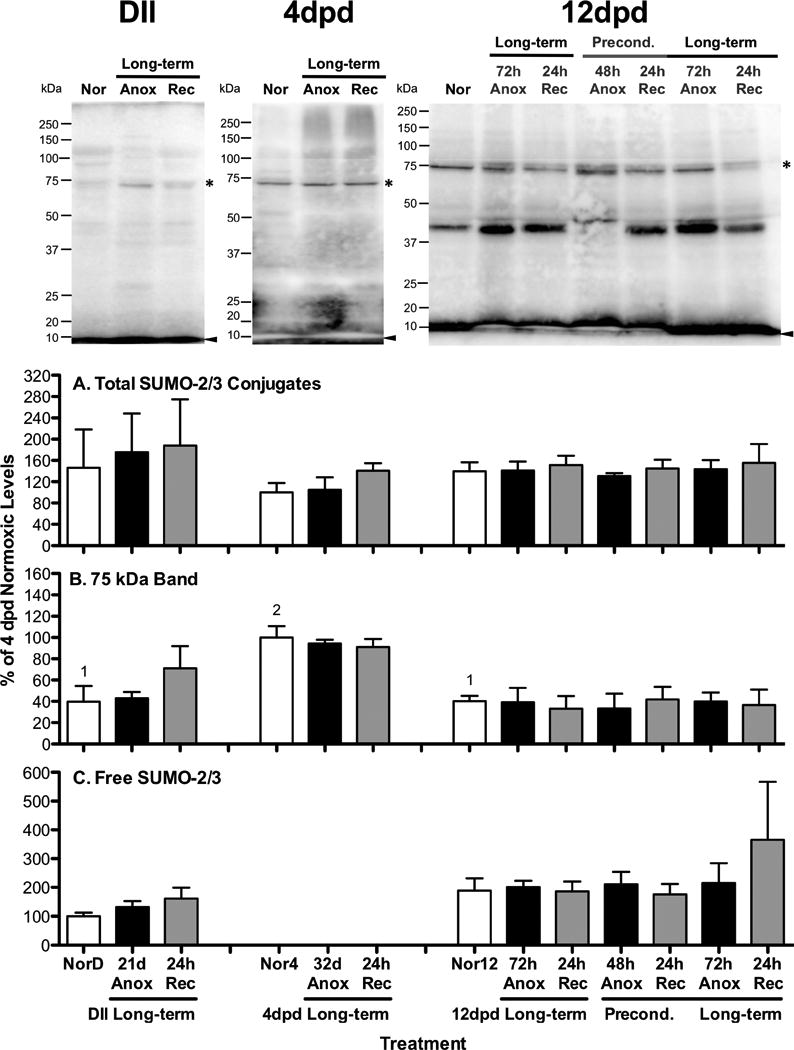
Total and free levels of SUMO-2/3 protein conjugates are similar in all developmental stages investigated (Fig 4A,C; ANOVA, p = 0.72). A characteristically strong band at around 75 kDa is present in significantly higher levels in embryos at 4 dpd than in either diapause II or 12 dpd embryos (Fig 4B; ANOVA, Student Newman-Keuls, p = 0.04).
Figure 4. SUMO-2/3 conjugate levels in embryos of A. limnaeus exposed to anoxia.
Representative blots for each embryonic stage investigated (DII = diapause II, dpd = days post-diapause II) are presented at the top of the figure. Bar charts represent quantification of SUMO-2/3 conjugated proteins. Total SUMO-2/3 conjugates (A) were quantified between 20 and 250 kDa. The 75 kDa band quantified (B) is denoted with an asterisk in the gel images, while free SUMO-2/3 bands (C) are identified by an arrowhead in the gel images. Each lane was loaded with 10 μg total protein. All quantifications were normalized between blots to a control sample loaded onto each gel and expressed as a percentage of the average levels in 4 dpd normoxic embryos. Bars represent means ± S.E.M. (n=3). Different letters above bars denote means that are statistically different (Student Newman-Keuls, p < 0.05, see text for details) between treatments within each developmental stage. Different numbers above normoxic bars indicate means that are statistically differrent between different developmental stages under normoxic conditions (Student Newman-Keuls, p < 0.05, see text for details). NorD = normoxic diapause II embryos; Nor4 = normoxic embryos for 4 dpd embryos; Nor12 = normoxic 12 dpd embryos. Times for exposure to long-term anoxia are equal to one half of the LT50 for anoxia in days as detailed in Table 1.
