Abstract
Cyclosporine A (CsA) use is associated with several side effects, the most important of which is nephrotoxicity that includes, as we previously showed, tubular injury and interstitial fibrosis. Recently, many researchers have been interested in minimizing these effects by pharmacological interventions. To do this, we tested whether the administration of a red wine polyphenol, Provinol (PV), prevents the development of CsA-induced nephrotoxicity. Rats were treated for 21 days and divided into four groups: control; group treated with PV (40 mg/kg/day by oral administration in tap water); group treated with CsA (15 mg/kg/day by subcutaneous injection); group treated with CsA plus PV. CsA produced a significant increase of systolic blood pressure; it did not affect urinary output, but caused a significant decrease in creatinine clearance. These side effects were associated with an increase in conjugated dienes, which are lipid peroxidation products, inducible NO-synthase (iNOS), and nuclear factor (NF)-kB, which are involved in antioxidant damage. However, PV prevented these negative effects through a protective mechanism that involved reduction of both oxidative stress and increased iNOS and NF-kB expression induced by CsA. These results provide a pharmacological basis for the beneficial effects of plant-derived polyphenols against CsA-induced renal damage associated with CsA.
Keywords: fibrosis, kidney, Provinol, ROS
Cyclosporine A (CsA), a fungal undecapeptide, is the most common immunosuppressive drug used in organ transplantation and autoimmune diseases. However, clinical use is often limited by nephrotoxicity, which remains a major problem. Our previous articles showed that nephrotoxicity was related to tubulointerstitial fibrosis and glomerular vasoconstriction. In fact, tubulointerstitial fibrosis was mainly observed in proximal tubules with respect to distal tubules, suggesting that the proximal tubules were involved in CsA-induced nephrotoxicity (Rezzani 2004; Rezzani et al. 2005). Consequently, pharmacological interventions may be necessary to minimize renal side effects. The molecular mechanisms of CsA nephrotoxicity are not well characterized, but more recent studies suggest an increase of angiotensin and endothelin-1, a downregulation of heme oxygenase, and an enhancement of reactive oxygen species (ROS) in this toxicity (Rezzani et al. 2001, 2005; Chander et al. 2004). In particular, because ROS overproduction, lipid peroxidation increase, and oxidative stress can be involved in CsA nephrotoxicity, the antioxidant therapy is the real focus of this work. Several lines of evidence have shown that different agents potentially help to attenuate or prevent CsA-induced damage in different experimental models (Durak et al. 1998; Parra et al. 1998a; 1998b; L'Azou et al. 1999; Tariq et al. 1999). Recently, it has been reported that diet rich in natural substances reduces the risk of diseases associated with an increase in oxidative stress. The beneficial effects of fruits, vegetables, or red wine may be in part explained by the presence of polyphenols, which have a multitude of biological activities, including antioxidant and free radical-scavenging properties, antiaggregatory platelet property, and inhibition of vascular smooth muscle cell proliferation (Middleton et al. 2000). Accordingly, the polyphenolic compounds of wine could be implicated in enhancing the antioxidant system, because they behave as ROS scavenger, metal chelators, and enzyme modulators (Pietta et al. 1998). Clinical studies have shown a significant rise in serum antioxidant capacity after consumption of red wine (Whitehead et al. 1995; Durak et al. 1999), an effect also reported in rats (Rodrigo et al. 2002), which reinforces the systemic endogenous antioxidant defense system and helps to modulate renal lipid peroxidation (Rodrigo et al. 2005). Moreover, it has been suggested that polyphenols indirectly function as antioxidants downregulating “pro-oxidant” enzymes, such as inducible NO-synthase (iNOS), lipoxygenases, xanthine oxidase, and inhibiting nuclear factor-kB (NF-kB) (Frei and Higdon 2003). In agreement with the data cited previously, we showed that a red wine polyphenolic compound, Provinol (PV), prevents the development of hypertension, myocardial fibrosis, aortic wall thickening, and vascular dysfunction in different experimental models of hypertension (Bernatova et al. 2002; Pechanova et al. 2004). These effects are associated with reduction of oxidative stress and increase of the expression of protective genes, such as endothelial NO synthase.
The present study was designed to test whether the administration PV might prevent the development of nephrotoxicity induced by chronic treatment of rats with CsA. Moreover, we evaluated by: (1) morphological method, picrosirius staining, the CsA-induced alterations in renal cytoarchitecture underlying the increase of interstitial fibrosis induced by the drug; (2) immunohistochemical and biochemical analysis, the iNOS and nuclear factor (NF)-kB expression, as markers of oxidative damage; and (3) biochemical assay, the lipid peroxidation products, such as conjugated dienes.
Materials and Methods
Animal and Experimental Design
Forty adult male Wistar rats weighing 200-300 g were obtained from Harlan Laboratories (Udine, Italy). They were housed in individual cages at a constant temperature with a 12-hour dark/light cycle and fed with a standard diet. All experiments were performed in accordance with Institutional guidelines for the ethical care of animals. An adaptation period of 2 weeks was allowed before the treatments.
The rats were randomly divided into four groups (n = 10 in each group) and treated for 21 days. Group I (control rats) received olive oil, the CsA vehicle, with SC injections; group II rats were treated with oral administration of PV alone (40 mg/kg/day diluted in tap water); group III rats were treated with CsA (15 mg/kg/day in olive oil, SC); group IV rats were treated simultaneously with PV and CsA at the same dose previously reported. PV, dry powder from red wine, was provided by Mr. D. Ageron (Société Francaise de Distillerie; Vallont Pont d'Arc, France). The composition of PV has been determined as follows: (in mg/g of dry powder): proanthocyanidins 480, total anthocyanins 61, free anthocyanins 19, catechin 38, hydroxycinnamic acid 18, and flavonols 14. The administration of CsA (purchased from Novartis, Basel, Switzerland) was made considering the weight of the animal. To make sure that each animal received the complete dose of PV, the calculated amount of PV was given to each rat in the appropriate volume of water (0.2 mg/ml). Daily water consumption was estimated individually for every animal 1 week before the experiment. During the experiment, water consumption was controlled, with graduated Richter tubes, and PV concentration in the drinking fluid was adjusted, if necessary.
Body weight and systolic blood pressure (SBP), measured an average of 3-5 times by the noninvasive method of tailcuff plethysmography in each conscious rats, were recorded before and after the treatments. The animals were placed in individual metabolic cages after the last dose of drug(s) to collect urine output for 24 hours. The urine output were expressed in milliliters, according to Padi and Chopra (2002). On Day 22, all animals were killed by decapitation and the kidneys were removed.
