Abstract
Fibrosing alveolitis is a disease with inflammatory, proliferative, and fibrotic components. In different models, it has been shown that the cytokine interleukin-10 (IL-10) plays a conflicting role in inflammation-associated fibrotic processes, inasmuch as it is an antiinflammatory cytokine but also a TH2 cytokine with inherent pro-fibrotic effects. IL-10 is produced primarily by inflammatory cells. In this report, we show in a rat model of radiation-induced fibrosing alveolitis that IL-10 is also produced by type I alveolar epithelial cells in both normal and fibrotic lungs. The total amount of IL-10 in the lung is increased after irradiation, but type I pneumoyctes contain less IL-10. The R3/1 permanent type I pneumocyte cell line also contains IL-10, which is reduced after irradiation. Whereas in the normal lung, the entire alveolar surface is covered by IL-10—producing pneumocytes, this continuity is interrupted in fibrotic lungs, because type I pneumocytes lack full differentiation and thus full spreading over the alveolar surface. The exposure of the IL-10—negative epithelial basal membrane may allow for an easier attachment of inflammatory cells such as alveolar macrophages. These cells have the potential to act in a pro-inflammatory way by tumor necrosis factor α and also in a pro-fibrotic way by activating TH2 cytokines.
Keywords: interleukin-10, type I pneumocytes, fibrosing alveolitis, radiation damage
Irradiation of the thorax leads to fibrosing alveolitis. Although in the late stage of the disease, fibrosis is dominant, an inflammatory component is almost always seen, especially in the early phase. Several pro-inflammatory cytokines have been described in the process of the disease, including tumor necrosis factor α (TNFα), transforming growth factor β (TGFβ), and interleukin-1 (IL-1) (Kovacs 1991; Kovacs and DiPietro 1994). The pro-inflammatory cytokine IL-6, which is induced in pulmonary fibrosis, stimulates proliferation of fibroblasts (Haase et al. 2003; Olman et al. 2004). One immunomodulatory cytokine that has a potential role in pulmonary fibrosis is IL-10. However, the function of IL-10 in the development of fibrosing processes such as radiation-induced pulmonary fibrosis is controversial.
On the one hand, IL-10 is an anti-inflammatory cytokine, because it downregulates TNFα activity and inhibits long-term IL-6 production (Marshall et al. 1996; Huaux et al. 1999). By acting as a TH2 cytokine, however, it generally protects against the action of TH1 cytokines (Zhou et al. 2004). There are several pathways that may be responsible for the anti-inflammatory function of IL-10. The TNF pathway itself has a negative feedback loop via TNF receptor—associated factor 3 (TRAF3). TFAF3 is necessary for IL-10 production, because TRAF3-deficient mice display defective IL-10 production and overproduce inflammatory cytokines (Hacker et al. 2006). In IL-10 knockout mice, TGFβ/Smad-mediated degradation of Toll-like receptor 2 (TLR2) is inhibited, which leads to an increased activation of the pro-inflammatory nuclear factor (NF)-κB pathway (Ruiz et al. 2006). In bleomycin-induced fibrosis models, IL-10 in vivo gene delivery attenuates pulmonary fibrosis, possibly by interfering with pro-inflammatory cytokines such as TNFα (Arai et al. 2000) or TGFβ (Arai et al. 2000; Nakagome et al. 2006). However, in a model of bleomycin-induced fibrosis with IL-10—deficient mice, it was shown that IL-10 inhibits inflammation but not fibrosis (Kradin et al. 2004). In a patient with interstitial pulmonary fibrosis (IPF), a polymorphism in the 3′-untranslated region of IL-10 was found that resulted in a decreased production of IL-10 (Whittington et al. 2003). Taken together, the above-mentioned facts clearly demonstrate that IL-10 is an anti-inflammatory cytokine that might have an inhibitory effect on the inflammatory component of pulmonary fibrosis.