SUMO-2/3 protein modifications in response to anoxia
Overall levels of total SUMO-2/3 conjugated proteins (Fig 4A) and levels of free SUMO-2/3 (Fig 4C) are unaffected by exposure to anoxia and anoxic preconditioning (ANOVA, p > 0.3). Levels of SUMO-2/3 conjugated proteins in the 75 kDa range do not appear to change in response to anoxia (Fig 4B; ANOVA, p = 0.4).
Discussion
This is the first study to examine patterns of protein modifications associated with embryonic diapause and exposure to anoxia in embryos of A. limnaeus. The experimental design of this study allows the interpretation of protein modifications in response to anoxia, anoxic preconditioning, and dormancy associated with embryonic diapause. The discussion below suggests that protein modifications via ubiquitylation and SUMOylation are likely to play a key role in regulatory events supporting metabolic depression and tolerance of anoxia in this species.
Protein ubiquitylation associated with exposure to anoxia and anoxic preconditioning
Protein ubiquitylation can have manifold effects on protein function such as regulating activity, determining subcellular localization, and targeting proteins for degradation (Meller 2009). Ubiquitylation and ubiquitin-dependent proteolysis play key roles in regulating survival of ischemia and oxygen deprivation in mammalian tissues (Hochrainer et al. 2012; Wojcik and Di Napoli 2004; Meller et al. 2008; Meller 2009; Liu et al. 2004). While ubiquitylation of specific targets can induce endogenous protective mechanisms associated with preconditioning, the accumulation of large quantities of ubiquitin-conjugated proteins is typically associated with cellular damage and often leads to cell death (Meller 2009). In embryos of the brine shrimp Artemia franciscana, ubiquitin conjugation of proteins is arrested during exposure to anoxia (Anchordoguy and Hand 1994, 1995; van Breukelen and Hand 2000). Taken together, these studies suggest that cellular survival of anoxia likely requires a down-regulation of the ubiquitin-dependent proteolysis system (Hand and Hardewig 1996). Patterns of ubiquitin-conjugation in embryos of A. limnaeus exposed to anoxia suggest that there may be multiple ways to regulate ubiquitin-dependent proteolysis during exposure to anoxia. Diapause II embryos accumulate a significant amount of ubiquitin-conjugated proteins during long-term anoxia while 4 dpd embryos do not, yet these embryonic stages both exhibit extreme tolerance of anoxia. Accumulation of ubiquitin-conjugates in diapause II embryos does not necessarily indicate increased protein damage, but rather could represent a steep down-regulation of proteasome activity as has been shown in mammalian hibernators (Van Breukelen and Carey 2002; Velickovska et al. 2005; Velickovska and van Breukelen 2007). Down-regulation of protein degradation would be consistent with prolonged survival in dormancy despite the low levels of protein synthesis exhibited during diapause (Podrabsky and Hand 2000). The lack of accumulation of ubiquitin-conjugates in 4 dpd embryos could represent regulation of proteolysis at the level of protein ubiquitylation in this stage, rather than at the level of the proteasome. In fact, the significant increase in ubiquitin-conjugates during recovery from anoxia may support this theory, as do reports from mammalian systems detailing an increase in ubiquitin conjugates during reperfusion of tissues following ischemia (Hochrainer et al. 2012). Late post-diapause II embryos (12 dpd) respond to anoxia with a significant reduction in levels of total ubiquitin conjugates. This pattern may suggest yet another response to anoxia that includes blockage of protein ubiquitylation, but an incomplete blockage of proteolysis. Levels of total ubiquitin conjugates were similar in long-term anoxic and anoxic preconditioned 12 dpd embryos. Additional studies using inhibitors of the ubiquitin-dependent proteolytic system will be required to better understand the importance of the ubiquitin-conjugation patterns associated with exposure to anoxia in embryos of A. limnaeus.
Interestingly, there was a specific band at around 23 kDa that was consistently absent in anoxic, but present in normoxic post-diapause II embryos. This same protein band is absent in normoxic diapause II embryos. This pattern suggests that diapause II embryos could be pre-adapted for exposure to anoxia. Identification of this small protein modified by ubiquitylation (likely mono-ubiquitylation) may identify a new regulator of anoxia tolerance in these embryos.