Renal function was assessed by colorimetric assay of urinary creatinine. Creatinine clearance was calculated using standard formulae according to Shi et al. (2004).
Morphology
Renal tissue samples were isolated immediately after the sacrifice of the animals and washed in ice-cold saline. They were fixed in 10% buffered formalin (Nova Chimica; Milan, Italy), embedded in paraffin and serially sectioned at 5 μm by a microtome. The sections were first deparafinized, rehydrated, immersed in water, and then stained with hematoxylin and eosin according to standard procedures and with Picrosirius Red. For the Picrosirius Red method, the sections were stained for 30 min with phosphomolybdic acid 0.1%, washed in water, and then immersed in Picrosirius Red (Sirius Red 0.1% in picric acid) for 60 min. Then, the sections were washed in water and then rapidly dehydrated, cleared in xylene and mounted. Collagen fibers were detected by polarized light microscopy (Olympus; Milan, Italy). Under these conditions, type I collagen fibers were stained from yellow to red, whereas the type III collagen fibers appeared green (Vranes et al. 1999).
Findings ascribed to tubular injury included cellular vacuolization and tubular distension. For tubular injury, the quantitative scorings used were similar to those reported by Shi et al. (2004) and they were ranged from 0 (no tubular injury) to 3 (>65% of tubules injured).
The findings of interstitial fibrosis consisted of matrixrich expansion of the interstitium with distortion and collapse of the tubules. Interstitial fibrosis was estimated by counting the percentage of injured areas per field and was scored quantitatively (Shi et al. 2004) from 0 = normal interstitium, to 3 (>45%).
Immunohistochemistry
Sections, obtained from each renal sample, fixed in formalin, and embedded in paraffin, were treated for iNOS and NF-kB immunohistochemical analysis. Briefly, sections were first deparaffinized and rehydrated and then immersed in 3% hydrogen peroxide in methanol for 30 min to block the endogenous peroxidase activity. Sections were then incubated with goat serum (diluted 1:5; Dakopatts, Milan, Italy) for 60 min, and serially treated with iNOS (rabbit polyclonal, diluted 1:50; Santa Cruz Biotechnology; Santa Cruz, CA) and NF-kB (rabbit polyclonal, diluted 1:50; Santa Cruz Biotechnology) at 4C overnight. The sections were washed in Tris buffer solution 0.1M pH 7.4 and sequentially incubated with proper biotinylated secondary antibody and avidin-biotin horseradish peroxidase complex according to the manufacturer's instructions (ABC kit; Dakopatts). The sections were stained by immersing in a solution of 0.05% 3, 3-diaminobenzidine tetrahydrochloride and 0.03% hydrogen peroxide. All slides were counterstained with hematoxylin, dehydrated, and mounted. Control reactions were performed in the absence of the primary antibodies.
Western Blot Analysis
Samples of kidneys (80 mg of wet tissue) were homogenized in 25 mmol/1 Tris-HCl, pH 7.4, containing 5 mmol/1 EDTA, 50 mmol/1 NaCl, 1 μmol/1 leupeptin, 0.3 μmol/1 aprotinin, 0.1 mmol/1 PMSF, 1 mmol/1 pepstatin, and 1% SDS. After the centrifugation (15,000 × g, 20 min, twice) supernatants were subjected to SDS-PAGE using 10% gels. Following the electrophoresis, proteins were transferred to nitrocellulose membranes and were probed with a polyclonal rabbit anti-iNOS antibodies (Alexis Biochemicals; Grunberg, Germany) and a polyclonal rabbit anti-NF-kB antibody (Santa Cruz Biotechnology). Bound antibodies were detected using a secondary peroxidase-conjugated anti-rabbit antibody (Alexis Biochemicals). The bands were visualized using the enhanced chemiluminescence system (Amersham; Buckinghamshire, United Kingdom) and analyzed densitometrically using Photo-Capt V.99 software (Vilber Lourmat; Marne-la-Vallée Cedex, France).
Determination of Conjugated Diene Concentration
The concentration of conjugated diene (CD) was determined in lipid extracts of the kidney homogenates according to Kogure et al. (1982). Briefly, after chloroform evaporation under the inert atmosphere and addition of cyclohexane, CD concentration was determined spectrophotometrically [Λ=233 nm; Bio-Rad, GBC 911A (Hercules, CA)].
Statistical Analysis
Samples were analyzed and scored blindly. The results are presented as means ± SEM. Statistical significance of differences between the experimental groups was estimated using the ANOVA and Bonferroni test, with p<0.05 considered significant.
Results
Effect of PV on Body Weight, SBS, and Urine Output in CsA-treated Rats
Body weight and SBP were not significantly different in rats before the beginning of the treatment. CsA treatment reduced the gain of body weight compared with either control or PV-treated rats (p<0.05). Interestingly, PV treatment prevented this effect of CsA (Table 1). CsA treatment produced a significant increase of SBP compared with control and PV-treated rats. SBP rise was prevented by concomitant treatment of the rat with CsA plus PV. In contrast to body weight and SBP, CsA treatment did not affect urinary output in rats that drank the same amount of water or water plus PV. However, CsA caused a significant decrease in creatinine clearance that was markedly improved by concomitant treatment with PV.
Table 1.
Effect of Provinol (PV) (40 mg/kg diluted in tap water) on body weight, urine output, systolic blood pressure (SBP), and creatinine clearance in cyclosporine A (CsA)-treated rats (15 mg/kg/day SC)
| Treatment | Body weight (g) | Urine output 24 hr (ml) | SBP (mmHg) | Creatinine clearance (ml/min/kg) |
| Control (olive oil) | 290 ±9.2 | 8.9 ± 0.73 | 115 ± 5.1 | 2.9 ± 0.16 |
| PV alone | 295 ± 10.1 | 8.4 ± 0.41 | 112 ± 3.8 | 3.0 ± 0.22 |
| CsA | 190 ± 6.3∗ | 9.3 ± 0.51 | 225 ± 3.2∗ | 1.3 ± 0.09∗ |
| CsA + PV | 300 ± 7.9+ | 10.4 ± 0.89 | 130 ± 5.1+ | 3.2 ± 0.35+ |
| Values are means ± SEM. |
p≤0.05 vs control; + p≤0.05 CsA + PV vs CsA-treated animals.
