Abstract
CD74 is known as the major histocompatibility complex (MHC) class II-associated invariant chain (Ii) that regulates the cell biology and functions of MHC class II molecules. Class II MHC and Ii expression was believed to be restricted to classical antigen-presenting cells (APC); however, during inflammation, other cell types, including mucosal epithelial cells, have also been reported to express class II MHC molecules. Given the importance of Ii in the biology of class II MHC, we sought to examine the expression of Ii by gastric epithelial cells (GEC) to determine whether class II MHC molecules in these nonconventional APC cells were under the control of Ii and to further support the role that these cells may play in local immune and inflammatory responses during Helicobacter pylori infection. Thus we examined the expression of Ii on GEC from human biopsy samples and then confirmed this observation using independent methods on several GEC lines. The mRNA for Ii was detected by RT-PCR, and the various protein isoforms were also detected. Interestingly, these cells have a high level expression of surface Ii, which is polarized to the apical surface. These studies are the first to demonstrate the constitutive expression of Ii by human GEC.
Keywords: antigen presentation, CD74, invariant chain, mucosal immunity
Helicobacter pylori infects the human gastric epithelium and is associated with peptic and duodenal ulcers, as well as gastric carcinoma. Because not everyone who is infected develops clinically significant disease, host factors are suspect in the pathogenesis. CD4+ T cells are rarely found within the uninfected gastric mucosa, but are substantially increased during infection and are predominantly Th1 cells (Bamford et al. 1998). These Th1 cells are regarded as contributors to the tissue damage. Interestingly, the antigen-presenting cells (APC) involved in the activation of these T cells are uncharacterized.
The expression of class II major histocompatibility complex (MHC) molecules is characteristic of professional APCs, such as dendritic cells, macrophages, and B cells. The role of class II MHC molecules on APCs is to present peptides from endocytosed antigens to CD4+ T cells before the initiation of an immune response. The human gastric epithelium has been noted to constitutively express class II MHC molecules, in addition to the costimulatory molecule CD86 and antigen-processing enzymes (Ye et al. 1997; Barrera et al. 2001; Barrera et al. 2002). In Helicobacter pylori infection CD4+ T cells are found adjacent to the gastric epithelium; therefore, gastric epithelial cells have been suspected of acting as APCs locally. In APCs, the assembly and intracellular transport of class II MHC are influenced by their association with an invariant chain protein (Ii, CD74) (Bakke and Dobberstein 1990; Lotteau et al. 1990). Because of the presence of a targeting signal sequence in the cytoplasmic domain of Ii, Ii and the associated class II MHC molecules are able to intersect the endocytic pathway. Recent studies have also implicated an isoform of Ii in regulating the action of endocytic proteases, which may in turn influence the formation of peptides that may become selected for presentation by class II MHC molecules (Fiebiger et al. 2002).
Because Ii plays an important role in APC function, in this study we sought to characterize the expression of Ii by gastric epithelial cells to establish whether class II MHC function in these cells is under the same regulatory control as in conventional APCs (Pieters 2000). The constitutive expression of Ii by polarized cells that line the gastric mucosa is of considerable importance to understand the immunological events that are elicited in response to a common human pathogen, such as H. pylori. The expression of class II MHC by other epithelial cells has been noted during various inflammatory conditions such as autoimmunity and organ transplantation; however, the expression of class II MHC by gastric epithelial cells is constitutive and increases during infection with H. pylori. Although the role of gastric epithelial cells, or other epithelial cells that express class II MHC in immunity, is largely unexplored, in this report, we show that gastric epithelial cells express this important regulatory molecule of APC function known as Ii. The expression of Ii by the gastric epithelium is constitutive in vivo and was readily detected in established gastric epithelial cell lines. Interestingly, the expression of Ii by gastric epithelial cells is polarized to the apical surface. In addition, the studies presented in this report show that gastric epithelial cells also express the various iso-forms of Ii that are present in conventional APCs.
Materials and Methods
Gastric Biopsy Staining
Gastric antrum biopsy tissues were obtained from consenting donors undergoing gastroesophageal duodenoscopy at the University of Texas Medical Branch. The biopsies were obtained from grossly inflamed areas of the antrum and from regions that appeared uninvolved. The tissue was acquired after receiving informed consent from patients and volunteers, and with prior approval of the institutional review board. The tissue samples were fixed in 10% neutral buffered solution and then embedded in paraffin for immunohistochemistry. Four μm thick sections of the paraffin-embedded tissue blocks were mounted on plus (coated) glass slides and deparaffinized in xylene, followed by rehydration in ethanol. Endogenous peroxidase was blocked by incubation in 3% methanol peroxide for 10 min. For Ii immuno-histochemical staining, antigen retrieval was done by pre-treating the sections with citrate buffer (0.01 ml/L citric acid at pH 6.0) for 20 min at 99C in a microwave oven and then allowed to cool for 20 min at room temperature. Nonspecific endogenous proteins were blocked using the avidin-biotin blocking kit (Vector, Burlingame, CA) followed by 30 min incubation at room temperature with biotinylated, mouse, anti-human Ii antibody clone LN-2 (Dako; Carpinteria, CA) at 1:200 dilution. The sections were stained with a streptavidin-biotin peroxidase detection kit (Dako), incubated with 3,3'-diaminobenzidine, and counterstained with hematoxylin followed by cover-slipping. The immunostaining reactions were examined by light microscopy.
Cell Lines and Culture Conditions
Jesthom, an HLA-DR1 homozygous EBV-transformed cell line, was used as prototypic APC. KATO III and N87 gastric epithelial cell lines were from ATCC (Manassas, VA). The cell lines were grown in RPMI 1640 (Gibco BRL; Gaithersburg, MD) medium supplemented with 10% FCS (Atlanta Biologicals; Atlanta, GA), glutamine (1 mM), penicillin (1 U/ml), and streptomycin (100 μg/ml). Confluent KATO III and N87 cells were collected using EDTA in PBS at 37C, followed by shaking. After collection, the cells were washed with PBS and their concentration adjusted in the indicated buffer before using them.
