Abstract
Because the development and activity of osteoclasts in bone remodeling is critically dependent on cell-cell and cell-matrix interactions, we used laser confocal microscopy to study the response of osteoclasts to lipopolysaccharide (LPS; 10 μg/ml), parathyroid hormone (PTH; 10−8 M), and bisphosphonates (BPs; 1–25 μM clodronate or 0.1–2.5 μM risedronate) in cultured neonatal calvaria. Following treatment with LPS or PTH (<48 hr), osteopontin (OPN) and the αvβ3 integrin were found colocalized with the actin ring in the sealing zone of actively resorbing osteoclasts. In contrast, non-resorbing osteoclasts in BP-treated cultures showed morphological abnormalities, including retraction of pseudopods and vacuolization of cytoplasm. In the combined presence of LPS and BP, bone-resorbing osteoclasts were smaller and the sealing zone diffuse, reflecting reduced actin, OPN, and β3 integrin staining. Depth analyses of calvaria showed that the area of resorbed bone was filled with proliferating osteoblastic cells that stained for alkaline phosphatase, collagen type I, and bone sialoprotein, regardless of the presence of BPs. These studies show that confocal microscopy of neonatal calvaria in culture can be used to assess the cytological relationships between osteoclasts and osteoblastic cells in response to agents that regulate bone remodeling in situ, avoiding systemic effects that can compromise in vivo studies and artifacts associated with studies of isolated osteoclasts.
Keywords: calvarial cells, bone remodeling, bisphosphonates, osteopontin, αvβ3 integrin, confocal microscopy
The modeling and remodeling that occurs throughout the growth and development of bones requires a close coordination between the activities of the bone-forming osteoblasts and the bone-resorbing osteoclasts. Loss of cooperativity between these cells can result in the development of an abnormal skeleton and, in the adult, decreased (osteoporosis) or increased (osteopetrosis) bone mass. Regulation of bone remodeling involves systemic control by hormones such as parathyroid hormone (PTH) and 1, 25 di-hydroxyvitamin D3 (vitamin D3), and cytokines displaying autocrine and paracrine functions that provide local communication between osteoblasts and osteoclasts. Remodeling of adult bone occurs continuously as a requirement of functional adaptation and for calcium homeostasis. In response to decreased serum calcium, PTH and vitamin D3 restore calcium levels through their effects on the intestine, kidney, and bone. Both PTH and vitamin D3 increase bone resorption by osteoclasts to release calcium through the dissolution of the hydroxyapatite crystals. However, these effects are largely mediated by osteo-blastic cells, which express cell surface and steroid receptors for the PTH and vitamin D3, respectively. The activity of osteoclasts is also targeted directly and indirectly by inflammatory mediators during fracture repair and in the formation of bone metastases, as well as in response to bacterial infection. Lipopolysaccharides (LPS) produced by bacteria are potent stimulators of osteoclastic resorption, functioning through toll-like receptor 4, which are expressed by osteoblasts as well as by both osteoclast precursors and mature osteoclasts. Stimulation of osteoclastogenesis by LPS, however, appears to involve direct effects on osteoblastic cells, which respond by parallel increases in RANKL expression and the suppression of osteoprotegerin (OPG), which is mediated by prostaglandin E2 production (Suda et al. 2004).
Bone resorption initially involves the differentiation of large multinucleated tartrate-resistant acid phosphatase (TRACP)-positive osteoclasts from the fusion of TRACP-negative monocytic precursors, stimulated by receptor activator of nuclear factor B-ligand (RANK-L) and macrophage colony-stimulating factor (M-CSF) (Lacey et al. 1998; Yasuda et al. 1998). Mature osteoclasts are highly motile cells that form a tight attachment to the mineralized bone surface to be resorbed through a sealing zone, which involves the rearrangement of the cytoskeleton so that actin forms a dense belt-like structure, the “actin ring” (Lakkakorpi et al. 1989). The part of the plasma membrane enclosed by the actin enlarges into the highly convoluted ruffled border through which protons and liposome proteases such as cathepsin K, a cysteine protease highly active in digesting native collagen fibers, are transported (Vaananen et al. 2000). Formation of the ruffled border and the intravesicular transport of degradation products are modulated by TRACP (Hollberg et al. 2002), whereas the attachment of osteoclasts to the bone surface involves ligation of osteoclast integrins with extracellular matrix proteins within the bone matrix.
Results of numerous studies of osteoclasts and osteoblasts have been obtained from cells cultured on artificial substrates, such as plastic or glass. However, two-dimensional monolayer cultures are limited by distortions introduced by the cells having to adapt to artificial flat and rigid surfaces (Themistocleous et al. 2004). Studies using three-dimensional culture systems, which have the potential to simulate cell-cell interactions that take place in tissues under physiological and pathophysiological conditions, have been used successfully in the investigation of complex biological processes such as angiogenesis, wound healing, tumor invasion, and metastasis (Edelman and Keefer 2005). Consequently, the importance of studying cells within their natural environment is being increasingly recognized.