Effect of PV on CsA-induced Renal Morphological Changes
Morphological data obtained from control and PV-treated rats were not significantly different. Thus only pictures taken from control rats are shown in Figure 1. Kidneys from control rat displayed normal cortex and medulla features. The cortex showed a number of proximal and distal tubules located around the glomerula (Figure 1A). The medulla showed the different portions of Henle loops and a number of peritubular capillaries (vasa recta) (Figure 1B). Both cortex and medulla showed very little amount of collagen, stained by Sirius red method, distributed throughout anatomical structures (Figures 1C and 1D). Under a polarized light microscope, these fibers appeared as green color because of the presence of type III collagen (Figures 1E and 1F).
Figure 1.
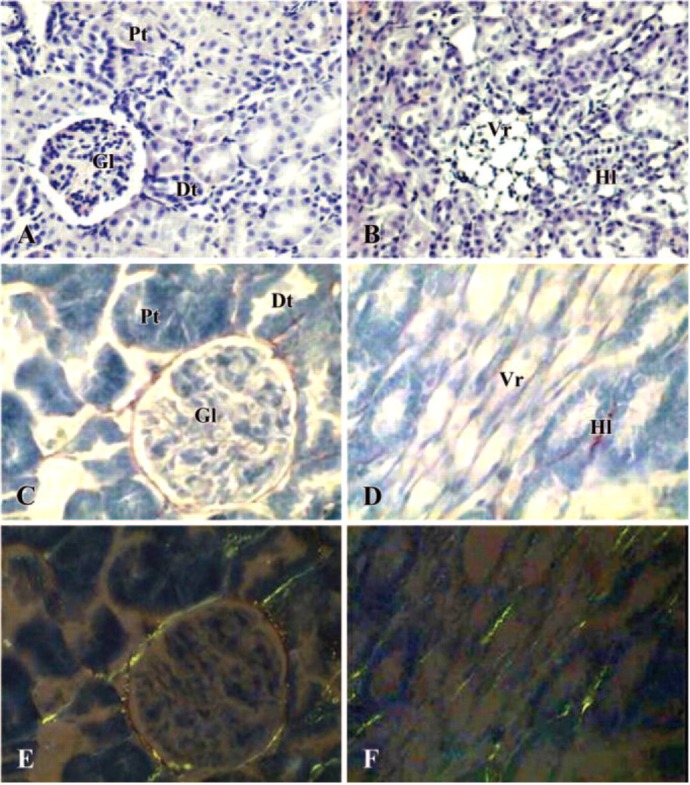
Photomicrographs of kidney in control rat. (A, B) Hematoxylin-eosin staining (×200); (C, D) Sirius Red staining under light microscope (×400); (E, F) Sirius Red staining under polarized light microscope (×400). (A, C, E) cortex; (B, D, F) medulla. Gl = glomerula; Pt = proximal tubules; Dt = distal tubules; Vr = vasa recta; Hl = Henle loops.
In contrast, animals treated with CsA showed characteristic renal morphological changes such as those described for chronic human lesion (Rezzani 2004). They displayed marked histological alterations in cortex and outer medulla. The cortical changes consisted in tubular and glomerular injury with dilation of the lumen and Bowman's capsule, respectively. The Bowman's capsule dilation was sometimes associated with arteriolar constriction (Figure 2A). Alterations of the medulla showed vasa recta disruption (Figure 2B). In particular, marked interstitial fibrosis was seen both around tubules and glomerula in the cortex (Figure 2C) and around damaged vasa recta in the medulla (Figure 2D). The extracellular matrix deposition around glomerula, tubular structures, and vasa recta were observed under polarized light microscope; this fibrosis showed mainly type I collagen indicated by yellow/red fibers (Figures 2E and 2F). Quantitative scoring of both tubular injury and tubulo-interstitial fibrosis showed significant increases of these structural abnormalities in kidneys taken from CsA-treated rats (Table 2).
Figure 2.
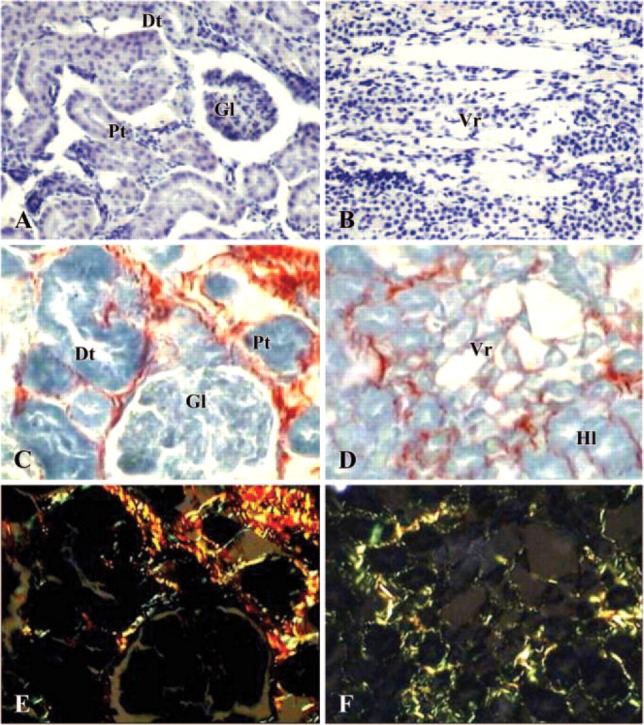
Photomicrographs of kidney in a cyclosporin A-treated rat. (A, B) Hematoxylin-eosin staining (×200); (C, D) Sirius Red staining under light microscope (×400); (E, F) Sirius Red staining under polarized light microscope (×400). (A, C, E) cortex; (B, D, F) medulla. Gl = glomerula; Pt = proximal tubules; Dt = distal tubules; Vr = vasa recta; Hl = Henle loops.
Table 2.
Quantitative scores of tubular injury and tubulointerstitial fibrosis of cyclosporin A (CsA)-treated group versus control and CsA + Provinol (PV) (CsA+PV) groups vs CsA-treated group
| Treatment | Tubular injury (0-3+) | Interstitial fibrosis (0-3+) | |
| Control (olive oil) | 0.5 ± 0.06 | 0.2 ± 0.02 | |
| PV alone | 0.6 ± 0.03 | 0.3 ± 0.02 | |
| CsA | 2.3 ± 0.13∗ | 3.0 ± | 0.28∗ |
| CsA + PV | 0.4 ± 0.03+ | 0.5 ± 0.03+ |
Values are means ± SEM.
p≤0.05 vs control; + p≤0.05 CsA + PV vs CsA-treated animals.
