Abstract
In the physiology of placental blood circulation, nitric oxide (NO) synthases seem to play important roles, although their expression in pathological placentas and their role is still unclear. In addition, NO synthase activation seems to be related to estrogen receptor expression. Therefore, the aims of this study were to investigate the expression of estrogen receptors alpha (ERα), estrogen receptor beta (ER and the endothelial NO synthase (eNOS), and inducible NO synthase (iNOS) in intrauterine growth-restricted (IUGR) placentas, preeclamptic placentas, and in normal healthy control placentas. Slides of paraffin-embedded placental tissue were obtained after delivery from patients diagnosed with IUGR, preeclampsia, and normal term placentas and analyzed for eNOS, iNOS as well as ERα and ERβ expression. Intensity of immunohistochemical reaction was analyzed using a semiquantitative score and statistical analysis was performed. In addition, Western blot experiments were performed for comparison of staining intensities obtained by immunohis-tochemistry and western blot. Expression of eNOS, iNOS, and ERβ is significantly reduced in trophoblast cells of placentas with IUGR. However, preeclamptic placentas demonstrated a significant elevated expression intensity of these proteins compared with normal controls. A different expression of eNOS, iNOS, ERα, and ERβ by human trophoblast cells seems to results in lower NO output and impaired trophoblast invasion. Results obtained in our study provide evidence that reduced expression of the investigated proteins is related to IUGR.
Keywords: nitric oxide synthases, estrogen receptor alpha/beta, intrauterine growth restriction, preeclampsia
During establishment of fetoplacental circulation, uterine spiral arteries undergo remodeling: spiral artery endothelial cells are replaced by endovascular extravillous trophoblasts cells (EVT) and the arterial smooth muscle and elastic is lost and replaced by fibrinoid (Brosens et al. 1967; Pijnenborg et al. 1983). This process terminates in low-resistance, high-output vessels. Placental blood flow thereafter is dependent on humoral and endothelial derived factors because placental tissue and vessels lack autonomic innervation (Nasiell et al. 1998). A decrease in uterine impedance parallels advancing gestational age up to the 22nd week of pregnancy and remains stable until delivery (Yagel et al. 1999; Papageorghiou et al. 2001,2002; Di Paolo et al. 2003).
Failure of this process has been associated with complications of pregnancy such as preeclampsia (PE), intrauterine growth restriction (IUGR), and, in severe cases, second-trimester miscarriage (Pijnenborg et al. 1991).
IUGR is diagnosed either by intrauterine growth assessment (sonography) showing an estimated weight below the 5th centile for gestational age or postnatal showing a birth weight below the third centile (Chatelain 2000). Small fetuses resulting from IUGR are at higher risk for poor perinatal and long-term outcome (Baschat and Hecher 2004).
Preeclampsia is associated with significant maternal and perinatal morbidity in those patients who suffer early onset of PE (Myatt and Miodovnik 1999). Patients with chronic hypertension, pregestational diabetes, or multifetal gestation are at risk for developing preeclampsia as are nulliparous women, but factors defining the risk for multiparous women are yet to be defined. Although factors such as callicrein-creatinine, coagulation, and vascular function tests and oxidant stress parameters as well as placental peptide hormones have been identified as potential markers for patients at risk for PE prospective and longitudinal studies are mandatory to verify the data (Myatt and Miodovnik 1999).
Nitric oxide (NO) as a potent vasodilator is thought to contribute to the phenomenon of decreasing vascular resistance in uterine circulation, but still its role in normal pregnancy and pregnancies complicated by PE or IUGR is controversial and remains to be clarified (Nasiell et al. 1998). Izumi and coworkers report on vasorelaxation in the human umbilical artery: their data suggest that NO synthase (NOS) is stimulated in endothelial cells and the derived NO activates guanylate cyclase to produce cyclic guanosine monophosphate in umbilical smooth muscle cells (Izumi et al. 1995). The NO systems also seems to be mainly involved in regulation of uterine quiescence during pregnancy and initiation of labor: Buhimshi et al. demonstrated an NO-cyclic guanosine monophosphate reaction pathway in the human uterus (Buhimschi et al. 1995).
The question if impaired trophoblast invasion is related to different expression of endothelial NOS (eNOS) and inducible NOS (iNOS) and results in lower NO output or if the remaining elevated impedance at the uterine and spiral arteries causes elevated compensatory NO production is topic of ongoing discussions. Purcell and colleagues showed changing concentrations of iNOS in rat placentas during the course of pregnancy with a decrease after day 16 to day 22 before labor and during delivery (Purcell et al. 1997). The reported data suggest a paracrine role for nitric oxide in regulation of uterine contractility, blood flow and immunosuppression, which are all requested for pregnancy maintenance (Purcell et al. 1997).