Antibodies
Anti-human Ii monoclonal antibody VicY-1 to an epitope in the cytoplasmic tail was a gift from Dr. Walter Knapp (Institute of Immunology; University of Vienna, Vienna, Austria). The PIN 1.1 antibody, which also recognizes an epitope within the amino terminal portion of Ii, was a gift from Dr. Peter Creswell (Yale University; New Haven, CT). The anti-human Ii (clone BU-45) antibody to an exomembranous epitope was from Serotec (Raleigh, NC). The anti-human epithelial antigen (Clone Ber-EP4) was from Dako. The anti-Zonula occludens-1 (ZO-1) antibody was from BD Transduction Laboratories (Franklin Lakes, NJ).
Flow Cytometry
A total of 106 gastric epithelial cells or control B cells were grown in medium alone or supplemented, when indicated, with IFN-γ (100 U/ml) for 48 hr. Some samples were treated with 2 units of chondroitinase ABC (Sigma-Aldrich; St. Louis, MO) for 4 hr at 37C and washed with ice-cold PBS to stop the reaction to detect the extent of Ii modified by chon-droitin sulfate (CS) that is detected by anti-Ii after CS removal. Cells were incubated with anti-Ii BU-45 monoclonal antibody (1 μg) in PBS buffer for 30 min on ice. The cells were washed three times with ice-cold PBS and then incubated with FITC-conjugated, goat anti-mouse IgG (1 μg) for an additional 30 min on ice. After incubation, the cells were washed and fixed with 1% paraformaldehyde in PBS. Then, 10,000 events per sample were collected for further analysis. The flow cytometry analysis was done on a FACScan cytometer (Becton Dickinson; Mountain View, CA) and the data analysis was done using WinMDI version 2.8 software (freeware written by Dr. Joseph Trotter; The Scripps Institute, La Jolla, CA).
Confocal Microscopy
N87 cells were grown on polycarbonate filter inserts 6.5 mm in diameter with a 3.0-μm pore size (Costar; Cambridge, MA) to near confluency where they were treated with interferon γ (100 U/ml) for 48 hr. Cells were washed with PBS three times and blocked by incubating with horse serum for 1 hr at room temperature. Three more washes were administered, followed by 1-hr incubation at 20C with mouse anti-human Ii BU-45 antibody or mouse anti-human decay accelerating factor antibody (a kind gift from Dr. Bogdan Nowicki; University of Texas Medical Branch, Galveston, TX), which was used to detect an apical marker control (Peiffer et al. 2000). After three washes, secondary goat anti-mouse IgG conjugated with Alexa 568 (Molecular Probes; Eugene, OR) was added to chamber inserts and to wells outside the chamber and incubated for 1 hr at room temperature. As staining controls, anti-epithelial antigen FITC-conjugated IgG (Dako) was used on a separate insert, as were the cells stained with anti-ZO-1 and the anti-Ii antibody, PIN 1.1, that recognizes the cytoplasmic tail of Ii. Cells were then washed three times, and fixed with 2% paraformaldehyde in PBS for 15 min. To stain the cells with anti-ZO-1 and PIN 1.1, the cells were permeabilized with Cytofix/Cytoperm Solution (BD Biosciences; Franklin Lakes, NJ) and washed with Perm/Wash solution (BD Biosciences) before the addition of the corresponding antibodies. After washing three times, secondary goat anti-mouse IgG conjugated with Alexa 568 (Molecular Probes) was added. The filters were cut out of the inserts, mounted, cover-slipped, and sealed for microscopy. The samples were analyzed using a Zeiss laser-scanning microscope, model 510, equipped with Argon2 and HeNe1 lasers, and a 63 × oil objective. Images were analyzed using Zeiss LSM Image Browser 5 (Carl Zeiss; Jena, Germany).
Metabolic Labeling
Stimulated gastric epithelial cells and B cells in the log phase of growth were incubated for 1 hr at a density of 107/ml in methionine- or sulfate-free medium, depending on whether they were labeled with [35S] methionine or [35SO4] sulfate, respectively. At the end of the deprivation period, the cells were supplemented with the radiolabeled amino acid or SO4 (0.5 mCi/108 cells). After 6 hr of incubation, the cells were washed three times with PBS. Then, cells were hypotonically lysed with 10 mM Tris-HCl buffer, pH 8.1, containing 10 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 2 mM N-ethyl malemide. Nuclei were removed by centrifugation at 10,000 × g for 10 min and the supernatants were centrifuged at 100,000 × g for 45 min to obtain the microsomal membranes. Microsomal membrane proteins were solubilized in ice-cold, 10 mM Tris-HCl, pH 8.1, containing 0.5% Triton X-100. Unsolubilized material was removed by centrifugation at 100,000 × g for 1 hr and then the samples were ready to apply to prepared Immunoadsorbent or to the DEAE-Sephacel, anion-exchange resin for Ii-CS enrichment (described in the following section).
Immunoprecipitation and Electrophoresis
Solubilized membrane samples were precleared with normal rabbit serum and formalized Staphylococcus aureus, Cowen strain A (Chemicon; El Segundo, CA). Immunoadsorbents were prepared by incubating 100 μl of protein A-Sepharose beads (Sigma Chemical Co.; St Louis, MO) with ∼1 μg of each antibody, as previously described (Reyes et al. 1991). After 16 hr of incubation at 4C with solubilized proteins, the immunoadsorbents were washed five times with immunoadsorbent buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, and 0.02% NaN3) and eluted with SDS sample buffer (62.5 mM Tris HCl, pH 6.8, 10% [w/v] glycerol, 5% 2-mercaptoethanol, and 2.3% SDS). The proteins were then separated on SDS/PAGE by one dimension. In the case of two-dimensional, nonequilibrium pH gradient electrophoresis, samples were eluted in isoelectrofocusing sample buffer (9.0 M urea, 4% NP40, 2% ampholytes, 1% dithiothreitol), separated by nonequilibrium pH gradient electrophoresis, followed by SDS-PAGE and then autoradiographed.