Coadministration of CsA and PV greatly improved the alterations of renal morphology both in cortex and medulla; in fact, the observed structures were not significantly different from those seen in kidney from either control or PV-treated rats. Figure 3A shows glomeruli with normal Bowman's capsule and tubular structures. In the medulla, some damaged structures could still be observed around the vasa recta (Figure 3B). In conjunction with the former data, fibrosis was not present in the cortex and a little fibrosis persisted around the damaged vasa recta in the medulla (Figures 3C and 3D). Under polarized light microscope, type III collagen, indicated by green fibers, was observed in the cortex, whereas a number of yellow/red spots of type I collagen were seen in the medulla (Figures 3E and 3F). As reported in Table 2, both tubular injury and interstitial fibrosis scoring were greatly improved in kidney of CsA plus PV-treated rats.
Figure 3.
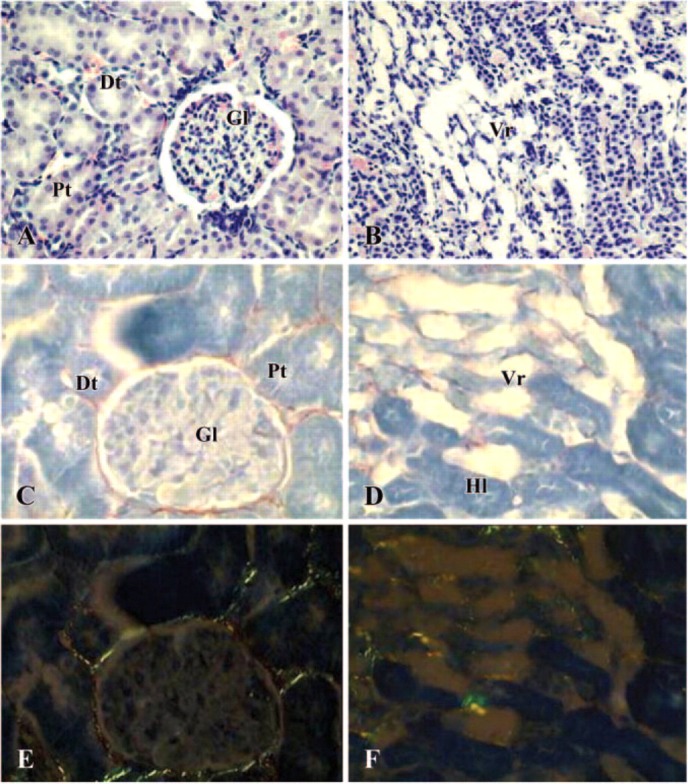
Photomicrographs of kidney in a cyclosporin A plus Provinol-treated rat.(A, B) Hematoxylin-eosin staining (×200); (C, D) Sirius Red staining under light microscope (×400); (E, F) Sirius Red staining under polarized light microscope (×400). (A, C, E) cortex; (B, D, F) medulla. Gl = glomerula; Pt = proximal tubules; Dt = distal tubules; Vr = vasa recta; Hl = Henle loops.
Effect of PV on Expression of iNOS and NF-kB Proteins in CsA-treated Animals
Immunohistochemical studies showed that no detectable iNOS and NF-kB was observed both in cortical and medullar structures of kidney taken from control rats (Figures 4A, 4B, 5A, and 5B). In the renal cortex of CsA treated animals, diffuse, moderate and strong iNOS and NF-kB staining was seen in all the proximal and distal tubules. Low iNOS and NF-kB staining was observed in the glomeruli (Figures 4C and 5C). It should be noted that the staining was mainly distributed in the basal part of the tubular cells where numerous mitochondria are distributed. The Henle loops and vasa recta in outer medulla from CsA-treated rat showed marked iNOS and NF-kB labeling (Figures 4D and 5D).
Figure 4.
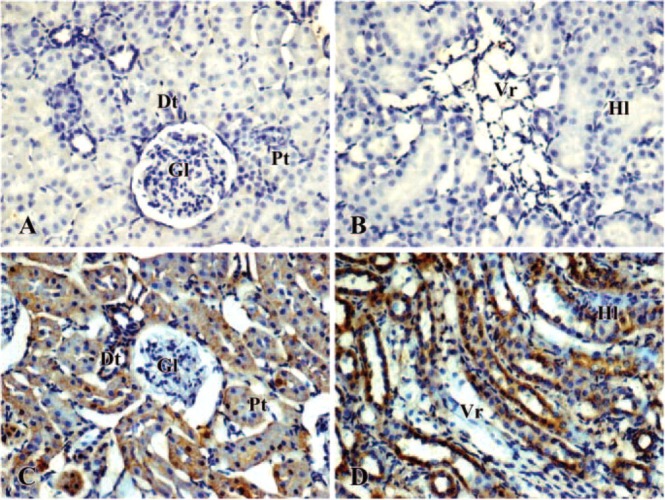
Immunolocalization of iNOS in kidney of control (A, B) and cyclosporin A-treated (C, D) rat. Brown color indicates immunopositivity (×200). (A, C) cortex; (B, D): medulla. Gl = glomerula; Pt = proximal tubules; Dt = distal tubules; Vr = vasa recta; Hl = Henle loops.
Figure 5.
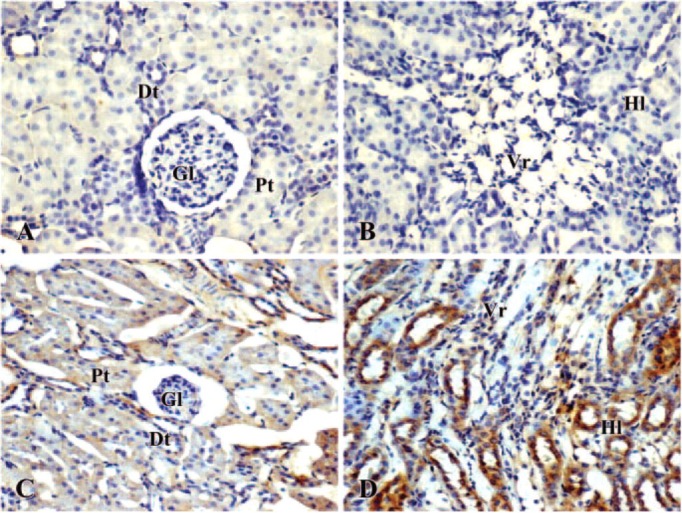
Immunolocalization of nuclear factor-kB in kidney of control (A, B) and cyclosporin A-treated (C, D) rat. Brown color indicates immunopositivity (×200). (A, C) cortex; (B, D) medulla. Gl = glomerula; Pt = proximal tubules; Dt = distal tubules; Vr = vasa recta; Hl = Henle loops.