Myatt et al. examined placental villous tissue from normal, PE, and IUGR pregnancies by investigating the expression of eNOS (Eis et al. 1995; Myatt et al. 1997). They found increased eNOS expression in IUGR, which they interpreted as a possible adaptive response to increased resistance and poor perfusion in these pathological pregnancies (Eis et al. 1995; Myatt et al. 1997). Nasiell investigated expression of endothelial constitutive NOS (Nasiell et al. 1998). Total nuclei acids were prepared and a hybridization technique was used for mRNA analysis. The mRNA expression was significantly higher in pathological groups (IUGR, IUGR+PE, and PE) compared with normal placentas, which might reflect a compensatory mechanism in the disturbed uterine circulation seen in PE or IUGR (Nasiell et al. 1998). Within the three pathologic groups investigated, no significant differences in elevated endothelial constitutive NOS expression in IUGR, IUGR + PE, and PE alone was found. Based on these findings, elevated NO concentrations in venous umbilical blood in placentas of IUGR have also been reported, suggesting a compensatory response to improve placental circulation (Lyall et al. 1996; Macara et al. 1996). Additionally, elevated NO concentrations could also play a role in limiting platelet adhesion aggregation (Lyall et al. 1996). In contrast, Beinder and coworkers found that NOS activity from patients with preeclampsia was significantly lower in the uterine placental bed (Beinder et al. 1999).
Based on the hypothesis that heat exposure disrupts placental structure and reduces placental eNOS protein expression, Galan et al. described reduced eNOS protein content in the hyperthermic group (Galan et al. 1999,2001). Myatt et al. showed that eNOS is expressed by the syncytiotrophoblast (Myatt et al. 1993). Trophoblast differentiation is associated with expression of estrogen receptors (ER)α and ERβ (Bukovsky et al. 2003a,b). Interestingly, the ERα expression has been associated with a inhibition of angiogenesis in cancer cells (Ali et al. 2000), but if this also is the case in human pregnancy remains unknown (Ali et al. 2000). We therefore combined the immunohistochemical detection of eNOS and iNOS with investigations on ERα and ERβ expression in normal, PE, and IUGR placentas.
The aims of this study were (a) clarifying the relation between expression of iNOS/eNOS and (b) assessing the expression of ERα and ERβ in normal, preeclamptic and growth restricted human placental tissue by immunohistochemistry and Western blot experiments.
Materials and Methods
Tissue Samples
Placental tissues were obtained from 22 women who underwent delivery at the 1st Department of Obstetrics and Gynecology of the LMU Munich. Specimens were collected immediately after delivery from six patients with IUGR (mean date of delivery: 33 × 3 weeks of gestation), eight patients with preeclampsia (mean date of delivery: 33 × 3.2 weeks of gestation), and eight patients after a normal course of pregnancy (mean date of delivery: 38.2 × 3 weeks of gestation) after delivery. The study had the approval of the local ethical committee of the LMU Munich, Germany (No. 158/00) and informed consent from the patients was obtained.
Immunohistochemistry
Immunohistochemistry on paraffin sections (7 μm) of the different specimens was done by incubating the slides in methanol/H2O2 (30 min) to inhibit endogenous peroxidase activity, followed by washing in PBS (5 min) and treating with goat serum (20 min, 22C) to reduce nonspecific background staining. Incubation with the primary antibody (Table 1) was done overnight at 4C. Sections were then thoroughly incubated with the biotinylated secondary anti-mouse or anti-rabbit antibody (1 hr, 22C) and avidin-biotinylated peroxidase (45 min, RT). Between each step, sections were washed with PBS (pH 7.4). Peroxidase staining reaction was done with diaminobenzidine/H2O2 (1 mg/ml; 5 min) and stopped in tap water (10 min). Sections were counterstained in hemalum (1 min) and then cover-slipped. In controls, the primary antibody was replaced with preimmune mouse serum with positive and negative controls being included. From each section, five digital images were obtained with a 3CCD color camera (JVC; Victor Company of Japan, Japan) and a Leitz (Wetzlar, Germany) microscope.
Table 1.
Antibodies used for the study
| Antigen | Antibody | Isotype | Dilution | Source | |
| iNOS | PA3-030A | Rabbit IgG | 1:1500 | Dianova | |
| eNOS | PA1-037 | Rabbit | IgG | 1:300 | Dianova |
| ERα | ER 1D5 | Mouse IgG1 | 1:150 | Immunotech | |
| ERβ | PPG5/10 | Mouse IgG2a | 1:700 | Serotec |
Immunochemical Detection of eNOS, iNOS, ERα, and ERβ in Villous Trophoblast Cell Lysates on Blots
Villous trophoblast cells (600 μg) of normal, PE, and IUGR placental tissue were lysed in 400 μl Laemmli sample buffer for 5 min at 100C. Lysate proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 4% BSA in TBS/Tween (20 mM Tris/HCl, pH 7.2, 1 M NaCl, 0.05% Tween 20), blots were incubated with 2 μg/ml antibodies (Table 1) with 1% BSA for 16 hr at 6C. Blots were washed and incubated with goat anti-rabbit IgG-alkaline phosphatase conjugate (diluted 1:500 in TBS/Tween with 1% BSA) for 2 hr or with goat anti-mouse IgG-alkaline phosphatase conjugate (diluted 1:500 in TBS/Tween with 1% BSA) for 2 hr. Staining was performed with 5-bromo-4-chloro-3-indolyle phosphate/nitroblue-tetrazolium chloride in 0.1 M Tris-HCl, 0.15 M NaCl, pH 9.5
Immunohistochemical Evaluation and Statistical Analysis
The intensity and distribution patterns of the staining reaction was evaluated by two blinded, independent observers, including a gynecological pathologist (PH), using a semi-quantitative score (graded as 0 = no, 1 = weak, 2 = moderate, and 3 = strong staining) and without knowing the pathological evaluation, the diagnosis, or the standard performed hematoxylin reaction of each specimen. The SPSS/PC software package, version 6.01 (SPSS; Munich, Germany), was used for collection, processing, and statistical analysis of all data. Statistical analysis was performed using the non-parametrical Wilcoxon's signed rank tests for comparison of the means. The Spearman rho coefficient was used to assess any significant correlations between the analyzed substances within the distinct groups. p<0.05 values were considered statistically significant.