On the other hand, several reports exist that classify IL-10 as a pro-fibrotic cytokine that is associated with a role in the TH2 immune response (Barbarin et al. 2005). IL-10 stimulates cell proliferation by activation of phosphoinositol-3-kinase and p70S6 kinase (Crawley et al. 1996). There are several clinical studies on the role of IL-10 in interstitial lung disease. An increased production of IL-10 by alveolar macrophages has been demonstrated in interstitial lung disease (Martinez et al. 1997). The levels of IL-2, −8, −10, and −12 are higher in sera of patients with IPF compared with those of controls (Tsoutsou et al. 2006). The bronchoalveolar lavage fluid of sarcoidosis patients contains a higher level of IL-10 compared with that of control patients (Barbarin et al. 2003), possibly due to increased release from alveolar macrophages (Oltmanns et al. 2003). Higher IL-10 mRNA and protein levels have been observed in lung tissue of patients with idiopathic pulmonary fibrosis (Bergeron et al. 2003). Cultured alveolar macrophages from patients with IPF show increased production of IL-10 mRNA after induction with lipopolysaccharide (Freeburn et al. 2005). IL-10 deficiency results in an increased production of B7 costimulatory molecules on alveolar macrophages (Soltys et al. 2002). Similar results have been observed in animal experiments. The production of TGFβ depends on IL-10, as shown in IL-10 knockout mice (Ruiz et al. 2005). IL-10 knockout mice display a lower expression of the pro-fibrotic cytokine TGFβ, of cyclooxygenase-2, and of anti-fibrotic cytokine prostaglandin E2 (Barbarin et al. 2004). Fibroblasts are able to stimulate IL-10 production in blood monocytes (Vancheri et al. 2001).
IL-10 has been described to be produced by cells of the immune system such as TH0 and TH2 cells, monocytes, macrophages, and B-lymphocytes. IL-10 is also produced by mast cells and acts in an autocrine manner, thereby activating the cells (Thompson-Snipes et al. 1991; Lin and Befus 1997). It increases the production of mast cell proteases, such as mouse mast cell protease 1 (Ghildyal et al. 1992b). Only a few reports have detected the presence of IL-10 in epithelial cells. Bronchial epithelial cells have been described as containing IL-10 (Dosanjh et al. 2001; Bonfield et al. 1995). One in vivo study described that bronchial epithelial cells did not contain IL-10 (Lim et al. 2004). Data in one study with human biopsy material suggest that type II pneumocytes contain IL-10, but the epithelial nature of the cells had not been verified in detail (Bergeron et al. 2003).
In this report, we show that the alveolar surface is covered by IL-10—positive type I pneumocytes, which may act as a protective shield. This continuity is disturbed in radiation-induced fibrosing alveolitis, which allows adhesion of inflammatory cells and exposure to inflammatory and pro-fibrotic cytokines. According to the dual role as an anti-inflammatory but also pro-fibrotic cytokine, it is discussed how IL-10 might be involved in radiation-induced fibrosing alveolitis in different cell types.
Materials and Methods
Cell Culture
The R3/1 rat lung epithelial cell line (Koslowski et al. 2004) has features primarily of type I pneumocytes (Barth et al. 2005). The cells were cultured in DMEM containing 10% fetal calf serum. Cells were irradiated using a Maxishot cabinet X-ray system (Yxlon; Copenhagen, Denmark) at a dose rate of 1.3 Gy/min.
Animal Model
The right lungs of female Fischer rats were irradiated with 20 Gy of ionizing radiation as previously described (Geyer 1999; Pauluhn et al. 2001; Haase et al. 2003). At least four animals were studied at each time point, as indicated in previous studies with this system (Haase et al. 2000, 2003). Lungs were removed at the indicated time points and either snap-frozen or fixed in Schaffer's solution (64% v/v methanol, 33% v/v of a 40% formaldehyde solution, 3% w/v glucose in PBS) for 24 hr. Animal housing and experiments were approved according to German and international animal welfare regulations.