Concentration of Ii-CS or Associated Proteins
The solubilized proteins were diluted in associative DEAE-buffer (0.05 M C2H3NaO2, 0.15 M NaCl, 0.5% Triton X-100, pH 6.0, 10 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride and 2 mM N-ethyl malemide) and were applied to the DEAE-sephacel anion exchange resin (Pharmacia, Inc.; Piscat-away, NJ) at a ratio of 1 × 107 cells/0.5 ml packed sephacel. Unbound material was collected and passed through the column twice. The DEAE-sephacel was then washed with ice-cold, associative buffer containing protease inhibitors until the counts per minute were close to background. Materials bound to the DEAE-sephacel column, which included Ii-CS and associated proteins, were eluted with DEAE buffer (0.8M NaCl, 0.05 M C2H3NaO2, 0.002% Triton X-100, pH 6.0). After the elution procedure, the samples were adjusted to pH 7.4 with 0.2 M NaCl, using Centricon 10 (Pierce Chemical; Rockford, IL), and then used in immunoprecipitation analysis.
RT-PCR
Total RNA was extracted from 1 × 106 gastric epithelial cells and B cells using Trizol reagent (Life Technologies Inc.; Grand Island, NY) according to the manufacturer's instructions. Gastric epithelial cells were treated with interferon-γ (100 U/ml) for 48 hr. Next, 5 μ RNA was reverse-transcribed using 0.1 μ of oligo dT primer, 1 mM of each dNTP, and 50 U MuLV reverse transcriptase (Perkin-Elmer; Branchburg, NJ), at 42C for 15 min as previously described (Barrera et al. 2001). The resulting cDNA was then amplified by PCR in a reaction mixture consisting of 1 mM MgCl2, 50 mM KCl, 0.2 mM of each dNTP, 0.4 μ of each primer, and 2.5 U Amplitaq DNA polymerase (Perkin Elmer). PCR for Ii was carried out in a programmed thermal cycler (Perkin Elmer Cetus; Emeryville, CA) at 30 cycles of 95C for 15 sec and 72C for 45 sec. The primers were synthesized by Sigma Genosys (Chicago, IL). The Ii primers were 5'-GAATGCTGACCCCCTGAAGGTGTA-3’ (sense) and 5'-GGGGGCTGAAGGGAGCAAGAAAGC-3’ (antisense). The expected products are 586 bp (p41) and 396 bp (p33). Amplified products were analyzed by 1.5% agarose gel electrophoresis and after electrophoresis the gels were stained with 0.5 μg/ml of ethidium bromide for 1 hr and washed before exposure to an ultraviolet Foto/Prep I (Fotodyne Inc.; New Berlin, WI) for visualization and photography. As an internal control, the RNA preparations were processed by RT-PCR to amplify the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. The glyceraldehyde-3-phosphate dehydrogenase primers were 5'-GTGAAGGTCGGAGTCA-ACG-3’ (sense) and 5'-GGTGAAGACGCCAGTGGAC-3’ (antisense) synthesized by Sigma Genosys and the expected product is of 340 bp.
Results
Human Gastric Epithelial Cell Lines Express Invariant Chain Proteins
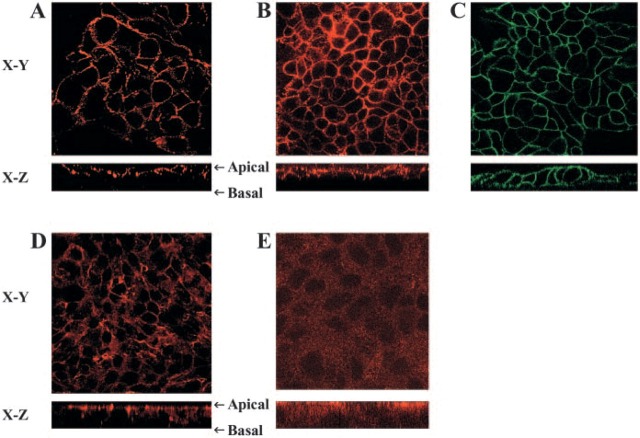
Because MHC class II Expression has been detected on gastric epithelial cells in biopsy samples and the expression increased during infection with H. pylori, we wanted to determine whether MHC class II Expression and function in these cells is influenced by Ii. To examine whether human gastric epithelial cells Express Ii, we performed immunohistochemistry on gastric biopsies from donors and from patients infected with H. pylori to examine the expression of Ii. Interestingly, the expression of Ii was evident in uninfected tissue, but the infected tissue showed a marked increase in Ii expression (Figure 1). This was a consistent observation in a limited number of samples (n = 10) examined in which there was inflammation (not shown) compared with a similar number of uninfected samples. As expected, the inflamed tissue also had other cells in the lamina propria that expressed Ii. To confirm the distribution of the surface expression of Ii under basal conditions and inflammatory conditions, we performed confocal microscopy studies on IFN-γ-treated N87 gastric epithelial cells. Specific staining using anti-human Ii BU45 antibodies showed that the highest density of Ii expression occurred along the apical side of the cells; faint staining was detected at the basolateral side (Figure 2B). These observations confirmed the immuno-histochemical staining pattern of epithelial cells in biopsies, which also seemed to be more intense along the apical surface. Staining with anti-human decay accelerating factor antibody was used as a positive apical control (Peiffer et al. 2000) and was restricted to the apical surface (Figure 2A). To assure that the antibodies were able to reach the apical as well as the basolateral side, N87 cells were also stained in parallel with anti-epithelial antigen antibodies. As expected, the anti-epithelial antigen antibody stained both basolateral and apical sides (Figure 2C). It was also important to confirm the characteristic location of apical junction complexes in polarized cells by staining for ZO-1 protein that was apical and junctional (Figure 2D). To confirm that the apical location of Ii, as detected with the BU-45 to an extracellular epitope, was not influenced by posttranslational modifications that could prevent recognition of some populations of Ii, we stained N87 cells that were fixed and permeabilized to detect Ii with an antibody, PIN 1.1, directed to the cytoplasmic domain (Cresswell 1996). This antibody detected intracellular pools of Ii in a vesicular pattern, as seen in the X-Y horizontal representative image, but also revealed in a reconstructed X-Z vertical image of the N87 cells, that most of the staining for Ii is toward the apical surface of the cells (Figure 2E).