Results
Immunohistochemical eNOS/iNOS Expression
We found a strong immunohistochemical expression of eNOS in normal syncytiotrophoblast cells (Figure 1A) and preeclamptic syncytiotrophoblast cells (Figure 1B). In IUGR placentas, the intensity of the eNOS expression was reduced (Figure 1C). However, a strong expression of eNOS in extravillous trophoblast cells of normal-term placentas (Figure 1D) and preeclamptic placentas (Figure 1E) could be demonstrated. In IUGR placentas, eNOS expression in extravillous trophoblasts cells is reduced (Figure 1F). The expression of iNOS is similar to eNOS. We found a strong expression of iNOS in normal syncytiotrophoblast cells (Figure 2A) and preeclamptic syncytiotrophoblast cells (Figure 2B). In IUGR placentas, expression of iNOS in the syncytiotrophoblast cells is reduced (Figure 2C). In addition, we found a strong expression of iNOS in extravillous trophoblast cells of normal term placentas (Figure 2D) and preeclamptic placentas (Figure 2E). In IUGR-placentas iNOS expression is reduced (Figure 2F) in extravillous trophoblast cells. The semiquantitative score of the eNOS/iNOS staining intensity is summarized in Figure 5. Differences in eNOS expression in the syncytiotrophoblast of normal and IUGR placentas and preeclamptic and IUGR placentas are statistically significant (p=0.039 and p=0.038).
Figure 1.
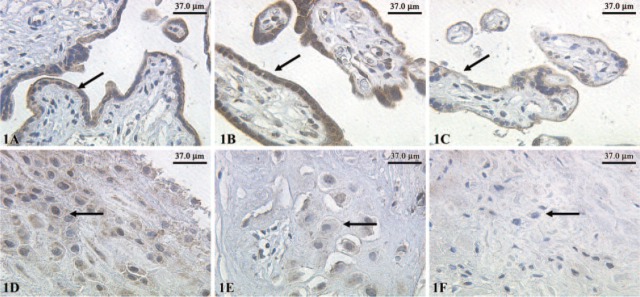
Expression of endothelial nitric oxide synthase (eNOS) in normal syncytiotrophoblasts indicated by arrows (A). Preeclamptic syncytiotrophoblast (B) and intrauterine growth restriction (C) detected with polyclonal rabbit antibodies of the IgG subtype and staining with diaminobenzidine; X25 lens. Expression of eNOS was also detected in extravillous trophoblast cells indicated by arrows of normal-term placentas (D), preeclamptic placentas (E), and intrauterine growth-restricted placentas (F); X25 lens.
Figure 2.
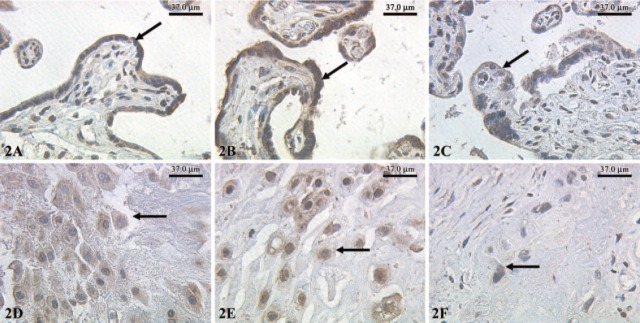
Expression of inducible nitric oxide synthase (iNOS) (indicated by arrows) in normal syncytiotrophoblasts (A), preeclamptic syncytiotrophoblast (B), and intrauterine growth restriction (C) detected with polyclonal rabbit antibodies of the IgG subtype and staining with diaminobenzidine; X25 lens. Expression of iNOS was also detected in extravillous trophoblast cells of normal-term placentas (D), preeclamptic placentas (E), and intrauterine growth-restricted placentas (F); X25 lens.
Figure 5.