Protein ubiquitylation associated with embryonic diapause
A down-regulation in the rate of protein synthesis coupled with reduced protein degradation is thought to be a hallmark of organisms that are able to enter into states of reversible metabolic arrest (Hand and Hardewig 1996). Diapause II embryos of A. limnaeus have greatly reduced rates of protein synthesis during dormancy associated with diapause even under aerobic conditions (Podrabsky and Hand 2000). Thus, maintenance of protein levels during long-term dormancy likely requires a concomitant down-regulation of protein degradation. The overall low levels of ubiquitin conjugates and extremely low levels of free ubiquitin in diapause II embryos compared to post-diapause II embryos suggests a lower activity of the ubiquitin conjugating systems during diapause II. Future studies on the activity of ubiquitin conjugating enzymes may help to clarify this theory.
SUMOylation patterns associated with exposure to anoxia and anoxic preconditioning
Previous studies have reported massive increases in both SUMO-1 and SUMO-2/3 conjugates following periods of ischemia in mammalian systems (Lee and Hallenbeck 2013; Wang et al. 2012; Silveirinha et al. 2013; Loftus et al. 2009; Yang et al. 2008b; Cimarosti et al. 2008). However, the role of these protein modifications remains uncertain, and there is some debate on whether these changes are an adaptive response or simply a marker of cell damage (Loftus et al. 2009). There is an accumulating body of convincing evidence that suggests protein modifications by SUMO-1 and SUMO-2/3 are a part of the endogenous neuroprotective response in mammalian brains associated with ischemic preconditioning (Ja Lee et al. 2009; Lee et al. 2011; Lee and Hallenbeck 2013). In addition, recent studies have led to the identification of the possible protein targets associated with SUMOylation in response to ischemia (Becker et al. 2013; Silveirinha et al. 2013). Our data support no change in total SUMOylation levels in response to anoxia or during recovery from anoxia in embryos of A. limnaeus. Further, anoxic preconditioning does not alter total levels of SUMO conjugates or the levels of any specific SUMO conjugated proteins. Thus, we conclude that changes in global SUMOylation are not required for survival of anoxia in this species. However, it is possible that these embryos have a high constitutive level of SUMOylated proteins even under aerobic conditions and it would be interesting to directly compare levels of SUMOylation in these embryos compared to mammalian brain tissue and to identify the proteins modified by SUMOylation in this species.
SUMOylation patterns associated with embryonic diapause
Protein SUMOylation may play a major regulatory role in metabolic depression. For example, hibernating ground squirrels accumulate large quantities of SUMO-1 and SUMO-2/3 conjugates in brain tissue during bouts of torpor (Lee et al. 2007). Interestingly, these conjugates appear to accumulate to highest levels late in a torpor bout and are cleared during euthermic periods between torpor bouts (interbout arousals). One interpretation of these data is that degradation of SUMO-conjugated proteins is reduced during torpor and they are accumulated until metabolic rates can support higher turnover during periods of euthermia. Our data on embryos of A. limnaeus suggest that total levels of SUMOylation are similar in active and dormant embryos, suggesting an arrest in the turnover of SUMO-conjugated proteins. However, there are some specific protein targets that may be regulated by SUMOylation that are associated with metabolic dormancy associated with diapause. Interestingly, several of these SUMO-1 conjugated proteins are differentially regulated in response to exposure to anoxia in embryos that have long-term tolerance of anoxia. Identification of these specific SUMO conjugates could shed light on the regulatory mechanisms that support metabolic dormancy and extreme anoxia tolerance in embryos of A. limnaeus.
Broader implications
Embryos of A. limnaeus are the most anoxia tolerant vertebrates. They exhibit a unique and unparalleled ability to survive environmental and cellular conditions that would be lethal to most other animals and all but a handful of other vertebrates. Future studies using this system to better understand the cellular mechanisms that support survival of anoxia promise to identify novel therapies for mediating damage caused by tissue ischemia in humans. In addition, the ability of these embryos to enter into metabolic depression associated with diapause makes this an excellent model for studies that focus on the control of cellular metabolism and proliferation in vertebrates cells. Perhaps new avenues for controlling cell proliferation can be identified and applied to the treatment of cancer and other diseases associated with altered states of cell growth and proliferation.
Acknowledgments
The SUMO-1 and SUMO-2/3 antibodies were a kind gift to R.M. from Jon Hallenbeck (NIH). This work was supported by the National Institutes of Health grants R01 HL095454 (JEP), R01 NS59588 (RM), and R01 NS39016 (RPS).