Immunohistochemistry
Paraffin sections were pretreated with microwave heating as described (Haase et al. 1997). For immunohistochemistry, the mouse monoclonal antibody to IL-10 (Clone A5-4; Beckton Dickinson, Heidelberg, Germany) was used at a dilution of 1:10. Signal amplification and color development were done with the VECTASTAIN Elite ABC Kit (Axxora; Grünberg, Germany) according to manufacturer's instructions. For immunofluorescence double labeling, the IL-10 antibody was used at a dilution of 1:100 and the rabbit polyclonal antibody to aquaporin-5 (Sigma-Aldrich; Taufkirchen, Germany) was used at a dilution of 1:70. Fluorescence labeling was performed using tyramide signal amplification kits (Molecular Probes/Invitrogen; Karlsruhe, Germany) according to the manufacturer's instructions. The kits contained Alexa 488 for green fluorescence and Alexa 594 for red fluorescence. Negative controls for both immunohistochemistry and immunofluorescence experiments were performed by replacing primary antibodies with non-immune serum of the appropriate species. Pictures were obtained with a confocal laser scanning microscope (Zeiss; Jena, Germany). Oil objectives (63× or 100×) were used for obtaining images. Detailed microscope settings are available upon request. Three sections at each time point were stained using each staining method. The whole sections were examined. For quantification of the alveolar area covered with IL-10-staining, 200 alveoli of contol lungs and lungs with fibrosing alveolitis were analyzed and the mean values were determined. Staining intensities were evaluated using a semi-quantitative score: negative (0), faintly positive (1), moderately positive (2), or strongly positive (3).
Western Blots
Protein extraction and Western blots were done as previously described (Haase et al. 2003). The anti—IL-10 antibody was used at a dilution of 1:1000, the peroxidase-labeled secondary antibody at 1:5000. Anti—β-actin (Sigma; dilution 1:20,000, peroxidase-labeled secondary antibody 1:30,000) served as loading control. Signals were detected using chemiluminescence (Super Signal West Dura Extended Duration Substrate; Pierce/KMF, Sankt Augustin, Germany) according to the manufacturer's instructions. Three independent experiments were performed.
Results
Quantification of IL-10 Protein Expression in the Rat Lung
In total protein extracts of the rat lung, IL-10 was expressed (Figure 1). Compared with the control lung (lane 1), IL-10 was constantly increased from 4 to 12 weeks after irradiation. As a next step, we wanted to characterize the cell types that express IL-10 in the lung.
Figure 1.
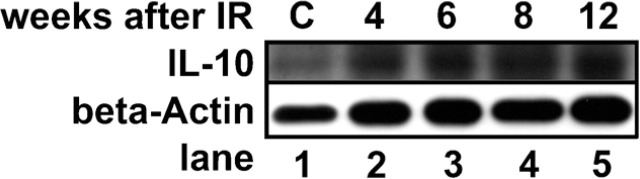
Interleukin-10 (IL-10) is induced after irradiation of the rat lung with 20 Gy. Cytoplasmic protein extracts of total lung lysates of normal and irradiated rat lungs were subjected to Western blotting. β-Actin served as a loading control. IL-10 protein level is induced between 4 and 12 weeks after irradiation. C, control cells.
Cellular Distribution of IL-10 Expression in the Rat Lung
In addition to inflammatory cells, IL-10 was expressed at the alveolar surface (Figure 2A), suggesting positivity of type I and type II pneumocytes. In rat lungs with fibrosing alveolitis, IL-10 was expressed by thickened cells of the alveolar surface (Figures 2B and 2C). Alveolar macrophages adhered to areas of the alveolar surface that lack IL-10 expression. Mast cells contained low amounts of IL-10 in this model, as judged by morphology (Haase et al. 2004). To verify that IL-10 is present in type I pneumocytes, immunofluorescence double staining with the type I pneumocyte marker aquaporin-5 was performed (Lee et al. 1997; Ramirez et al. 2000). Indeed, IL-10 was colocalized with aquaporin-5 (Figures 2D–2F) in the control lung. Type I pneumocytes form a thin layer covering the whole surface of the alveoli, with the exception of the regions where type II pneumocytes are localized. In the lung with fibrosing alveolitis, type I pneumocytes were not completely differentiated. They were thickened (Figure 2G), covered a smaller area than similar cells of the control lung, and contained less IL-10 (Figure 2H). Some areas of the alveolar surface of the lung with radiation-induced fibrosing alveolitis contained no pneumocytes (Figures 2K–2M). Quantification of the alveolar area covered by IL-10—positive cells revealed 97% in control cells versus 22% in lungs with radiation-induced fibrosing alveolitis (3 months after irradiation; see Table 1). The interstitial cells in the thickened alveolar walls also contained high amounts of IL-10. On the basis of these findings, together with the results of the Western blots (Figures 1 and 1), we conclude that IL-10 is downregulated in type I pneumocytes and upregulated in interstitial cells of the fibrotic lung.