Figure 1.
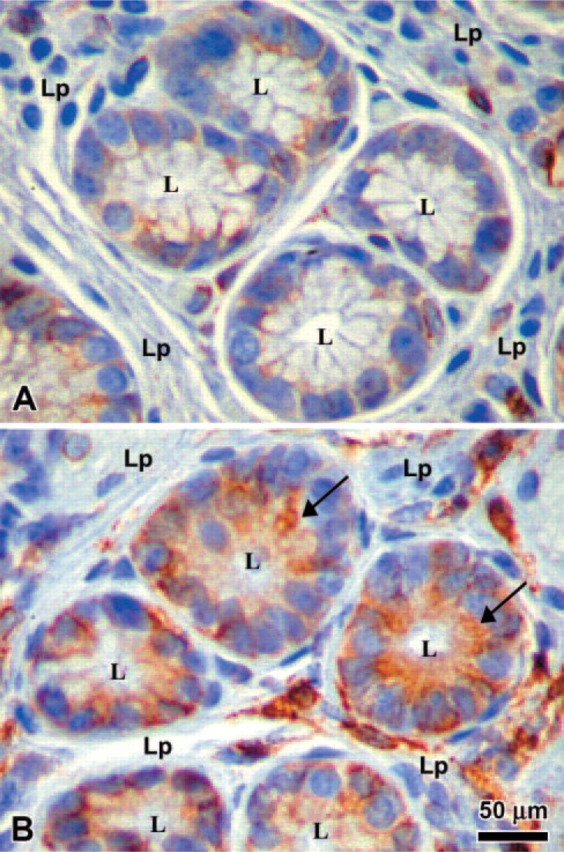
Immunohistochemistry of gastric biopsies. The expression of the invariant chain (Ii) was compared in gastric biopsies from Helicobacter pylori infected (B) vs noninfected donors (A). L = lumen; Lp = lamina propria. Arrow points to intense apical staining on epithelial cells.
Figure 2.
Surface distribution of invariant chain (Ii) on N87 cells grown on filter inserts. Confocal microscopy images are shown here of the horizontal interfaces (X-Y) and vertical interfaces (X-Z). (A) N87 cells stained with anti-DAF antibody (Alexa 568) as an apical marker, (B) N87 cells stained with anti-Ii antibody (Alexa 568), and (C) N87 cells stained with anti-epithelial antigen with apical and basal staining (FITC). Cells were stained with saturating concentration of antibodies, which reach both apical and baso-lateral sides of the cells. As additional controls, the cells were stained with antibodies to (D) the apical junctional complex protein Zona Occludens-1 and a secondary goat anti-mouse IgG conjugated with Alexa 568 and to (E) the cytoplasmic tail of Ii also with the same secondary antibody conjugated with Alexa 568.
Further analysis of Ii expression by gastric epithelial cells was performed by RT-PCR analysis to confirm the expression of the mRNA for Ii. Using a carefully designed set of primers, we were able to identify the mRNA for p41 and p33 Ii isoforms (Figure 3). IFN-γ appeared to have a marginal effect in increasing the mRNA for both isoforms of Ii. To confirm that the protein is expressed in these cells, we performed flow cytometric analysis to detect the expression of Ii in several gastric epithelial cell lines and compare the noted expression with that of a B-cell line, Jesthom, as a prototypic APC. The basal level of Ii expression in KATO III and N87 gastric epithelial cells is readily detectable, and when we added IFN-γ, which is actively produced in vivo by gastric T cells during infection with H. pylori, the level of Ii expression was enhanced (Figure 4).
Figure 3.
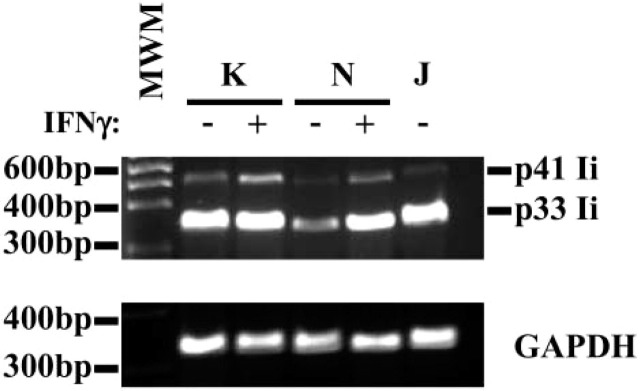
Invariant chain (Ii) mRNA detected in gastric epithelial cells by RT-PCR analysis. RNA extracted from KATO III and N87 gastric epithelial cells was reverse transcribed, amplified, and separated by electrophoresis. Specific products were detected in these cells by RT-PCR using the Ii chain primers described in Materials and Methods. As an internal control, B (Jesthom) cells showed the mRNA for those proteins. The amplification of housekeeping gene GAPDH was included as a control for the procedure. This is a representative of three experiments.
Figure 4.