The iNOS and NF-kB labeling was undetectable both in cortical and medullar areas after CsA plus PV treatment (data not shown).
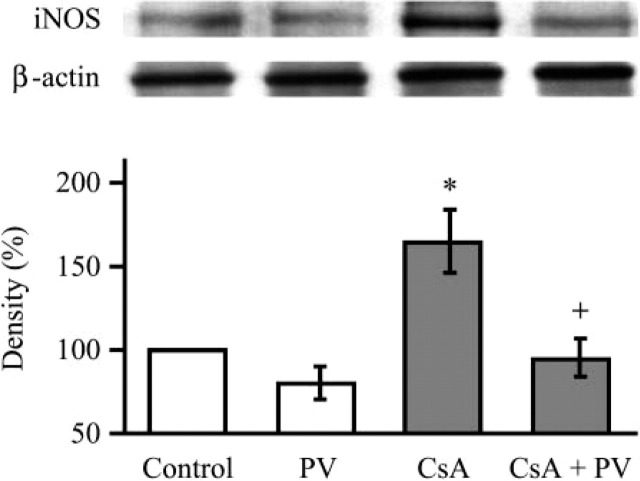
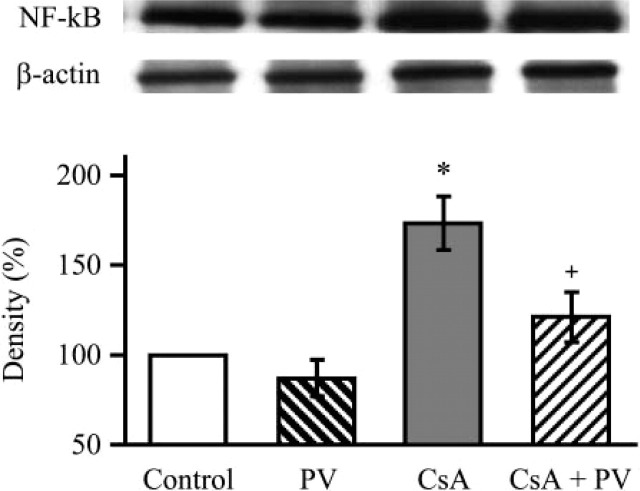
Western blot of iNOS showed enhanced expression of the enzyme in kidney taken from CsA-treated rats that was significantly reduced by concomitant treatment ment with PV (Figure 6). In conjunction with iNOS, CsA treatment produced an increase of NF-kB expression that was significantly reduced in kidney taken from PV plus CsA-treated rats (Figure 7).
Figure 6.
Western blot analysis demonstrating the effect of Provinol and cyclosporin A (CsA) treatment on inducible NO-synthase protein expression in the kidney (with β-actin as control). Representative blots with densitometric readings are expressed as percent of control. Significant differences: ∗ p<0.05 as compared with control, + p<0.05 as compared with CsA treated group (n=5).
Figure 7.
Western blot analysis demonstrating the effect of Provinol and cyclosporin A (CsA) treatment on nuclear factor-kB protein expression in the kidney (with β-actin as control). Representative blots with densitometric readings are expressed as percent of control. Significant differences: ∗ p<0.05 as compared with control, + p<0.05 as compared with CsA treated group (n=5).
Effect of PV on CsA-induced Increase on CD Concentration
The concentration of CD was significantly increased by CsA treatment in comparison with the control group (238 ± 16 nmol/g tissue vs 170 ± 11 nmol/g tissue, p<0.05, n=5). On the other hand, the concomitant treatment with CsA plus PV prevented the increase of CD concentration and showed values similar to those observed in control group (195 ± 13 nmol/g tissue vs 170 ± 11 nmol/g tissue, n=5) (Table 3).
Table 3.
Effect of chronic Provinol (PV) and cyclosporin A (CsA) treatment on renal conjugated diene (CD) concentration, which are lipid peroxidation products
| Treatment | CD (nmol/g tissue) |
| Control (olive oil) | 170 ± 11 |
| PV alone | 175 ± 10 |
| CsA | 238 ± 18∗ |
| CsA + PV | 195 ± 13+ |
Values are means ± SEM.
p≤0.05 vs control; + p≤0.05 CsA + PV vs CsA-treated animals.
Discussion
The present study provides evidence that PV prevents the increase of systolic blood pressure as well as structural and functional injuries of the kidney induced by CsA treatment. The immunosuppressor CsA has been associated to human endothelial dysfunction, accelerated atherosclerosis, and hypertension (Navarro-Antolin et al. 2002; Rezzani 2004). Accumulated evidence suggested that endothelial dysfunction coincides with an enhanced inducible nitric oxide expression (Polytarchou and Papadimitriou 2005) and oxidative stress (Bitar et al. 2005), which are considered markers of atherosclerosis and vascular damage. Because vascular diseases determine hypertension, we could propose that endothelial functions are very important to maintain normal blood pressure.
PV treatment was associated with a decreased tubular injury and interstitial fibrosis and was more pronounced in the glomeruli. Our data suggest that PV completely restored the alterations caused by CsA treatment in renal cortex but not in the medulla, in which we still observed fibrosis, especially around the vasa recta. Reduction of both oxidative stress and increased iNOS expression via the NF-kB pathway may be responsible for the protective effect of PV on CsA-induced nephrotoxicity.
The introduction of CsA to the transplant community had great importance primarily because of the improved cadaveric graft survival rates with corresponding decrease in rate of rejection episodes. However, the most serious and limiting effect associated with CsA is nephrotoxicity (Kahan 1989; Padi and Chopra 2002; Parra Cid et al. 2003). In the present study, CsA treatment induced marked functional and structural changes of the kidney. The former included reduced creatinine clearance, even though no change in urine output has been observed. The latter was associated with both tubular and glomerular injuries, including Bowman's capsule and vascular alterations linked to intimal thickening. Also, CsA treatment induced marked interstitial fibrosis with increased extracellular matrix deposition containing mainly type I collagen fibers. Nephrotoxicity induced by CsA at the dose used in this experimental model displays similar characteristics of structural and functional changes as observed in the clinical practice (Rezzani 2004). The protective effect of PV is associated with an improvement of creatinine clearance and therefore with renal function, which is crucial from a clinical point of view.