References
- Anchordoguy TJ, Hand SC. Acute blockage of the ubiquitin-mediated proteolytic pathway during invertebrate quiescence. Am J Physiol. 1994;267(2):R895–R900. doi: 10.1152/ajpregu.1994.267.4.R895. [DOI] [PubMed] [Google Scholar]
- Anchordoguy TJ, Hand SC. Reactivation of ubiquitination in Artemia franciscana embryos during recovery from anoxia-induced quiescence. J Exp Biol. 1995;198(6):1299–1305. doi: 10.1242/jeb.198.6.1299. [DOI] [PubMed] [Google Scholar]
- Becker J, Barysch SV, Karaca S, Dittner C, Hsiao HH, Diaz MB, Herzig S, Urlaub H, Melchior F. Detecting endogenous SUMO targets in mammalian cells and tissues. Nat Struct Mol Biol. 2013;20(4):525–531. doi: 10.1038/nsmb.2526. [DOI] [PubMed] [Google Scholar]
- Bohren KM, Nadkarni V, Song JH, Gabbay KH, Owerbach D. A M55V Polymorphism in a Novel SUMO Gene (SUMO-4) Differentially Activates Heat Shock Transcription Factors and Is Associated with Susceptibility to Type I Diabetes Mellitus. J Biol Chem. 2004;279(26):27233–27238. doi: 10.1074/jbc.M402273200. [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian Hibernation: Cellular and Molecular Responses to Depressed Metabolism and Low Temperature. Physiol Rev. 2003;83(4):1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79(1):13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Ciechanover A, Finley D, Varshavsky A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell. 1984;37(1):57–66. doi: 10.1016/0092-8674(84)90300-3. [DOI] [PubMed] [Google Scholar]
- Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. BioEssays. 2000;22(5):442–451. doi: 10.1002/(sici)1521-1878(200005)22:5<442::aid-bies6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Lindberg C, Bomholt SF, R‾ nn LCB, Henley JM. Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology. 2008;54(2):280–289. doi: 10.1016/j.neuropharm.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Culpepper KM, Podrabsky JE. Cell cycle regulation during development and dormancy in embryos of the annual killifish Austrofundulus limnaeus. Cell Cycle. 2012;11:1697–1704. doi: 10.4161/cc.19881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datwyler AL, Lattig-Tunnemann G, Yang W, Paschen W, Lee SLL, Dirnagl U, Endres M, Harms C. SUMO2/3 conjugation is an endogenous neuroprotectice mechanism. J Cereb Blood Flow Metab. 2011;31:2152–2159. doi: 10.1038/jcbfm.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson-Kolmes L, Podrabsky JE. Differential effects of anoxia on heart rate in developmental stages of the annual killifish Austrofundulus limnaeus that differ in their tolerance of anoxia. J Exp Zool. 2007;307A:419–423. doi: 10.1002/jez.395. [DOI] [PubMed] [Google Scholar]
- Girdwood DWH, Tatham MH, Hay RT. SUMO and transcriptional regulation. Sem Cell Devel Biol. 2004;15(2):201–210. doi: 10.1016/j.semcdb.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hand SC, Hardewig I. Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Annu Rev Physiol. 1996;58:539–563. doi: 10.1146/annurev.ph.58.030196.002543. [DOI] [PubMed] [Google Scholar]
- Hay RT. SUMO: A History of Modification. Mol Cell. 2005;18(1):1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Hochrainer K, Jackman K, Anrather J, Iadecola C. Reperfusion rather than ischemia drives the formation of ubiquitin aggregates after middle cerebral artery occlusion. Stroke. 2012;43(8):2229–2235. doi: 10.1161/STROKEAHA.112.650416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrbek T, Taphorn DC, Thomerson JE. Molecular phylogeny of Austrofundulus Myers (Cyprinodontiformes: Rivulidae), with revision of the genus and the description of four new species. Zootaxa. 2005;825:1–39. [Google Scholar]