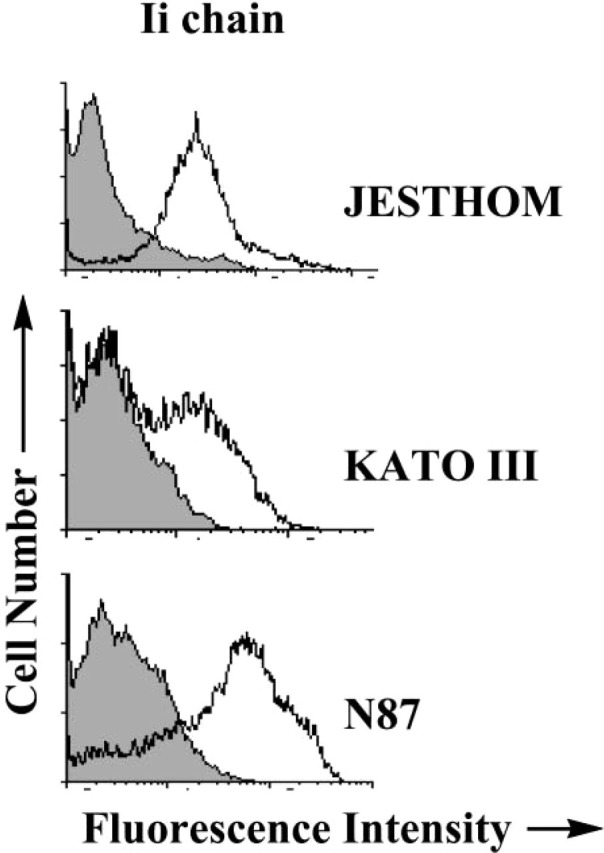
Invariant chain expression by gastric epithelial cells detected by flow cytometry. Ii chain molecules were expressed in gastric epithelial cells (A) KATO III and (B) N87 before and after IFNγ treatment. For comparison (C), the Jesthom B cell line was used as an internal positive control. The cells were stained with anti-Ii BU45 mAb. The shaded curve represents the staining with the isotype control antibody. The displacement of the fluorescence intensity curve to the right indicated an increase in expression level.
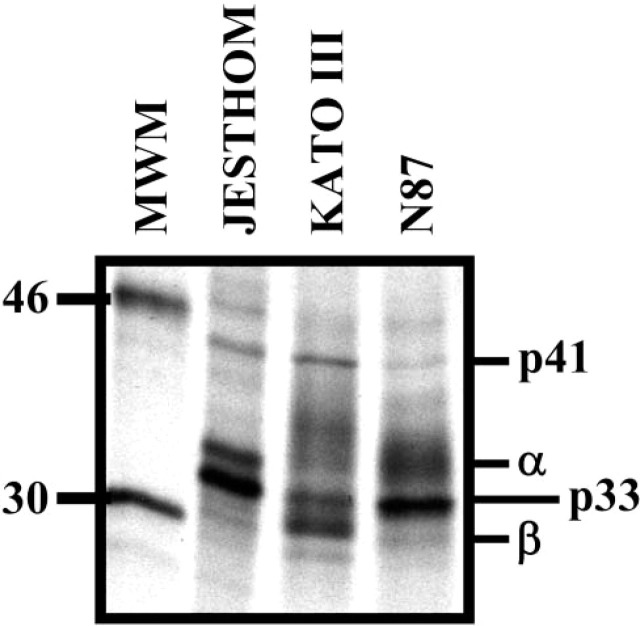
To establish the specificity of the antibodies used in immunohistochemistry, confocal microscopy, and flow cytometry studies, we immunoprecipitated Ii proteins from gastric epithelial cells that were metabolically labeled with [35S] methionine. The anti-Ii antibody, BU45, immunoprecipitated isoforms of Ii, 33 and 41 kDa, and the coassociated α and β chain of MHC class II (Figure 5). The molecular weights of these proteins are consistent with the molecular weights reported for the Ii isoforms in studies using conventional APCs (Kampgen et al. 1991; Sant and Miller 1994). These results also confirm the observations made using the other independent assays.
Figure 5.
Immunoprecipitation of invariant chain isoforms. One-dimensional gel electrophoresis of invariant chain (Ii) proteins from metabolically labeled Jesthom (B cells), KATO III, and N87 (gastric epithelial cells) demonstrates the expression of those isoforms. The Ii isoforms p33 and p41 are presented after immunoprecipitation with anti-BU45 mAb.
Ii Chain on Gastric Epithelial Cells is Coassociated with MHC Class II Molecules
The identity and expression of the various isoforms was confirmed by their migration in two-dimensional gel electrophoresis analysis (Figure 6). The spots that represent Ii chain proteins were consistent with those that have been reported from APCs (Reyes et al. 1991). In addition, two-dimensional gel analysis showed the coassociation of α and β chains of MHC class II with Ii, which is an indirect evidence for the biological role of Ii in assembly and transport of MHC class II molecules during the intracellular pathway.
Figure 6.
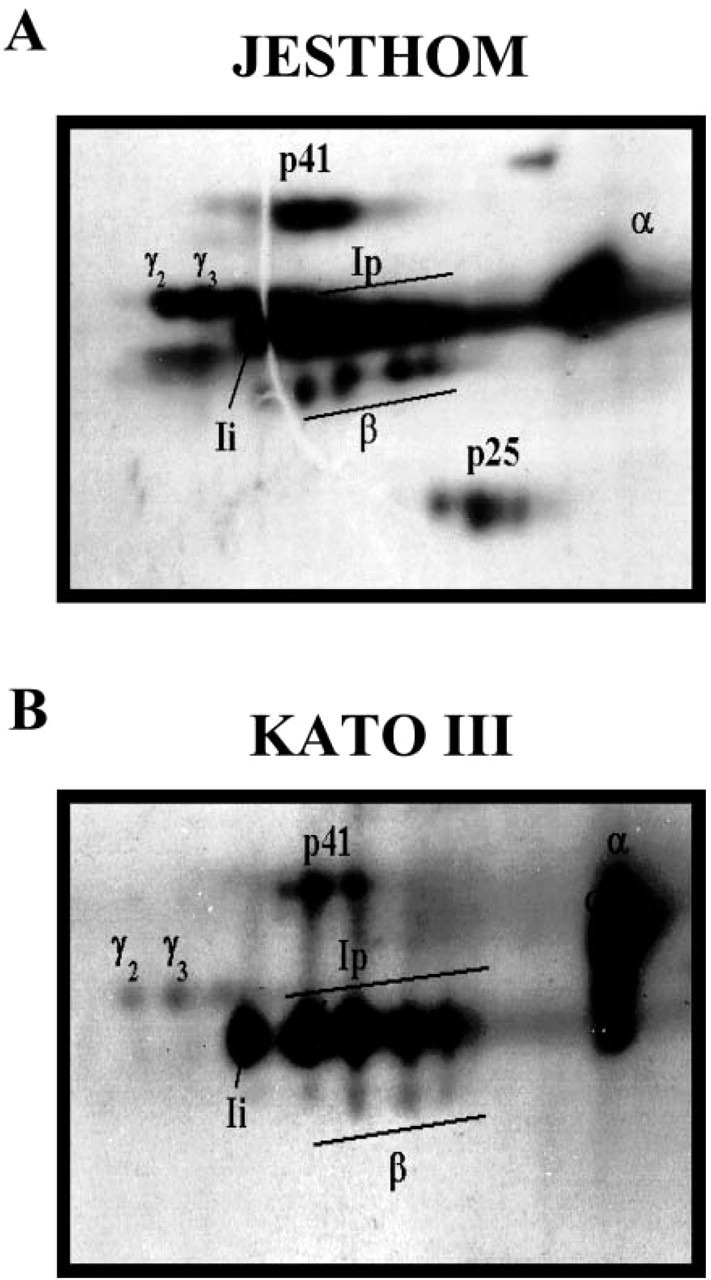
Microheterogeneity of class II MHC-associated invariant chain. Two-dimensional gel electrophoresis of invariant chain (Ii) proteins from metabolically labeled (A) Jesthom and (B) KATO III demonstrates the similarities in expression of all of the components. The α and β chains of MHC class II and different isoforms of Ii (Ii, Ip, p41, γ2, and γ3) are presented after immunoprecipitation with anti-BU45 mAb.