The mechanisms of CsA-induced nephrotoxicity are not fully elucidated, but several lines of evidence suggest an increased oxidative stress (Suleymanlar et al. 1994; Chen et al. 2002; Anjaneyulu et al. 2003; Esrefoglu et al. 2003). In vitro and in vivo studies have shown that CsA induces lipid peroxidation in rat kidney (Ahmed et al. 1993; Suleymanlar et al. 1994) and increases lipid peroxidation index in renal cortex homogenates, blood, and urine (Wang and Salahudeen 1995). In accordance with these data, we found that CsA treatment induced an increase of CD concentration in the kidney. Whether the increased oxidative stress was due to a direct production of ROS by CsA itself or its metabolism by cytochrome P450 system remains to be elucidated. Nevertheless, CsA-induced increase in CD is associated with enhanced expression of both the proinflammatory enzyme iNOS and the transcription factor NF-kB. Finally, it cannot be excluded that the increase of SBP induced by CsA treatment might be the consequence of enhanced oxidative stress. Moreover, regarding this latter issue, there are different hypotheses that could be discussed. One of these is that the oxidative stress is responsible for peroxynitrite formation, as previously reported in endothelial cells (Navarro-Antolin et al. 2002). It is known that the peroxynitrite selectively inactivate the prostacyclin receptor (Zou et al. 1999) and causes downregulation of prostacyclin-synthase through the activation of NF-kB (Cooke and Davidge 2002). The decreased protein levels of prostacyclin synthase produce inhibition of prostacyclin-mediated vasodilation while determining prostaglandin- and thromboxane-mediated vasoconstriction. The latter is responsible for blood pressure upregulation. Another hypothesis could be related to endothelin increase. In fact, in our previous work, we showed that CsA induced endothelin-1 upregulation (Rezzani et al. 2001). This increase could be stimulated by F2-isoprostane, which is another marker of oxidative stress, which might be increased after CsA treatment. The F2-isoprostane, increasing endothelin-1 expression in endothelial cells (Yura et al. 1999), could be responsible indirectly to hypertension.
In our previous study, PV accelerated blood pressure lowering or prevented the development of hypertension in a NO-deficient model of hypertension (Bernatova et al. 2002; Pechanova et al. 2004). These effects of PV were associated with improved structural and functional cardiovascular changes produced by chronic inhibition of NO synthesis. In the present study, we provide evidence that this dose of PV produced sufficient amounts of circulating polyphenols to prevent hypertension and structural and functional changes of the kidney induced by CsA treatment. Despite the fact that the precise composition of PV is still not clear, oligomeric-condensed tannins and anthocyanins could be responsible for its in vivo effects (Andriambeloson et al. 1998).
In the literature, there are few data reporting the importance of a reinforced and improved antioxidant system in the reduction of CsA-induced renal oxidative stress (Fryer 1997; Iqbal and Athar 1998; Pedraza-Chaverri et al. 2000; Rodrigo and Rivera 2002). The increased expression of superoxide dismutase significantly blocks formation of ROS and minimizes pathological alterations and inhibition of renal function caused by CsA (Zhong et al. 2001). Dietary supplementation with vitamin E, vitamin C, and melatonin corrected the CsA-induced nephrotoxicity (Longoni et al. 2002; Shin et al. 2002; Parra Cid et al. 2003; Durak et al. 2004). With regard to natural dietary polyphenols, tea polyphenols have been reported to significantly inhibit renal expression of TGF-b1 that might protect renal function and tissue structure in a rat model of CsA-induced toxicity both in kidney and other organs, such as the liver (Shi et al. 2004). Moreover, Ishikawa and Kitamura (2000) and Satyanarayana et al. (2001) reported that a red wine polyphenol, quercetin, exerts a cytoprotective action on glomerular mesangial cells and tubular structures.
In the present study, it was shown that PV minimized oxidative stress induced by CsA in the kidney, as illustrated by the reduced CD concentration. In our previous studies, reduced oxidative stress contributes to the antihypertensive effect of PV and to the protection against cardiovascular remodeling in several models of experimental hypertension (Bernatova et al. 2002; Pechanova et al. 2004). A similar mechanism may account for the prevention of CsA-induced increase in SBP resulting from improvement of endothelial function, decreased blood vessels stiffness, and fibrosis. These latter properties of PV may protect the kidney against CsA-induced alterations in renal blood flow and kidney architecture, including tubular injury and interstitial fibrosis, especially at the level of the cortex, and therefore prevent the decrease of creatinine clearance. Our results are in agreement with different studies showing that red wine polyphenols protect the kidney and renal damage mediated by oxidative stress, including ischemia-reperfusion (Giovannini et al. 2001) and myoglobinuric acute renal failure induced by rhabdomyolysis (Rodrigo et al. 2004).
Very recently, we have reported that one of the mechanisms involved in the reduction of oxidative stress by PV results from increased NO production by endothelial NO-synthase that could contribute to the anti-inflammatory and antiremodeling in vivo properties of PV. The activation of endothelial NO pathway may be involved in the regulation of inflammatory cytokines, adhesion molecules, and chemokine production by inhibition of either transcription NF-kB (Kitamoto et al. 2000) or transforming growth factor-β (Tomita et al. 1998). In agreement with this view, PV prevents the increase of renal NF-kB expression induced by CsA treatment.
NF-kB activation is upstream of the synthesis of inflammatory mediators. Among the genes positively regulated by NF-kB, there is iNOS. iNOS can produce a huge amount of NO that can combine with superoxide anions to release the cytotoxic compound peroxynitrite responsible for lipid peroxidation and interstitial fibrosis. In the present study, PV treatment reduced the increase of iNOS expression in the kidney of CsA-treated rats; this effect probably results from the reduction of NF-kB expression. The mechanism by which PV affects the NF-kB and iNOS pathways remains to be determined, but it may act either by decreasing the level of ROS or by acting on different intracellular kinases that alter their expression or activity. Nevertheless, it was reported that CsA-induced nephrotoxicity at least partially involved changes in iNOS in several experimental models (Amore et al. 1995). Reduction of the iNOS expression may account for the beneficial effect of PV on structural and functional renal damage induced by CsA.
In summary, PV protects against CsA-induced increase of systolic blood pressure and nephrotoxicity in the rat. Reduction of both oxidative stress and iNOS expression via the NF-kB pathway may be responsible for the protective effect of PV on CsA-induced structural and functional alterations of the kidney. The present data support the assumption about the beneficial effect of PV on blood pressure and renal damage associated with immunosuppressive agents.
Acknowledgments
This work was in part supported by the research grants VEGA - 2/3185/24, APVT - 51-017902.