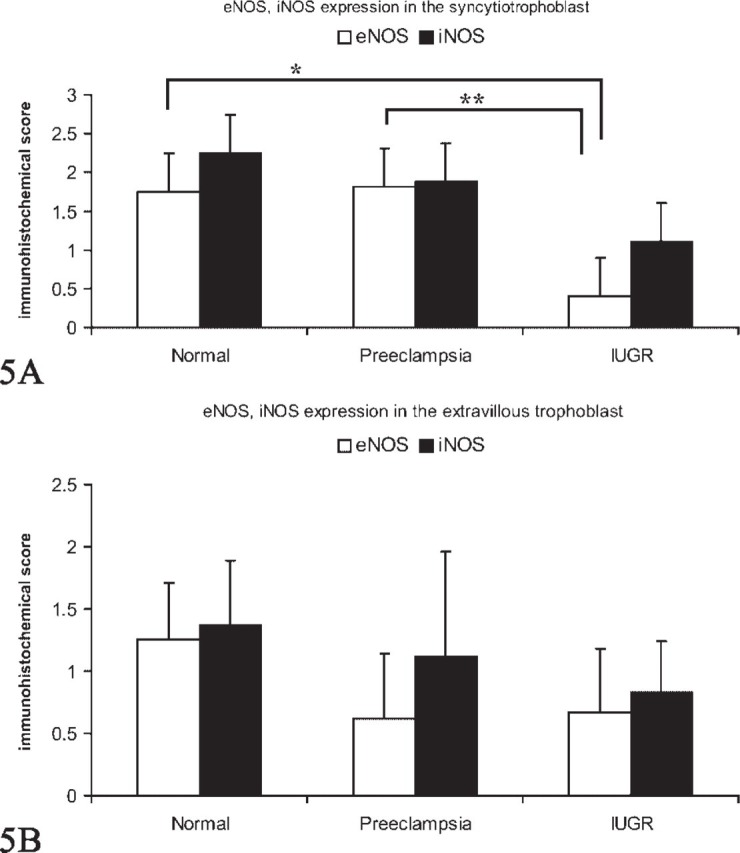
(A) Staining intensity of endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) in normal, preeclamptic, and intrauterine growth-restricted (IUGR) syncytiotrophoblast determined by a semiquantitative immunohistochemical score on the different tissue slides. Data shown represent mean × SEM. Differences in eNOS expression in normal and IUGR syncytiotrophoblast (∗) and preeclamptic and IUGR syncytiotrophoblast (∗∗) are statistically significant (p=0.039 and p=0.038). (B) Staining intensity of eNOS and iNOS in normal, preeclamptic, and IUGR extravillous trophoblast determined by a semiquantitative immunohistochemical score on the different tissue slides. Data shown represent mean × SEM.
ERα/ERβ Expression
A moderate expression of ERα in normal syncytiotrophoblast cells (Figure 3A), PE syncytiotrophoblast (Figure 3B) and IUGR syncytiotrophoblast cells (Figure 3C) was noted. Additionally, we also demonstrated a moderate expression of ERα in extravillous trophoblast cells of normal-term placentas (Figure 3D), PE placentas (Figure 3E), and IUGR-placentas (Figure 3F). The semiquantitative score of the ERα staining intensity showed no significant differences in all groups investigated. We found a low expression of ERβ in normal syncytiotrophoblast cells (Figure 4A) and a moderate expression in PE syncytiotrophoblasts (Figure 4B). In IUGR placentas, expression of ERβ in the syncytiotrophoblast cells is reduced (Figure 4C). In addition, we found a moderate expression of ERβ in extravillous trophoblast cells of normal-term placentas (Figure 4D) and PE placentas (Figure 4E). In IUGR placentas, ERβ expression in extravillous trophoblasts cells is almost not detectable (Figure 4F). The semiquantitative score of the eNOS/iNOS staining intensity is summarized in Figure 5, whereas the semiquantitative score of the ERα/ERβ staining intensity is summarized in Figure 6. Interestingly, a strong correlation between ERα expression and iNOS/eNOS could be demonstrated (p<0.05 and p<0.005, respectively) in the syncytiotrophoblast of normal placentas. However, no correlation between these factors was observed in placentas of IUGR and preeclamptic patients.
Figure 3.
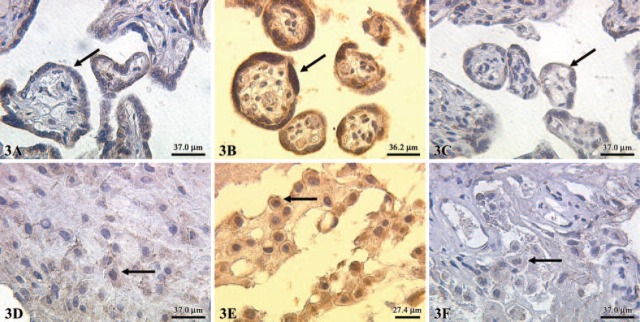
Expression of estrogen receptor (ER)α (indicated by arrows) in normal syncytiotrophoblasts (A), preeclamptic syncytiotrophoblast (B), and intrauterine growth restriction (C). Expression of ERα was also detected in extravillous trophoblast cells of normal term placentas (D) preeclamptic placentas (E) and intrauterine growth-restricted placentas (F); X25 lens.
Figure 4.