Gastric Epithelial Cells Express Ii-CS Isoform
An isoform of Ii that is modified by chondroitin sulfate has been previously shown to act as accessory molecule in T-cell activation. Ii-CS acts as a coreceptor for CD44. We confirmed the presence of Ii-CS on gastric epithelial cells by selectively isolating it onto a DEAE-Sepharose column. The gastric epithelial cells were metabolically labeled with [35SO4] and subsequently lysed before the enrichment of Ii-CS on an ion exchange column. To allow the visualization of Ii-CS isoform, the material was applied directly or after im-munoprecipitation with the anti-Ii antibodies PIN1.1 or VicY-1 to SDS/PAGE gels. We detected Ii-CS as a heterogeneous band in the range of 40-120 kDa on Jesthom B cells and 69-190 kDa on Kato III gastric epithelial cells (Figure 7A). The noted differences in the electrophoretic mobility of Ii-CS within cell phe-notypes were due to variations in the glycosylation processes within those cell types and not from differences in the core protein as previously examined by us (Barrera et al. 2002). To investigate the relative amount of Ii that was CS modified on N87 cells, anti-Ii BU-45 surface staining was performed for flow cytometric analysis. Because this antibody does not bind to chondroitin sulfate-modified Ii, staining was compared between untreated cells and chondroitinase-treated cells. As seen in Figure 7B, the increased staining seen with chondroitinase-treated cells over untreated cells was due to the portion of the cell surface expressed Ii that was chondroitin sulfate-modified. The mean fluorescence intensity of the chondroitinase-treated cells was 32% higher than the untreated cells, representing the pool of IiCS.
Figure 7.
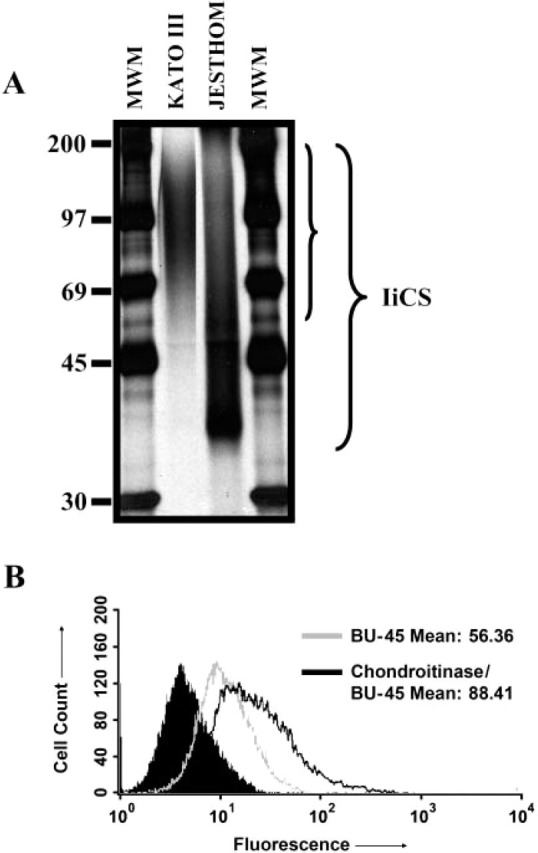
Characterization of invariant chain (Ii)-chondroitin sulfate (CS) in gastric epithelial cells. (A) One-dimensional gel electrophoresis showed expression of Ii-CS in KATO III gastric epithelial cells and Jesthom B-cell line controls. Ii-CS was enriched through a DEAE-sephacel column and then run on SDS/PAGE after immunoprecipitation with anti-Ii VicY-1 antibody. (B) Flow cytometric analysis of N87 cells stained for Ii with BU-45 antibodies after chondroitinase treatment to reveal the relative amount of Ii-CS. The solid peak is the isotype control.
Discussion
The cell biology that governs antigen processing and presentation, before T-cell activation, has been extensively examined with human and mouse B cells. The studies to date have underscored the importance of early or late endosomes in the trafficking of MHC class II-Ii complexes and antigen-loading compartments (Sanderson et al. 1994). Although many major advances have been made in the last decade in understanding the intracellular and molecular events that take place during processing and presentation of foreign antigens by APCs, the interactions at the mucosal surface level are poorly understood. Several lines of evidence have shown that nonprofessional APCs, such as gastric epithelial cells, express MHC class II molecules and other relevant molecules required for antigen processing and presentation, for instance CD80, CD86, and cathepsins (Ye et al. 1997; Barrera et al. 2001; Barrera et al. 2002). The expression of Ii by gastric epithelial cells should be considered as an essential requirement for gastric epithelial cells to present antigens to CD4+ T cells, given the documented importance of Ii in the biology of class II MHC.