Literature Cited
- Ahmed SS, Strobel HW, Napoli KL, Grevel J. (1993) Adrenochrome reaction implicates oxygen radicals in metabolism of cyclosporine A and FK-506 in rat and human liver microsomes. J Pharmacol Exp Ther 265: 1047–1054 [PubMed] [Google Scholar]
- Amore A, Gianoglio B, Ghigo D, Peruzzi L, Porcellini MG, Bussolino F, Costamagna C, et al. (1995) A possible role for nitric oxide in modulating the functional cyclosporine toxicity by arginine. Kidney Int 47: 1507–1514 [DOI] [PubMed] [Google Scholar]
- Andriambeloson E, Magnier C, Haan-Archipoff G, Lobstein A, Anton R, Beretz A, Stoclet JC, et al. (1998) Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J Nutr 128: 2324–2333 [DOI] [PubMed] [Google Scholar]
- Anjaneyulu M, Tirkey N, Chopra K. (2003) Attenuation of cyclosporine-induced renal dysfunction by catechin: possible antioxidant mechanism. Ren Fail 25: 691–707 [DOI] [PubMed] [Google Scholar]
- Bernatova I, Pechanova O, Babal P, Kysela S, Styrtina S, Andriantsitohaina R. (2002) Wine pholyphenols improve cardiovascular remodeling and vascular function in NO-deficient hypertension. Am J Physiol Heart Circ Physiol 282: 942–948 [DOI] [PubMed] [Google Scholar]
- Bitar MS, Wahid S, Mustafa S, Al-Seleh E, Dhaunsi GS, Al-Mulla F. (2005) Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol 511: 53–64 [DOI] [PubMed] [Google Scholar]
- Chander V, Singh D, Tirkey N, Chander H, Chopra K. (2004) Amelioration of cyclosporine nephrotoxicity by irbesartan, a selective AT1 receptor antagonist. Ren Fail 26: 467–477 [DOI] [PubMed] [Google Scholar]
- Chen HW, Chien CT, Yu SL, Lee YT, Chen WS. (2002) Cyclosporine A regulate oxidative stress-induced apoptosis in cardiomyocytes: mechanisms via ROS generation, iNOS and Hsp70. Br J Pharmacol 137: 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke CL, Davidge ST. (2002) Peroxynitrite increases iNOS through NF-kB and decreases prostacyclin synthase in endothelial cells. Am J Physiol Cell Physiol 282: 395–402 [DOI] [PubMed] [Google Scholar]
- Durak I, Karabacak HI, Buyukkocak S, Cimen MY, Kacmaz M, Omeroglu E, Ozturk HS. (1998) Impaired antioxidant defense system in the kidney tissues from rabbits treated with cyclosporine. Protective effects of vitamins E and C. Nephron 78: 207–211 [DOI] [PubMed] [Google Scholar]
- Durak I, Cimen MY, Buyuklocat S, Kacmaz M, Omeroglu E, Ozturk H. (1999) The effect of red wine on blood antioxidant potential. Cur Med Res Opin 15: 208–213 [DOI] [PubMed] [Google Scholar]
- Durak I, Ozbek H, Elgun S. (2004) Cyclosporine reduces hepatic antioxidant capacity: protective roles of antioxidants. Int Immunopharmacol 4: 469–473 [DOI] [PubMed] [Google Scholar]
- Esrefoglu M, Kurus M, Sahna E. (2003) The beneficial effect of melatonin on chronic cyclosporin A nephrotoxicity in rats. J Int Med Res 31: 42–44 [DOI] [PubMed] [Google Scholar]
- Frei B, Higdon JV. (2003) Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr 133:3275S–3284S [DOI] [PubMed] [Google Scholar]
- Fryer MJ. (1997) Vitamin E may slow kidney failure owing to oxidative stress. Redox Rep 3: 259–261 [DOI] [PubMed] [Google Scholar]
- Giovannini L, Migliori M, Longoni BM, Das DK, Bertelli AA, Panichi V, Filippi C, et al. (2001) Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharmacol 37: 262–270 [DOI] [PubMed] [Google Scholar]
- Iqbal M, Athar M. (1998) Attenuation of iron-nitrilotriacetate (Fe-NTA)-mediated renal oxidative stress, toxicity and hyperproliferative response by the prophylactic treatment of rats with garlic oil. Food Chem Toxicol 36: 485–495 [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Kitamura M. (2000) Anti-apoptotic effect of quercetin: intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int 58: 1078–1087 [DOI] [PubMed] [Google Scholar]
- Kahan BD. (1989) Cyclosporine. N Engl J Med 321: 1725–1738 [DOI] [PubMed] [Google Scholar]
- Kitamoto S, Egashira K, Kataoka C, Koyanagi M, Katoh M, Shimokawa H, Morishita R, et al. (2000) Increased activity of nuclear factor-kappaB participates in cardiovascular remodeling induced by chronic inhibition of nitric oxide synthesis in rats. Circulation 102: 806–812 [DOI] [PubMed] [Google Scholar]
- Kogure K, Watson BD, Busto R, Abe K. (1982) Potentiation of lipid peroxides by ischemia in rat brain. Neurochem Res 7: 437–454 [DOI] [PubMed] [Google Scholar]
- L'Azou B, Medina J, Frieauff W, Cordier A, Cambar J, Wolf A. (1999) In vitro models to study mechanisms involved in cyclosporine A-mediated glomerular contraction. Arch Toxicol 73: 337–345 [DOI] [PubMed] [Google Scholar]
- Longoni B, Migliori M, Ferretti A, Origlia N, Panichi V, Boggi U, Filippi C, et al. (2002) Melatonin prevents cyclosporine-induced nephrotoxicity in isolated and perfused rat kidney. Free Radic Res 36: 357–363 [DOI] [PubMed] [Google Scholar]
- Middleton E, Kandaswami C, Theoharides TC. (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev 52: 673–751 [PubMed] [Google Scholar]
- Navarro-Antolin J, Lopez-Munoz MJ, Soria J, Lamas S. (2002) Superoxide limits cyclosporine-A-induced formation of peroxynitrite in endothelial cells (2). Free Radic Biol Med 32: 702–711 [DOI] [PubMed] [Google Scholar]
- Padi SS, Chopra K. (2002) Salvage of cyclosporine A-induced oxidative stress and renal dysfunction by carvedilol. Nephron 92: 685–692 [DOI] [PubMed] [Google Scholar]
- Parra T, de Arriba G, Arribas I, Perez de Lema G, Rodriguez-Puyol D, Rodriguez-Puyol M. (1998a) Cyclosporine A nephrotoxicity: role of thromboxane and reactive oxygen species. J Lab Clin Med 131: 63–70 [DOI] [PubMed] [Google Scholar]