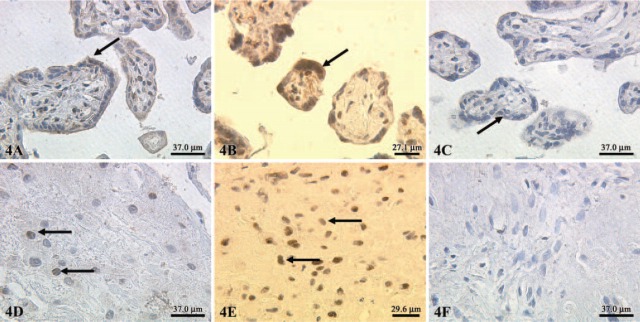
Expression of estrogen receptor (ER)β (indicated by arrows) in normal syncytiotrophoblasts preeclamptic (A), syncytiotrophoblast (B), and intrauterine growth restriction (C). Expression of ERβ was also detected in extravillous trophoblast cells of normal-term placentas (D), preeclamptic placentas (E), and intrauterine growth-restricted placentas (F); X25 lens.
Figure 6.
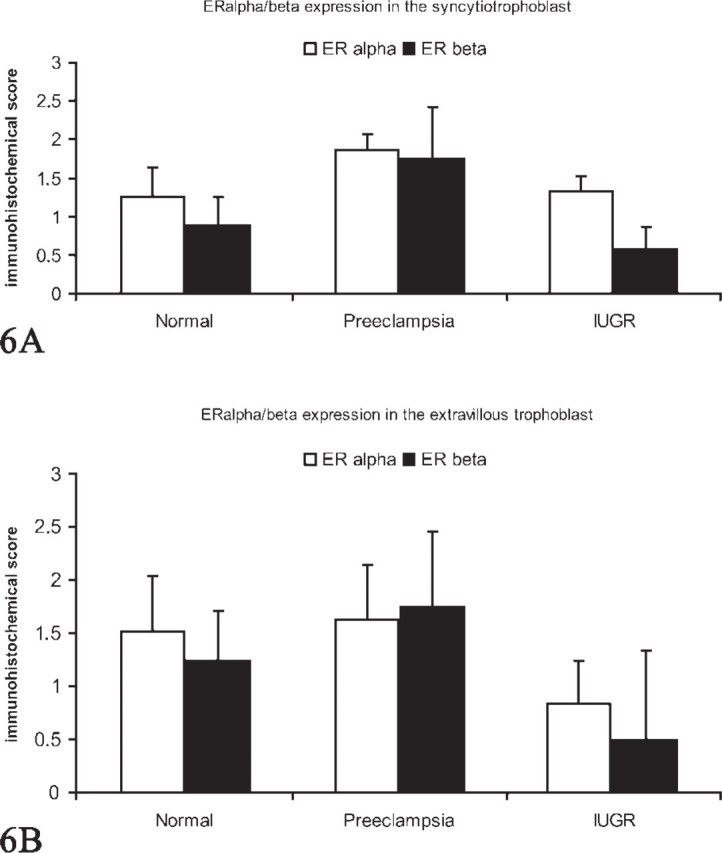
(A) Staining intensity of estrogen receptor (ER)α and ERβ in normal, preeclamptic, and intrauterine growth-restricted (IUGR) syncytiotrophoblast determined by a semiquantitative immunohistochemical score on the different tissue slides. Data shown represent mean × SEM. (B) Staining intensity of ERα and ERβ in normal, preeclamptic, and IUGR extravillous trophoblast determined by a semiquantitative immunohistochemical score on the different tissue slides. Data shown represent mean × SEM.
Immunochemical Detection of eNOS, iNOS, ERα, and ERβ in Villous Trophoblast Cell Lysates on Blots Results of immunochemical detection (Western blots) of eNOS in villous trophoblast cell lysates are shown in Figure 7A. The eNOS polyclonal antibody generates a main protein band in the 140 kDa molecular mass range. Normal villous trophoblast tissue (Lane 1) and PE trophoblast tissue (Lane 2) showed almost the same staining intensity, whereas, in IUGR tissue (Lane 3), eNOS expression is reduced.
Figure 7.
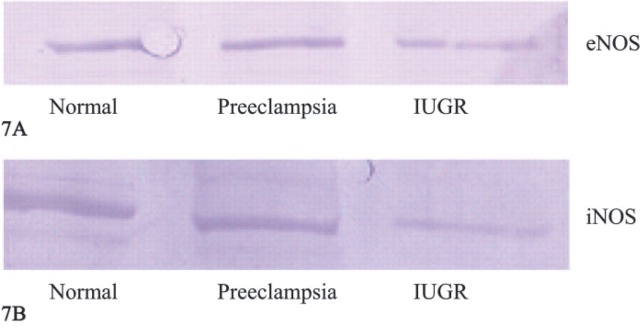
(A) Western blot of endothelial nitric oxide synthase detection in villous trophoblast cell lysate. Lane 1: normal placenta; Lane 2: preeclamptic placenta; Lane 3: intrauterine growth-restricted (IUGR) placenta. (B) Western blot of inducible nitric oxide synthase detection in villous trophoblast cell lysate. Lane 1: normal placenta; Lane 2: preeclamptic placenta; Lane 3: IUGR placenta.
Results of immunochemical detection (Western blots) of iNOS in villous trophoblast cell lysates are shown in Figure 7B. The iNOS polyclonal antibody generates a main protein band in the 130-kDa molecular mass range. Normal villous trophoblast tissue (Lane 1) and preeclamptic trophoblast tissue (Lane 2) showed almost the same staining intensity, whereas in IUGR tissue (Lane 3) iNOS expression was reduced.