Figure 2.
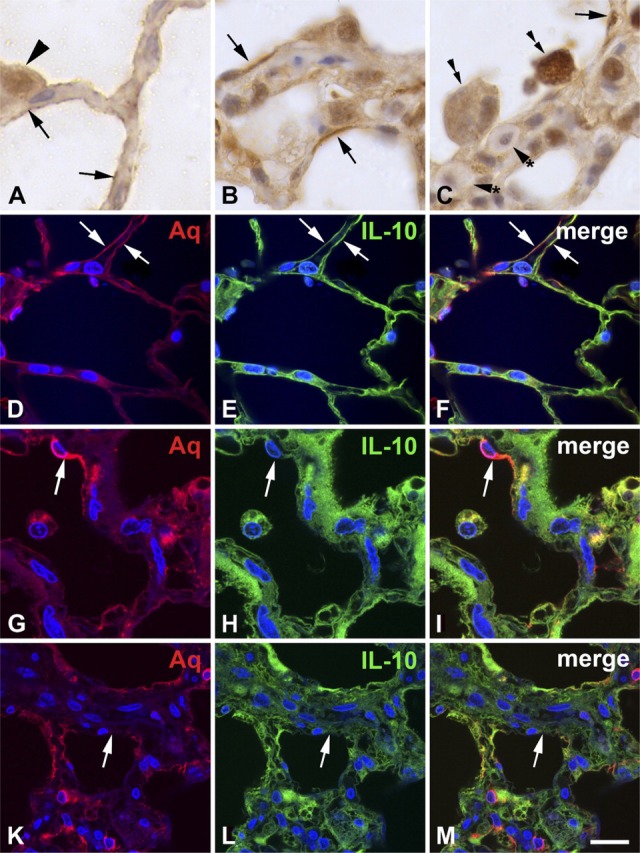
IL-10 is expressed in type I pneumocytes of the normal rat lung (A, D—F) and in the rat lung with radiation-induced fibrosing alveolitis 3 months after irradiation (B, C, G—M). Rat lungs were formalin-fixed and subjected to immunohistochemistry and immunofluorescence. IL-10 is expressed on the surface of the alveolar walls (arrows in A, E, F) of the normal lung, suggesting positivity of type I pneumocytes. Type II pneumocytes (arrowhead) are also positive, both in the cytoplasm and in the nucleus. In the lung with fibrosing alveolitis, the alveolar walls are thickened and the surface contains IL-10—positive cells that are thicker and do not cover the whole surface of the alveoli (arrows in B, C). Alveolar macrophages (double arrows in C) adhere to areas of the alveolar surface that lack IL-10. Mast cells (arrowheads with asterisk) contain low amounts of IL-10. Aquaporin-5 (Aq), a marker of type I pneumocytes, is colocalized with IL-10 in normal lung (arrows in D—F). In the lung with fibrosing alveolitis (G—M), partially differentiated type I pneumocytes cover a smaller surface than in the control lung, but they have a greater thickness and contain less IL-10 (arrows in H, I). There are areas of the alveolar surface that contain no type I pneumocytes (arrows in K—M). Bars: A = 6.4 μm; B, C = 12.9 μm; D—F = 16.75 μm; G—I = 16.9 μm; K—M = 25.8 μm.
Table 1.