- Parra T, de Arriba G, Conejo JR, Cantero M, Arribas I, Rodriguez-Puyol D, Rodriguez-Puyol M, et al. (1998b) Cyclosporine increases local glomerular synthesis of reactive oxygen species in rats: effect of vitamin E on cyclosporine nephrotoxicity. Transplantation 66: 1325–1329 [DOI] [PubMed] [Google Scholar]
- Parra Cid T, Conejo Garcia JR, Carballo Alvarez F, de Arriba G. (2003) Antioxidant nutrients protect against cyclosporine A nephrotoxicity. Toxicology 189: 99–111 [DOI] [PubMed] [Google Scholar]
- Pechanova O, Bernatova I, Babal P, Martinez MC, Kysela S, Styrtina S, Andriantsitohaina R. (2004) Red wine polyphenols prevent cardiovascular alterations in L-NAME-induced hypertension. J Hypertens 22: 1551–1559 [DOI] [PubMed] [Google Scholar]
- Pedraza-Chaverri J, Maldonado PD, Medina-Campos ON, Olivares-Corichi IM, Granados-Silvestre MA, Hernandez-Pando R, Ibarra-Rubio ME. (2000) Garlic ameliorates gentamicin nephrotoxicity: relation to antioxidant enzymes. Free Radic Biol Med 29: 602–611 [DOI] [PubMed] [Google Scholar]
- Pietta P, Simonetti P, Gardana C, Brusamolino A, Morazzoni P, Bombardeli E. (1998) Relationship between rate and extent of catechin absorption and plasma antioxidant status. Biochem Mol Biol Int 46: 895–903 [DOI] [PubMed] [Google Scholar]
- Polytarchou C, Papadimitriou E. (2005) Antioxidants inhibit human endothelial cell functions through down-regulation of endothelial nitric oxide synthase activity. Eur J Pharmacol 510: 31–38 [DOI] [PubMed] [Google Scholar]
- Rezzani R, Rodella L, Biachi R. (2001) Induction of endothelin in rat kidney after cyclosporine A treatment. Acta Histochem 103: 423–431 [DOI] [PubMed] [Google Scholar]
- Rezzani R. (2004) Cyclosporine A and adverse affects on organs: histochemical studies. Prog Histochem Cytochem 39: 85–128 [DOI] [PubMed] [Google Scholar]
- Rezzani R, Rodella L, Buffoli B, Goodman AA, Abraham NG, Lianos EA, Bianchi R. (2005) Change in renal heme oxygenase expression in cyclosporine A-induced injury. J Histochem Cytochem 53: 105–112 [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Rivera G. (2002) Renal damage mediated by oxidative stress: a hypothesis of protective effects of red wine. Free Radical Biol Med 33: 409–422 [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Rivera G, Orellana M, Araya J, Bosco C. (2002) Rat kidney antioxidant response to long-term exposure to flavonol rich red wine. Life Sci 71: 2881–2895 [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Bosco C, Herrera P, Rivera G. (2004) Amelioration of myoglobinuric renal damage in rats by chronic exposure to flavonol-rich red wine. Nephrol Dial Transplant 19: 2237–2244 [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Castillo R, Carrasco R, Huerta P, Moreno M. (2005) Diminution of tissue lipid peroxidation in rats is related to the in vitro antioxidant capacity of wine. Life Sci 76: 889–900 [DOI] [PubMed] [Google Scholar]
- Satyanarayana PS, Singh D, Chopra K. (2001) Quercetin, a bioflavonoid, protects against oxidative stress-related renal dysfunction by cyclosporine in rats. Methods Find Exp Clin Pharmacol 23: 175–181 [DOI] [PubMed] [Google Scholar]
- Shi SH, Zheng SS, Jia CK, Zhu YF, Xie HY. (2004) Inhibitory effect of tea polyphenols on transforming growth factor-β1 expression in rat with cyclosporine A-induced chronic nephrotoxicity. Acta Pharmacol Sin 25: 98–103 [PubMed] [Google Scholar]
- Shin YH, Lee SH, Mun KC. (2002) Effect of melatonin on the antioxidant enzymes in the kidneys of cyclosporine-treated rats. Transplant Proc 34: 2650–2651 [DOI] [PubMed] [Google Scholar]
- Suleymanlar G, Suleymanlar I, Shapiro JI, Chan L. (1994) Possible role of lipid peroxidation in cyclosporine nephrotoxicity in rats. Transplant Proc 26: 2888–2889 [PubMed] [Google Scholar]
- Tariq M, Morais C, Sobki S, Al Sulaiman M, Al Khader A. (1999) N-acetylcysteine attenuates cyclosporine-induced nephrotoxicity in rats. Nephrol Dial Transplant 14: 923–929 [DOI] [PubMed] [Google Scholar]
- Tomita H, Egashira K, Ohara Y, Takemoto M, Koyanagi M, Katoh M, Yamamoto H, et al. (1998) Early induction of transforming growth factor-beta via angiotensin II type 1 receptors contributes to cardiac fibrosis induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 32: 273–279 [DOI] [PubMed] [Google Scholar]
- Vranes D, Cooper ME, Dilley RJ. (1999) Cellular mechanisms of diabetic vascular hypertrophy. Microvasc Res 57: 8–18 [DOI] [PubMed] [Google Scholar]
- Wang C, Salahudeen AK. (1995) Lipid peroxidation accompanies cyclosporine nephrotoxicity: effects of vitamin E. Kidney Int 47: 927–934 [DOI] [PubMed] [Google Scholar]
- Whitehead TP, Robinson D, Allaway S, Syms J, Hale A. (1995) Effect of red wine ingestion on the antioxidant capacity of serum. Clin Chem 41: 32–35 [PubMed] [Google Scholar]
- Yura T, Fukunaga M, Khan R, Nassar GN, Badr KF, Montero A. (1999) Free-radical-generated F2-isoprostane stimulates cell proliferation and endothelin-1 expression on endothelial cells. Kidney Int 56: 471–478 [DOI] [PubMed] [Google Scholar]
- Zhong Z, Connor HD, Yin M, Wheeler MD, Mason RP, Thurman RG. (2001) Viral delivery of superoxide dismutase gene reduces cyclosporine A-induced nephrotoxicity. Kidney Int 59: 1397–1404 [DOI] [PubMed] [Google Scholar]
- Zou MH, Leist M, Ullrich V. (1999) Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am J Pathol 154: 1359–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]