Results of immunochemical detection (Western blots) of ERα in villous trophoblast cell lysates are shown in Figure 8A. The ERα monoclonal antibody generates a main protein band in the 67-kDa molecular mass range. Normal villous trophoblast tissue (Lane 1), preeclamptic trophoblast tissue (Lane 2), and IUGR tissue (Lane 3) showed almost the same staining intensity.
Figure 8.
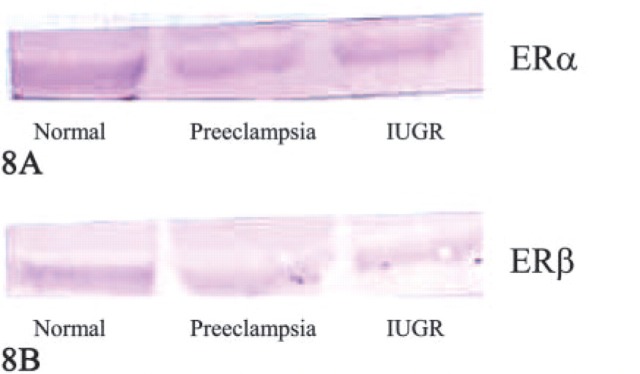
(A) Western blot of estrogen receptor (ER)α detection in villous trophoblast cell lysate. Lane 1: normal placenta; Lane 2: preeclamptic placenta; Lane 3: intrauterine growth-restricted (IUGR) placenta. (B) Western blot of ERβ detection in villous trophoblast cell lysate. Lane 1: normal placenta; Lane 2: preeclamptic placenta; Lane 3: IUGR placenta.
Results of immunochemical detection (Western blots) of ERβ in villous trophoblast cell lysates are shown in Figure 8B. The ERβ monoclonal antibody generates a main protein band in the 57-kDa molecular mass range. Normal villous trophoblast tissue (Lane 1) and PE trophoblast tissue (Lane 2) showed almost the same staining intensity, whereas in IUGR tissue (Lane 3), ERβ expression is reduced.
Discussion
We investigated the expression of ERα, ERβ, eNOS, and iNOS in IUGR, PE, and normal control placentas. In addition to previously published data, we found a significant lowered expression of iNOS and eNOS in placentas of growth-restricted fetuses, but not in PE placentas. We also identified a lowered expression of ERβ in IUGR placentas compared with normal and an elevated expression of ERβ in PE placentas compared with IUGR and normal placentas. To our best knowledge, there is no previous report on the interaction of these systems in the placenta in normal and pathologic pregnancy.
There are several reports on NO and its fetoplacental synthases (NOS) distribution in normal and preeclamptic placentas. PE is characterized by hypertension, edema, and proteinuria and affects ∼5–10% of all pregnancies (Bartl and Muller-Tyl 1985). PE is associated with IUGR and impaired uterine blood flow (Schonfelder et al. 2004; Takagi et al. 2004; Torry et al. 2004). IUGR, on the other hand, is not necessarily associated with PE symptoms such as hypertension and proteinuria. In PE, fetoplacental NOS activity and NO concentrations in the umbilical circulation are altered. Some studies have described a decreased or unchanged placental NOS activity in preeclampsia (Lee et al. 1997; Nasiell et al. 1998; Faxen et al. 2001; Schonfelder et al. 2004). Elevated nitrite/nitrate concentrations in umbilical vein blood from PE patients compared with control patients have also been demonstrated (Lyall et al. 1995; Norris et al. 1999), suggesting an increase of NO production in the fetoplacental unit in PE. NO in the fetoplacental circulation is derived from eNOS activity, found predominantly in the syncytiotrophoblast (Lyall et al. 1995; Norris et al. 1999; Ayuk et al. 2002). Ayuk and coworkers described investigations on l-arginine as precursor for NO synthesis. They found no differences of the transport systems of l-arginine in relation to NO production in normal-term pregnancies or those complicated by IUGR or PE (Ayuk et al. 2002; Speake et al. 2003). Their findings lead to speculate that decreased or lowered NO concentration is not the result of a reduced substrate concentration in PE or IUGR. In this study, we identified only moderate elevated eNOS expression in the syncytiotrophoblast of PE placentas, although differences were not significantly different from normal controls. Differences in eNOS expression were significant between IUGR placentas and normal controls and IUGR placentas and PE placentas. Both iNOS and eNOS expression is reduced in IUGR placentas, especially in the extravillous trophoblast (Figures 1F and Figure 2F). In addition, Witlin et al. demonstrated that L-NAME-treated rats show increased decidual necrosis and deficient fetal vessel development. Application of adrenomedullin did not ameliorate hypertension or growth restriction (Witlin et al. 2003).