- Ja Lee Y, Castri P, Bembry J, Maric D, Auh S, Hallenbeck JM. SUMOylation participates in induction of ischemic tolerance. J Neurochem. 2009;109(1):257–267. doi: 10.1111/j.1471-4159.2009.05957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73(1):355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of Proteins by Ubiquitin and Ubiquitin-Like Proteins. Annu Rev Cell Devel Biol. 2006;22(1):159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- Lee Yj, Hallenbeck JM. SUMO and Ischemic Tolerance. Neuromol Med. 2013:1–11. doi: 10.1007/s12017-013-8239-9. [DOI] [PubMed] [Google Scholar]
- Lee Yj, Mou Y, Maric D, Klimanis D, Auh S, Hallenbeck JM. Elevated global SUMOylation in Ubc9 transgenic mice protects their brains against focal cerebral ischemic damage. PLoS ONE. 2011;6(10):e25852. doi: 10.1371/journal.pone.0025852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Miyake SI, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM. Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab. 2007;27(5):950–962. doi: 10.1038/sj.jcbfm.9600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chen S, Kamme F, Hu BR. Ischemic preconditioning prevents protein aggregation after transient cerebral ischemia. Neuroscience. 2005;134(1):69–80. doi: 10.1016/j.neuroscience.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Martone ME, Hu BR. Protein Ubiquitination in Postsynaptic Densities After Transient Cerebral Ischemia. J Cereb Blood Flow Metab. 2004;24(11):1219–1225. doi: 10.1097/01.WCB.0000136706.77918.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus LT, Gala R, Yang T, Jessick VJ, Ashley MD, Ordonez AN, Thompson SJ, Simon RP, Meller R. Sumo-2/3-ylation following in vitro modeled ischemia is reduced in delayed ischemic tolerance. Brain Res. 2009;1272(0):71–80. doi: 10.1016/j.brainres.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller CL, Meller R, Simon RP, Culpepper KM, Podrabsky JE. Cell cycle arrest associated with anoxia-induced quiescence, anoxic preconditioning, and embryonic diapause in embryos of the annual killiifish Austrofundulus limnaeus. J Comp Physiol B. 2012;182:909–920. doi: 10.1007/s00360-012-0672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller R. The Role of the Ubiquitin Proteasome System in Ischemia and Ischemic Tolerance. The Neuroscientist. 2009;15(3):243–260. doi: 10.1177/1073858408327809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller R, Thompson SJ, Lusardi TA, Ordonez AN, Ashley MD, Jessick V, Wang W, Torrey DJ, Henshall DC, Gafken PR, Saugstad JA, Xiong ZG, Simon RP. Ubiquitin–Proteasome-Mediated Synaptic Reorganization: A Novel Mechanism Underlying Rapid Ischemic Tolerance. J Neurosci. 2008;28(1):50–59. doi: 10.1523/jneurosci.3474-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D, Riezman H. Proteasome-Independent Functions of Ubiquitin in Endocytosis and Signaling. Science. 2007;315(5809):201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- Podrabsky JE. Husbandry of the annual killifish Austrofundulus limnaeus with special emphasis on the collection and rearing of embryos. Env Biol Fish. 1999;54(4):421–431. [Google Scholar]
- Podrabsky JE, Carpenter JF, Hand SC. Survival of water stress in annual fish embryos: dehydration avoidance and egg envelope amyloid fibers. Am J Physiol. 2001;280(1):R123–R131. doi: 10.1152/ajpregu.2001.280.1.R123. [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Hand SC. Depression of protein synthesis during diapause in embryos of the annual killifish Austrofundulus limnaeus. Physiol Biochem Zool. 2000;73(6):799–808. doi: 10.1086/318106. [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Hrbek T, Hand SC. Physical and chemical characteristics of ephemeral pond habitats in the Maracaibo basin and Llanos region of Venezuela. Hydrobiologia. 1998;362(1-3):67–78. [Google Scholar]
- Podrabsky JE, Lopez JP, Fan TWM, Higashi R, Somero GN. Extreme anoxia tolerance in embryos of the annual killifish Austrofundulus limnaeus: Insights from a metabolomics analysis. J Exp Biol. 2007;210(13):2253–2266. doi: 10.1242/jeb.005116. [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Menze MA, Hand SC. Rapid Communication: Long-term survival of anoxia despite rapid ATP decline in embryos of the annual killifish Austrofundulus limnaeus. J Exp Zool A. 2012a;317:524–532. doi: 10.1002/jez.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podrabsky JE, Riggs CL, Duerr JM. Anoxia Tolerance During Vertebrate Development - Insights from Studies on the Annual Killifish Austrofundulus limnaeus. In: Padilla P, editor. Anoxia InTech. 2012b. pp. 3–24. [Google Scholar]