Quantification of staining intensities and alveolar area covered by IL-10 positivity in control lungs and 3 months after irradiation
| Control | 3 Months after irradiation | |
| Staining intensity of type I pneumocytes | 2 | 1 |
| Average alveolar area covered by IL-10 positivity | 97 | 22 |
| IL-10, interleukin-10. Semi-quantitative staining intensities were evaluated using the following score: negative, 0; weakly positive, 1; moderately positive, 2; strongly positive, 3. |
Presence of IL-10 in Cultured Type I Pneumocytes
To prove the identity of IL-10 in type I pneumocytes, Western blotting was performed with protein extracts of R3/1 cells that have features primarily of type I pneumocytes. In these cells, a characteristic 35-kDa IL-10 band could be detected (Figure 3). After irradiation of those cells with a single dose of 20 Gy of ionizing radiation, a decrease of IL-10 expression was detected.
Figure 3.
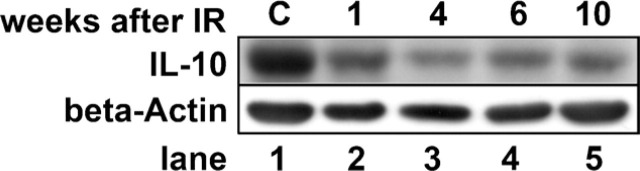
IL-10 is expressed in the type I pneumocyte cell line R3/1. R3/1 cells were either left untreated or irradiated with 20 Gy. Cytoplasmic protein extracts were subjected to Western blotting. β-Actin served as loading control. IL-10 expression in cultured type I pneumocytes is decreased from 1 to 10 weeks after irradiation. C, control cells.
Discussion
This study deals with the cell type—specific expression of IL-10 in the rat lung under normal conditions and in lungs with radiation-induced fibrosing alveolitis. There are conflicting results in the literature on the role of IL-10 in fibrosing alveolitis. These studies used bronchioloalveolar lavage fluid or total lung lysates and usually assumed that IL-10 expression is restricted to inflammatory cells. In this study, we show that IL-10 is expressed in type I pneumocytes of the alveolar surface. Because IL-10 is a molecule that suppresses acute inflammation, it may prevent the activation and the adhesion of inflammatory cells at the alveolar surface. Indeed, alveolar macrophages are rarely found to adhere to the alveolar surface. In contrast, many clusters of alveolar macrophages adhere to the alveolar surface in lungs with fibrosing alveolitis (Figure 2C), where the continuity of the IL-10—positive type I cell layer is interrupted. In addition, type I cells contain less IL-10 in these lungs. According to this model, type I pneumocytes might act as a protective sheath, not only mechanically but also by their expression of IL-10. In contrast, the thickened alveolar walls in lungs with fibrosing alveolitis containing proliferating fibroblasts and inflammatory cells are strongly IL-10 positive. Because IL-10 has been described as a pro-fibrotic TH2 cytokine, the increased expression of IL-10 in interstitial cells of fibrosing alveolar walls might have a pathogenetic role in this process.
Mast cells are present in huge numbers in the alveolar walls of lungs with radiation-induced fibrosing alveolitis. These cells have been described to contain IL-10 (Royer et al. 2001), which acts in an autocrine fashion. However, in our model, mast cells contain only relatively low amounts of IL-10 compared with surrounding cells. This may not contradict the results in the cited study, because the quantity of IL-10 in mast cells in that study was not quantified in relation to other cells. The great amount of IL-10 in the alveolar walls fits well with its role as a cytokine for mast cells (Thompson-Snipes et al. 1991; Ghildyal et al. 1992a, b), because in our model, the number of mast cells is 1400-fold induced in lungs with fibrosing alveolitis compared with control lungs (M. Haase, unpublished observation).
The conflicting role of IL-10 in the development of fibrosing diseases may be explained by its different roles in different cell types that were possibly underestimated previously. In addition, it is important to consider the localization of the protein within the cells (cytoplasm, surface), or of secreted IL-10, which has not been evaluated in this study.
The positivity of IL-10 was also detected in the permanent type I cell line R3/1 using Western blotting. As in the immunohistochemistry stainings, a decrease in IL-10 expression could be observed. This indicates that the downregulation of IL-10 might be a direct effect of irradiation of the cells.