Bukovsky and coworkers recently demonstrated both in vivo and in vitro that ERα+/ERβ- trophoblast cells differentiate into ERα+/ERβ+ in the first trimester of pregnancy, changing into ERα-/ERβ+ in mature states. They demonstrated a dynamic and maturity-dependent expression of ERα and ERβ (Bukovsky et al. 2003a,b). In the present study, we identified a raised expression of ERα/ERβ in PE placentas but a reduced ERβ expression in IUGR placentas compared with normal controls, but without statistical significance. ERα is known to play an important role in the proliferation and so does ERβ in the maturation of estrogen-dependent cells (Bukovsky et al. 2003a,b). Bukovsky et al. also stated that significantly enhanced expression of ERβ in differentiating trophoblast cells and stimulation of trophoblast differentiation by estrogens, indicates a unique role of the ERβ hormone-binding domain in the regulation of placental function (Bukovsky et al. 2003a,b). Because the trophoblast is a major source of placental hormones, ERβ expression by trophoblast cells may be involved in stimulation of placental hormonal production by estrogens.
In summary, we demonstrated reduced ERβ, iNOS, and eNOS expression in trophoblast cells in placentas of growth-restricted pregnancies. Regarding these two findings, one may speculate that ER trophoblast differentiation in pathological pregnancies is altered. Whether reduced iNOS/eNOS expression results in altered differentiation of ERα to ERβ or if an unchanged status of ER expression results in reduced expression of iNOS/eNOS merits further investigation. The significant association between ERα and NOS in normal pregnancies suggest an important role in the establishment of the fetoplacental unit and the physiological ongoing of pregnancy. This is underlined by the lack of association between IUGR and PE placentas and might suggest that during pathogenesis of IUGR and PE, the normal relation between ER and NOS is disrupted, although additional data are still needed.
Literature Cited
- Ali SH, O'Donnell AL, Balu D, Pohl MB, Seyler MJ, Mohamed S, Mousa S, et al. (2000) Estrogen receptor-alpha in the inhibition of cancer growth and angiogenesis. Cancer Res 60: 7094–7098 [PubMed] [Google Scholar]
- Ayuk PT, Theophanous D, D'Souza SW, Sibley CP, Glazier JD. (2002) L-arginine transport by the microvillous plasma membrane of the syncytiotrophoblast from human placenta in relation to nitric oxide production: effects of gestation, preeclampsia, and intrauterine growth restriction. J Clin Endocrinol Metab 87: 747–751 [DOI] [PubMed] [Google Scholar]
- Bartl W, Muller-Tyl E. (1985) Placental morphology and clinical correlations in pregnancies complicated by hypertension. Biol Res Pregnancy Perinatol 6: 173–176 [PubMed] [Google Scholar]
- Baschat AA, Hecher K. (2004) Fetal growth restriction due to placental disease. Semin Perinatol 28: 67–80 [DOI] [PubMed] [Google Scholar]
- Beinder E, Mohaupt MG, Schlembach D, Fischer T, Sterzel RB, Lang N, Baylis C. (1999) Nitric oxide synthase activity and Doppler parameters in the fetoplacental and uteroplacental circulation in preeclampsia. Hypertens Pregnancy 18: 115–127 [DOI] [PubMed] [Google Scholar]
- Brosens I, Robertson WB, Dixon HG. (1967), The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol 93:569–579 [DOI] [PubMed] [Google Scholar]
- Buhimschi I, Yallampalli C, Dong YL, Garfield RE. (1995) Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am J Obstet Gynecol 172: 1577–1584 [DOI] [PubMed] [Google Scholar]
- Bukovsky A, Caudle MR, Cekanova M, Fernando RI, Wimalasena J, Foster JS, Henley DC, et al. (2003a) Placental expression of estrogen receptor beta and its hormone binding variant—comparison with estrogen receptor alpha and a role for estrogen receptors in asymmetric division and differentiation of estrogen-dependent cells. Reprod Biol Endocrinol 1: 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovsky A, Cekanova M, Caudle MR, Wimalasena J, Foster JS, Henley DC, Elder RF. (2003b) Expression and localization of estrogen receptor-alpha protein in normal and abnormal term placentae and stimulation of trophoblast differentiation by estradiol. Reprod Biol Endocrinol 1: 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain P. (2000) Children born with intra-uterine growth retardation (IUGR) or small for gestational age (SGA): long term growth and metabolic consequences. Endocr Regul 34: 33–36 [PubMed] [Google Scholar]
- Di Paolo S, Volpe P, Grandaliano G, Stallone G, Schena A, Greco P, Resta L, et al. (2003) Increased placental expression of tissue factor is associated with abnormal uterine and umbilical Doppler waveforms in severe preeclampsia with fetal growth restriction. J Nephrol 16: 650–657 [PubMed] [Google Scholar]
- Eis AL, Brockman DE, Pollock JS, Myatt L. (1995) Immunohistochemical localization of endothelial nitric oxide synthase in human villous and extravillous trophoblast populations and expression during syncytiotrophoblast formation in vitro. Placenta 16: 113–126 [DOI] [PubMed] [Google Scholar]