- Saitoh H, Hinchey J. Functional Heterogeneity of Small Ubiquitin-related Protein Modifiers SUMO-1 versus SUMO-2/3. J Biol Chem. 2000;275(9):6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- Scheschonka A, Tang Z, Betz H. Sumoylation in neurons: nuclear and synaptic roles? Trends Neurosci. 2007;30(3):85–91. doi: 10.1016/j.tins.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Silveirinha V, Stephens GJ, Cimarosti H. Molecular targets underlying SUMO mediated neuroprotection in brain ischemia. J Neurochem. 2013 doi: 10.1111/jnc.12347. [DOI] [PubMed] [Google Scholar]
- Van Breukelen F, Carey H. Ubiquitin conjugate dynamics in the gut and liver of hibernating ground squirrels. J Comp Physiol B. 2002;172(3):269–273. doi: 10.1007/s00360-002-0252-5. [DOI] [PubMed] [Google Scholar]
- van Breukelen F, Hand SC. Characterization of ATP-dependent proteolysis in embryos of the brine shrimp, Artemia franciscana. J Comp Physiol B. 2000;170(2):125–133. doi: 10.1007/s003600050267. [DOI] [PubMed] [Google Scholar]
- Velickovska V, Lloyd BP, Qureshi S, van Breukelen F. Proteolysis is depressed during torpor in hibernators at the level of the 20S core protease. J Comp Physiol B. 2005;175(5):329–335. doi: 10.1007/s00360-005-0489-x. [DOI] [PubMed] [Google Scholar]
- Velickovska V, van Breukelen F. Ubiquitylation of proteins in livers of hibernating golden-mantled ground squirrels, Spermophilus lateralis. Cryobiology. 2007;55(3):230–235. doi: 10.1016/j.cryobiol.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang R, Sheng H, Sheng S, Paschen W, Yang W. Transient ischemia induces massive nuclear accumulation of SUMO2/3-conjugated proteins in spinal cord neurons. Spinal Cord. 2012;51(2):139–143. doi: 10.1038/sc.2012.100. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Berti R, Dave JR, Elliot PJ, Adams J, Tortella FC. Delayed Treatment of Ischemia/Reperfusion Brain Injury. Extended Therapeutic Window with the Proteosome Inhibitor MLN519. Stroke. 2004;35(5):1186–1191. doi: 10.1161/01.STR.0000125721.10606.dc. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Hale SL, Moffett JR, Dave JR, Elliott PJ, Adams J, Tortella FC. Delayed Treatment With MLN519 Reduces Infarction and Associated Neurologic Deficit Caused by Focal Ischemic Brain Injury in Rats via Antiinflammatory Mechanisms Involving Nuclear Factor-kappaB Activation, Gliosis, and Leukocyte Infiltration. J Cereb Blood Flow Metab. 2003;23(1):75–87. doi: 10.1097/01.WCB.0000039285.37737.C2. [DOI] [PubMed] [Google Scholar]
- Wilson VG, Rosas-Acosta G. Wrestling with SUMO in a New Arena. Sci STKE. 2005;2005(290):pe32. doi: 10.1126/stke.2902005pe32. [DOI] [PubMed] [Google Scholar]
- Wojcik C, MD, PhD, DSc, Di Napoli M., MD Ubiquitin-Proteasome System and Proteasome Inhibition: New Strategies in Stroke Therapy. Stroke. 2004;35(6):1506–1518. doi: 10.1161/01.STR.0000126891.93919.4e. [DOI] [PubMed] [Google Scholar]
- Wourms JP. The developmental biology of annual fishes III. Pre-embryonic and embryonic diapause of variable duration in the eggs of annual fishes. J Exp Zool. 1972;182(3):389–414. doi: 10.1002/jez.1401820310. [DOI] [PubMed] [Google Scholar]
- Yang W, Sheng H, Warner DS, Paschen W. Transient focal cerebral ischemia induces a dramatic activation of small ubiquitin-like modifier conjugation. J Cereb Blood Flow Metab. 2008a;28(5):892–896. doi: 10.1038/sj.jcbfm.9600601. [DOI] [PubMed] [Google Scholar]
- Yang W, Sheng H, Warner DS, Paschen W. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab. 2008b;28(2):269–279. doi: 10.1038/sj.jcbfm.9600523. [DOI] [PubMed] [Google Scholar]