Taken together, the results seen in the present study show that IL-10 is expressed in the epithelial cells of the alveolar wall. Possible mechanisms of IL-10 involvement in radiation-induced fibrosing alveolitis in different cell types have been discussed.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grant MU 1299/1-1) and the Bundesministerium für Bildung und Forschung (grants BMBF 01ZZ9604 and BMBF 03ZIK041 (Nils Cordes, OncoRay) as well as a grant from the Faculty of Medicine of the Dresden University of Technology (to MGH).
The authors thank Daniela Tschuck for excellent technical assistance. The R3/1 cell line was kindly provided by Michael Kasper (Dresden, Germany).
Literature Cited
- Arai T, Abe K, Matsuoka H, Yoshida M, Mori M, Goya S, Kida H, et al. (2000) Introduction of the interleukin-10 gene into mice inhibited bleomycin-induced lung injury in vivo. Am J Physiol Lung Cell Mol Physiol 278: L914–922 [DOI] [PubMed] [Google Scholar]
- Barbarin V, Arras M, Misson P, Delos M, McGarry B, Phan SH, Lison D, et al. (2004) Characterization of the effect of interleukin-10 on silica-induced lung fibrosis in mice. Am J Respir Cell Mol Biol 31: 78–85 [DOI] [PubMed] [Google Scholar]
- Barbarin V, Petrek M, Kolek V, Van Snick J, Huaux F, Lison D. (2003) Characterization of p40 and IL-10 in the BALF of patients with pulmonary sarcoidosis. J Interferon Cytokine Res 23: 449–456 [DOI] [PubMed] [Google Scholar]
- Barbarin V, Xing Z, Delos M, Lison D, Huaux F. (2005) Pulmonary overexpression of IL-10 augments lung fibrosis and Th2 responses induced by silica particles. Am J Physiol Lung Cell Mol Physiol 288: L841–L848 [DOI] [PubMed] [Google Scholar]
- Barth K, Reh J, Sturrock A, Kasper M. (2005) Epithelial vs myofibroblast differentiation in immortal rat lung cell lines—modulating effects of bleomycin. Histochem Cell Biol. 124: 453–464 [DOI] [PubMed] [Google Scholar]
- Bergeron A, Soler P, Kambouchner M, Loiseau P, Milleron B, Valeyre D, Hance AJ, et al. (2003) Cytokine profiles in idiopathic pulmonary fibrosis suggest an important role for TGF-β and IL-10. Eur Respir J 22: 69–76 [DOI] [PubMed] [Google Scholar]
- Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M. (1995) Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol 13: 257–261 [DOI] [PubMed] [Google Scholar]
- Crawley JB, Williams LM, Mander T, Brennan FM, Foxwell BM. (1996) Interleukin-10 stimulation of phosphatidylinositol 3-kinase and p70 S6 kinase is required for the proliferative but not the antiinflammatory effects of the cytokine. J Biol Chem 271: 16357–16362 [DOI] [PubMed] [Google Scholar]
- Dosanjh A, Morris RE, Wan B. (2001) Bronchial epithelial cell-derived cytokine IL-10 and lung fibroblast proliferation. Transplant Proc 33: 352–354 [DOI] [PubMed] [Google Scholar]
- Freeburn RW, Armstrong L, Millar AB. (2005) Cultured alveolar macrophages from patients with idiopathic pulmonary fibrosis (IPF) show dysregulation of lipopolysaccharide-induced tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10) inductions. Eur Cytokine Netw 16: 5–16 [PubMed] [Google Scholar]
- Geyer P. (1999) An accelerator technique for the irradiation of the right lungs of rats. Experimentelle Strahlentherapie und Klinische Strahlenbiologie 8: 75–77 [Google Scholar]
- Ghildyal N, McNeil HP, Gurish MF, Austen KF, Stevens RL. (1992a) Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J Biol Chem 267: 8473–8477 [PubMed] [Google Scholar]