Although the expression of MHC class II molecules by gastric epithelium is constitutive, that expression is upregulated during infection with H. pylori (Maekawa et al. 1997). In addition to MHC class II-increased levels, the expression of CD86 by the gastric epithelium is increased (Ye et al. 1997), and there is also an increased number of CD4+ T cells between and adjacent to the epithelium during this infection (Bamford et al. 1998). These CD4+ T cells express markers of activation, suggesting that there is local antigen presentation in the tissue area.
The present studies provide direct evidence that gastric epithelial cells express the various isoforms of Ii, which confirms that the cell biology and function of MHC class II molecules in these cells are also regulated by Ii and further strengthens the notion that the gastric epithelium may actively regulate mucosal immunity against H. pylori infection.
The Ii chain proteins expression was initially observed by immunohistochemistry of gastric biopsies from noninfected donors and patients infected with H. pulori. The presence of Ii was characterized and confirmed by several independent approaches, which include RT-PCR, flow cytometry, immunoprecipitation and affinity purification. The mRNA for the p31 and p41 Ii isoforms were detected by RT-PCR using a set of primers carefully designed to include both isoforms. The p31 include exons 4, 5, 6, 7 and 8 while p41 include exons 4, 5, 6, 6b, 7 and 8. We confirmed the expression of p41 on gastric epithelial cells, which results from alternative splicing of an additional exon (6B) on the Ii gene (O'Sullivan et al. 1987). Then, the translated proteins of Ii were detected by flow cytometry analysis, and the specificity of those antibodies was confirmed by immunoprecipitation. Polyacrylamide gel electrophoresis of the immunoprecipitated samples revealed the presence of the p33 and p41 isoforms of Ii, previously described on APCs (Strubin et al. 1986; O'Sullivan et al. 1987; Koch et al. 1989), and the coassociated MHC class II molecules. The electrophoretic mobility of these proteins differs within cell types, suggesting differences in molecular weight. Such differences in molecular weight of MHC class II molecules from B cells and gastric epithelial cells were carefully examined and attributed to differences in glycosylation on those cell types (Barrera et al. 2002).
As previously mentioned, Ii is required for the appropriate assembly of α and β chains of MHC class II, as well as for targeting this complex to the endosomal compartments. The structural microheterogeneity of this complex expressed by gastric epithelial cells has been the subject of studies done to understand their role in mucosal immunity (Barrera et al. 1998). Using two-dimensional gel analysis, we identified the complexes of the coassociated α and β chains of MHC class II and Ii isoforms. Those results suggested that, in gastric epithelial cells, Ii plays an important role in the biological functions of MHC class II, such as the trafficking of the complex to the endosomal compartments. Interestingly, although in previous studies, we demonstrated that gastric epithelial cells express functional endosomal proteases, the immuno-histochemical studies and flow cytometry suggest that these cells express a higher density of Ii in their surface when compared with B cells, where Ii is largely degraded in endosomes. This difference in Ii expression by gastric epithelial cells may be because these are polarized cells and class II MHC and Ii traffic may be governed differently than in B cells. Alternatively, Ii traffic in gastric epithelial cells may be delayed, leading to cell surface accumulation. In fact, the confocal studies together with the immunohistochemistry analysis of biopsy samples allowed us to confirm the surface expression of Ii and their distribution on gastric epithelial cells largely on the apical surface. Although the exact mechanism(s) responsible for the noted increased expression of Ii in the infected tissue is not clear, the local production of IFN-γ to which gastric epithelial cells respond, as shown by us and others, may be involved (Karttunen et al. 1995; Bamford et al. 1998). A correlation between the infection status and the level of Ii expression may require a larger sample size. However, because chondroitin sulfate-modified isoform Ii-CS of Ii is the only isoform previously noted to be on the surface of APCs (Sant et al. 1985), an initial consideration was that the Ii detected in the surface of gastric epithelial cells is Ii-CS, but the epitope recognized by the anti-Ii antibodies used in flow is masked by CS. That population of CS modified Ii only became evident after chondroitinase treatment.
Interestingly, in addition to the role of Ii in antigen processing and presentation, a novel role of Ii has been proposed, which involves T-cell activation. The Ii-CS isoform, which is coexpressed with class II MHC at the cell surface and functions as an accessory molecule, either to facilitate T-cell and APC interactions or to directly signaling to CD44 on T cells (Aruffo et al. 1990). Because intraepithelial lymphocytes are CD44+ and the expression of Ii-CS by mucosal epithelial cells could be an important factor in intraepithelial lymphocyte functions. The noted expression of Ii-CS isoform on gastric epithelial cells may highlight their importance in local T-cell functions. Because of the predominant apical expression of Ii with only low levels of expression in the lateral surfaces of gastric epithelial cells, the only T-cell population that could receive this costimulation are intraepithelial lymphocytes.
Macrophage migration inhibitory factor (MIF) has emerged as an important mediator of innate immunity. MIF is a key and powerful cytokine that induces proinflammatory responses. MIF was recently shown to be produced in the gastric mucosa during H. pylori infection (Xia et al. 2004). Interestingly, Ii was shown to be the receptor for MIF (Leng et al. 2003). Activation of ERK1/ERK2 by MIF requires Ii. MIF promotes the expression of a panel of cytokines, which include several cytokines that have been reported to be produced by the epithelium during H. pylori infection. Thus Ii expression by the epithelium could contribute to the overall epithelial cytokine response during the infection by binding locally produced MIF.
Because the epithelium represents the most abundant cell phenotype in the mucosal surface with characteristics of APC, Ii expression by gastric epithelia cells could be an important regulatory element in determining the outcome of oral immunization or oral tolerance induction. Studies provide evidence that Ii expression is required in the induction of oral tolerance because Ii-deficient mouse could not be tolerized by intragastric administration of an antigen, whereas wild-type or heterozygous mice became tolerant to the administered antigen (Desvignes et al. 1996). Although Ii expression by other gastrointestinal epithelial cells has been detected, this expression has been noted during inflammatory conditions or after manipulation to induce its expression. The studies presented here are the first ones that confirm the constitutive expression of Ii in human gastric mucosal epithelial cells as a potential regulator of local immune responses.