- Faxen M, Nisell H, Kublickiene KR. (2001) Altered mRNA expression of ecNOS and iNOS in myometrium and placenta from women with preeclampsia. Arch Gynecol Obstet 265: 45–50 [DOI] [PubMed] [Google Scholar]
- Galan HL, Hussey MJ, Barbera A, Ferrazzi E, Chung M, Hobbins JC, Battaglia FC. (1999) Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol 180: 1278–1282 [DOI] [PubMed] [Google Scholar]
- Galan HL, Regnault TR, Le Cras TD, Tyson RW, Anthony RV, Wilkening RB, Abman SH. (2001) Cotyledon and binucleate cell nitric oxide synthase expression in an ovine model of fetal growth restriction. J Appl Physiol 90: 2420–2426 [DOI] [PubMed] [Google Scholar]
- Izumi H, Makino Y, Shirakawa K, Garfield RE. (1995) Role of nitric oxide on vasorelaxation in human umbilical artery. Am J Obstet Gynecol 172: 1477–1484 [DOI] [PubMed] [Google Scholar]
- Lee CN, Chang SW, Cho NH, Cho SH. (1997) Nitrous oxide synthase expression in placenta of preeclampsia. J Korean Med Sci 12: 532–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall F, Greer IA, Young A, Myatt L. (1996) Nitric oxide concentrations are increased in the feto-placental circulation in intrauterine growth restriction. Placenta 17: 165–168 [DOI] [PubMed] [Google Scholar]
- Lyall F, Young A, Greer IA. (1995) Nitric oxide concentrations are increased in the fetoplacental circulation in preeclampsia. Am J Obstet Gynecol 173: 714–718 [DOI] [PubMed] [Google Scholar]
- Macara L, Kingdom JC, Kaufmann P, Kohnen G, Hair J, More IA, Lyall F, et al. (1996) Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta 17: 37–48 [DOI] [PubMed] [Google Scholar]
- Myatt L, Brockman DE, Eis AL, Pollock JS. (1993) Immunohistochemical localization of nitric oxide synthase in the human placenta. Placenta 14: 487–495 [DOI] [PubMed] [Google Scholar]
- Myatt L, Eis AL, Brockman DE, Greer IA, Lyall F. (1997) Endothelial nitric oxide synthase in placental villous tissue from normal, pre-eclamptic and intrauterine growth restricted pregnancies. Hum Reprod 12: 167–172 [DOI] [PubMed] [Google Scholar]
- Myatt L, Miodovnik M. (1999) Prediction of preeclampsia. Semin Perinatol 23: 45–57 [DOI] [PubMed] [Google Scholar]
- Nasiell J, Nisell H, Blanck A, Lunell NO, Faxen M. (1998) Placental expression of endothelial constitutive nitric oxide synthase mRNA in pregnancy complicated by preeclampsia. Acta Obstet Gynecol Scand 77: 492–496 [PubMed] [Google Scholar]
- Norris LA, Higgins JR, Darling MR, Walshe JJ, Bonnar J. (1999) Nitric oxide in the uteroplacental, fetoplacental, and peripheral circulations in preeclampsia. Obstet Gynecol 93: 958–963 [DOI] [PubMed] [Google Scholar]
- Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH. (2001) Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol 18: 441–449 [DOI] [PubMed] [Google Scholar]
- Papageorghiou AT, Yu CK, Cicero S, Bower S, Nicolaides KH. (2002) Second-trimester uterine artery Doppler screening in un-selected populations: a review. J Matern Fetal Neonatal Med 12: 78–88 [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A. (1991) Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 98: 648–655 [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Brosens I. (1983) Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 4: 397–413 [DOI] [PubMed] [Google Scholar]
- Purcell TL, Buhimschi IA, Given R, Chwalisz K, Garfield RE. (1997) Inducible nitric oxide synthase is present in the rat placenta at the fetal-maternal interface and decreases prior to labour. Mol Hum Reprod 3: 485–491 [DOI] [PubMed] [Google Scholar]
- Schonfelder G, Fuhr N, Hadzidiakos D, John M, Hopp H, Paul M. (2004) Preeclampsia is associated with loss of neuronal nitric oxide synthase expression in vascular smooth muscle cells of the human umbilical cord. Histopathology 44: 116–128 [DOI] [PubMed] [Google Scholar]
- Speake PF, Glazier JD, Ayuk PT, Reade M, Sibley CP, D'Souza SW. (2003) L-Arginine transport across the basal plasma membrane of the syncytiotrophoblast of the human placenta from normal and preeclamptic pregnancies. J Clin Endocrinol Metab 88: 4287–4292 [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nikaido T, Toki T, Kita N, Kanai M, Ashida T, Ohira S, et al. (2004) Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch 444: 49–55 [DOI] [PubMed] [Google Scholar]
- Torry DS, Hinrichs M, Torry RJ. (2004) Determinants of placental vascularity. Am J Reprod Immunol 51: 257–268 [DOI] [PubMed] [Google Scholar]
- Witlin AG, Gangula PR, Wimalawansa SJ, Grafe M, Grady JJ, Yallampalli C. (2003) Adrenomedullin requires an intact nitric oxide system to function as an endogenous vasodilator in rat gestation. Hypertens Pregnancy 22: 9–24 [DOI] [PubMed] [Google Scholar]
- Yagel S, Anteby EY, Shen O, Cohen SM, Friedman Z, Achiron R. (1999) Placental blood flow measured by simultaneous multigate spectral Doppler imaging in pregnancies complicated by placental vascular abnormalities. Ultrasound Obstet Gynecol 14: 262–266 [DOI] [PubMed] [Google Scholar]