- Ghildyal N, McNeil HP, Stechschulte S, Austen KF, Silberstein D, Gurish MF, Somerville LL, et al. (1992b) IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J Immunol 149: 2123–2129 [PubMed] [Google Scholar]
- Haase M, Geyer P, Appold S, Schuh D, Kasper M, Muller M. (2000) Down-regulation of SP1 DNA binding activity in the process of radiation-induced pulmonary fibrosis. Int J Radiat Biol 76: 487–492 [DOI] [PubMed] [Google Scholar]
- Haase MG, Klawitter A, Baretton GB. (2004) IκBγ is expressed in mast cells. Virchows Arch 445: 515–520 [DOI] [PubMed] [Google Scholar]
- Haase MG, Klawitter A, Geyer P, Alheit H, Baumann M, Kriegel TM, Kasper M, et al. (2003) Sustained elevation of NF-κB DNA binding activity in radiation-induced lung damage in rats. Int J Radiat Biol 79: 863–877 [DOI] [PubMed] [Google Scholar]
- Haase M, Koslowski R, Lengnick A, Hahn R, Wenzel KW, Schuh D, Kasper M, et al. (1997) Cellular distribution of c-Jun and c-Fos in rat lung before and after bleomycin induced injury. Virchows Arch 431: 441–448 [DOI] [PubMed] [Google Scholar]
- Hacker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, Kamps MP, et al. (2006) Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439: 204–207 [DOI] [PubMed] [Google Scholar]
- Huaux F, Arras M, Vink A, Renauld JC, Lison D. (1999) Soluble tumor necrosis factor (TNF) receptors p55 and p75 and interleukin-10 downregulate TNF-α activity during the lung response to silica particles in NMRI mice. Am J Respir Cell Mol Biol 21: 137–145 [DOI] [PubMed] [Google Scholar]
- Koslowski R, Barth K, Augstein A, Tschernig T, Bargsten G, Aufderheide M, Kasper M. (2004) A new rat type I-like alveolar epithelial cell line R3/1: bleomycin effects on caveolin expression. Histochem Cell Biol 121: 509–519 [DOI] [PubMed] [Google Scholar]
- Kovacs EJ. (1991) Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today 12: 17–23 [DOI] [PubMed] [Google Scholar]
- Kovacs EJ, DiPietro LA. (1994) Fibrogenic cytokines and connective tissue production. FASEB J 8: 854–861 [DOI] [PubMed] [Google Scholar]
- Kradin RL, Sakamoto H, Jain F, Zhao LH, Hymowitz G, Preffer F. (2004) IL-10 inhibits inflammation but does not affect fibrosis in the pulmonary response to bleomycin. Exp Mol Pathol 76: 205–211 [DOI] [PubMed] [Google Scholar]
- Lee MD, King LS, Nielsen S, Agre P. (1997) Genomic organization and developmental expression of aquaporin-5 in lung. Chest 111: 111S–113S [DOI] [PubMed] [Google Scholar]
- Lim S, Caramori G, Tomita K, Jazrawi E, Oates T, Chung KF, Barnes PJ, et al. (2004) Differential expression of IL-10 receptor by epithelial cells and alveolar macrophages. Allergy 59: 505–514 [DOI] [PubMed] [Google Scholar]
- Lin TJ, Befus AD. (1997) Differential regulation of mast cell function by IL-10 and stem cell factor. J Immunol 159: 4015–4023 [PubMed] [Google Scholar]
- Marshall JS, Leal-Berumen I, Nielsen L, Glibetic M, Jordana M. (1996) Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J Clin Invest 97: 1122–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JA, King TE, Jr, Brown K, Jennings CA, Borish L, Mortenson RL, Khan TZ, et al. (1997) Increased expression of the interleukin-10 gene by alveolar macrophages in interstitial lung disease. Am J Physiol 273: L676–L683 [DOI] [PubMed] [Google Scholar]