Acknowledgments
Supported in part by NIH grants DK-50669 and DK-56338. Support was also provided through the Texas Gulf Coast Digestive Diseases Center.
Literature Cited
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. (1990) CD44 is the principal cell surface receptor for hyaluronate. Cell 61: 1303–1313 [DOI] [PubMed] [Google Scholar]
- Bakke O, Dobberstein B. (1990) MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell 63: 707–716 [DOI] [PubMed] [Google Scholar]
- Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, et al. (1998) Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114: 482–492 [DOI] [PubMed] [Google Scholar]
- Barrera C, Ye G, Espejo R, Gunasena S, Almanza R, Leary J, Crowe S, et al. (2001) Expression of cathepsins B, L, S, and D by gastric epithelial cells implicates them as antigen presenting cells in local immune responses. Hum Immunol 62: 1081–1091 [DOI] [PubMed] [Google Scholar]
- Barrera C, Espejo R, Reyes VE. (2002) Differential glycosylation of MHC class II molecules on gastric epithelial cells: implications in local immune responses. Hum Immunol 63: 384–393 [DOI] [PubMed] [Google Scholar]
- Barrera CA, Almanza RJ, Ogra PL, Reyes VE. (1998) The role of the invariant chain in mucosal immunity. Int Arch Allergy Immunol 117: 85–93 [DOI] [PubMed] [Google Scholar]
- Cresswell P. (1996) Invariant chain structure and MHC class II function. Cell 84: 505–507 [DOI] [PubMed] [Google Scholar]
- Desvignes C, Bour H, Nicolas JF, Kaiserlian D. (1996) Lack of oral tolerance but oral priming for contact sensitivity to dinitrofluorobenzene in major histocompatibility complex class II-deficient mice and in CD4+ T cell-depleted mice. Eur J Immunol 26: 1756–1761 [DOI] [PubMed] [Google Scholar]
- Fiebiger E, Maehr R, Villadangos J, Weber E, Erickson A, Bikoff E, Ploegh HL, et al. (2002) Invariant chain controls the activity of extracellular cathepsin L. J Exp Med 196: 1263–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampgen E, Koch N, Koch F, Stoger P, Heufler C, Schuler G, Romani N. (1991) Class II major histocompatibility complex molecules of murine dendritic cells: synthesis, sialylation of invariant chain, and antigen processing capacity are down-regulated upon culture. Proc Natl Acad Sci USA 88: 3014–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karttunen R, Karttunen T, Ekre HP, MacDonald TT. (1995) Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut 36: 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N, Lipp J, Pessara U, Schenck K, Wraight C, Dobberstein B. (1989) MHC class II invariant chains in antigen processing and presentation. Trends Biochem Sci 14: 383–386 [DOI] [PubMed] [Google Scholar]
- Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, et al. (2003) MIF signal transduction initiated by binding to CD74. J Exp Med 197: 1467–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, Quaranta V, et al. (1990) Intracellular transport of class II MHC molecules directed by invariant chain. Nature 348: 600–605 [DOI] [PubMed] [Google Scholar]
- Maekawa T, Kinoshita Y, Matsushima Y, Okada A, Fukui H, Waki S, Kishi K, et al. (1997) Helicobacter pylori induces proinflammatory cytokines and major histocompatibility complex class II antigen in mouse gastric epithelial cells. J Lab Clin Med 130: 442–449 [DOI] [PubMed] [Google Scholar]
- O'Sullivan DM, Noonan D, Quaranta V. (1987) Four Ia invariant chain forms derive from a single gene by alternate splicing and alternate initiation of transcription/translation. J Exp Med 166: 444–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer I, Guignot J, Barbat A, Carnoy C, Moseley SL, Nowicki BJ, Servin AL, et al. (2000) Structural and functional lesions in brush border of human polarized intestinal Caco-2/TC7 cells infected by members of the Afa/Dr diffusely adhering family of Escherichia coli. Infect Immun 68: 5979–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters J. (2000) MHC class II-restricted antigen processing and presentation. Adv Immunol 75: 159–208 [DOI] [PubMed] [Google Scholar]
- Reyes VE, Lu S, Humphreys RE. (1991) Cathepsin B cleavage of Ii from class II MHC alpha- and beta-chains. J Immunol 146: 3877–3880 [PubMed] [Google Scholar]
- Sanderson F, Kleijmeer MJ, Kelly A, Verwoerd D, Tulp A, Neefjes JJ, Geuze HJ, et al. (1994) Accumulation of HLA-DM, a regulator of antigen presentation, in MHC class II compartments. Science 266: 1566–1569 [DOI] [PubMed] [Google Scholar]
- Sant AJ, Schwartz BD, Cullen SE. (1985) Cellular distribution of the Ia-associated chondroitin sulfate proteoglycan. J Immunol 135: 408–415 [PubMed] [Google Scholar]
- Sant AJ, Miller J. (1994) MHC class II antigen processing: biology of invariant chain. Curr Opin Immunol 6: 57–63 [DOI] [PubMed] [Google Scholar]
- Strubin M, Long EO, Mach B. (1986) Two forms of the Ia antigen-associated invariant chain result from alternative initiations at two in-phase AUGs. Cell 47: 619–625 [DOI] [PubMed] [Google Scholar]
- Xia HH, Lam SK, Huang XR, Wong WM, Leung SY, Yuen ST, Lan HY, et al. (2004) Helicobacter pylori infection is associated with increased expression of macrophage migratory inhibitory factor—by epithelial cells, T cells, and macrophages—in gastric mucosa. J Infect Dis 190: 293–302 [DOI] [PubMed] [Google Scholar]
- Ye G, Barrera C, Fan X, Gourley WK, Crowe SE, Ernst PB, Reyes VE. (1997) Expression of B7-1 and B7-2 costimulatory molecules by human gastric epithelial cells: potential role in CD4+ T cell activation during Helicobacter pylori infection. J Clin Invest 99: 1628–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]