- Nakagome K, Dohi M, Okunishi K, Tanaka R, Miyazaki JI, Yamamoto K. (2006) In vivo IL-10 gene delivery attenuates bleomycin induced pulmonary fibrosis by inhibiting the production and activation of TGF-β in the lung. Thorax 61: 886–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olman MA, White KE, Ware LB, Simmons WL, Benveniste EN, Zhu S, Pugin J, et al. (2004) Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1 β-induced IL-6 expression. J Immunol 172: 2668–2677 [DOI] [PubMed] [Google Scholar]
- Oltmanns U, Schmidt B, Hoernig S, Witt C, John M. (2003) Increased spontaneous interleukin-10 release from alveolar macrophages in active pulmonary sarcoidosis. Exp Lung Res 29: 315–328 [DOI] [PubMed] [Google Scholar]
- Pauluhn J, Baumann M, Hirth-Dietrich C, Rosenbruch M. (2001) Rat model of lung fibrosis: comparison of functional, biochemical, and histopathological changes 4 months after single irradiation of the right hemithorax. Toxicology 161: 153–163 [DOI] [PubMed] [Google Scholar]
- Ramirez MI, Chung UI, Williams MC. (2000) Aquaporin-5 expression, but not other peripheral lung marker genes, is reduced in PTH/PTHrP receptor null mutant fetal mice. Am J Respir Cell Mol Biol 22: 367–372 [DOI] [PubMed] [Google Scholar]
- Royer B, Varadaradjalou S, Saas P, Gabiot AC, Kantelip B, Feger F, Guillosson JJ, et al. (2001) Autocrine regulation of cord blood-derived human mast cell activation by IL-10. J Allergy Clin Immunol 108: 80–86 [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Shkoda A, Kim SC, Sartor RB, Haller D. (2005) IL-10 gene-deficient mice lack TGF-β/Smad signaling and fail to inhibit proinflammatory gene expression in intestinal epithelial cells after the colonization with colitogenic Enterococcus faecalis. J Immunol 174: 2990–2999 [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Shkoda A, Kim SC, Sartor RB, Haller D. (2006) IL-10 gene-deficient mice lack TGF-β/Smad-mediated TLR2 degradation and fail to inhibit proinflammatory gene expression in intestinal epithelial cells under conditions of chronic inflammation. Ann N Y Acad Sci 1072: 389–394 [DOI] [PubMed] [Google Scholar]
- Soltys J, Bonfield T, Chmiel J, Berger M. (2002) Functional IL-10 deficiency in the lung of cystic fibrosis (cftr(-/-)) and IL-10 knockout mice causes increased expression and function of B7 costimulatory molecules on alveolar macrophages. J Immunol 168: 1903–1910 [DOI] [PubMed] [Google Scholar]
- Thompson-Snipes L, Dhar V, Bond MW, Mosmann TR, Moore KW, Rennick DM. (1991) Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med 173: 507–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoutsou PG, Gourgoulianis KI, Petinaki E, Germenis A, Tsoutsou AG, Mpaka M, Efremidou S, et al. (2006) Cytokine levels in the sera of patients with idiopathic pulmonary fibrosis. Respir Med 100: 938–945 [DOI] [PubMed] [Google Scholar]
- Vancheri C, Mastruzzo C, Tomaselli V, Sortino MA, D'Amico L, Bellistri G, Pistorio MP, et al. (2001) Normal human lung fibroblasts differently modulate interleukin-10 and interleukin-12 production by monocytes: implications for an altered immune response in pulmonary chronic inflammation. Am J Respir Cell Mol Biol 25: 592–599 [DOI] [PubMed] [Google Scholar]
- Whittington HA, Freeburn RW, Godinho SI, Egan J, Haider Y, Millar AB. (2003) Analysis of an IL-10 polymorphism in idiopathic pulmonary fibrosis. Genes Immun 4: 258–264 [DOI] [PubMed] [Google Scholar]
- Zhou P, Streutker C, Borojevic R, Wang Y, Croitoru K. (2004) IL-10 modulates intestinal damage and epithelial cell apoptosis in T cell-mediated enteropathy. Am J Physiol Gastrointest Liver Physiol 287: G599–604 [DOI] [PubMed] [Google Scholar]