Organ cultures of fetal and neonatal bones, in which the patency of local interactions between osteoblastic and osteoclastic cells is retained within the context of a three-dimensional bone matrix (Stern and Krieger 1983), are frequently used for the biochemical analysis of bone remodeling. However, few studies have examined the cellular responses to factors that modulate bone cell activity. Therefore, we have used laser scanning confocal microscopy to study the effects on bone cells of PTH and LPS, which stimulate bone resorption, and bisphosphonates (BPs) that suppress bone resorption in cultured neonatal mouse calvaria. Responses were determined by fluorescence staining of calvaria for TRACP activity, F-actin, β3 integrin, osteopontin (OPN), alkaline phosphatase (ALP) activity, collagen type I, bone sialoprotein (BSP), and bone mineral.
Materials and Methods
Calvaria Culture
Organ culture of neonatal mouse calvaria has been described in detail previously (Stern and Krieger 1983). Briefly, calvaria were dissected from 4- to 6-day-old ddY mice (Japan SLC Co.; Shizuoka, Japan) and cultured free-floating in roller tubes in Dulbecco's minimal essential medium supplemented with 15% heat-inactivated horse serum, 10 U/ml of heparin, and 100 U/ml of penicillin. Calvaria were analyzed after a culture period of 24 hr, or after 48 hr in which case the medium containing fresh reagents was changed after 24 hr. All cultures were carried out in the presence of LPS (0 or 10 μg/ml) or PTH (0 or 10-8 M) to stimulate osteoclastic bone resorption. The effects of the following BPs were studied: risedronate (Procter and Gamble Pharmaceutics, Wood Corners Laboratories; Norwich, NY) at concentrations of 0, 0.1, 0.5, or 2.5 μM; clodronate (Procter and Gamble Pharmaceutics) at 0, 1, 5, or 25 μM. At the end of culture, calvaria were fixed in 0.01 M PBS containing 4% paraformaldehyde and stained using various fluorescent dyes for histochemical examination. All experiments were performed in accordance with Showa University Animal Care and Use Committee guidelines (#13029).
Fluorescent Staining of Calvaria
Calvaria were cut into four pieces along the sutures producing one frontal, two parietal, and one occipital bone. Only parietal bones were used in these experiments. Bone pieces were washed twice with PBS, blocked with 2% BSA-0.01% Triton X-100 in PBS (BSA buffer) for 1 hr, and incubated with primary antibodies (diluted 1:100 in BSA buffer) overnight at 4C. After washing with BSA buffer for 2 hr, bones were incubated with secondary antibodies (diluted 1:200 in BSA buffer) for 2 hr, washed with BSA buffer for 2 hr, and mounted on glass slides using a PermaFluor (ThermoShandon; Pittsburgh, PA) aqueous mounting medium. Rabbit anti-human OPN (LF123), which cross-reacts with murine OPN, was generously provided by Dr. L.W. Fisher (National Institutes of Health; Bethesda, MD). Hamster anti-mouse β3 integrin monoclonal antibody (clone 2C9.G2), diluted 1:100, was from BD Biosciences (San Jose, CA). Alexa Fluor 488- or rhodamine-conjugated phalloidin was from Molecular Probes (Eugene, OR). Rabbit anti-rat type I collagen, which cross-reacts with murine type I collagen, was from LSL Co. Ltd. (Tokyo, Japan). Expression of BSP was determined using an affinity-purified antibody raised in rabbits against the N-terminal peptide of rat BSP. This antibody is specific for bone cells and cross-reacts with mouse BSP.
TRACP activity was detected as described previously (Mostafa et al. 1982) but using naphthol AS-MX phosphate as a substrate, and ALP activity was detected by an ELF 97 endogenous phosphatase detection kit (Molecular Probes). Mineral was stained by incubation with Alizarin Red S diluted at 10 mg/ml in 0.25% ammonia solution for 10 min (Burdi 1965).
Osteoclast Culture and Fluorescent Staining
Bone marrow cells obtained from tibias and femurs of 5- to 7-week-old ddY mice were treated with 0.83% NH4Cl in 10 mM of Tris-HCl buffer (pH 7.4) for 20 min on ice to hemolyze red blood cells. The remaining cells were suspended in 0.5-1.0 ml of α-minimal essential medium (α-MEM) with 10% FCS adjusted to pH 7.0 after adding FCS and applied onto a Sephadex G-10 (Amersham Pharmacia Biotech; Uppsala, Sweden) column (bed volume 5-10 ml) and incubated for 45 min at 37C to remove macrophages and stromal cells. Non-adherent cells were collected by eluting with culture media and plated onto Lab-Tek chamber slides (eight-well glass slide; Nalge Nunc International, Naperville, IL) at a density of 5 × 105 cells/cm2 and then cultured in α-MEM with 10% FCS for 3 days in the absence or presence of 25 μM clodronate or 2.5 μM risedronate. The Lab-Tek chamber slides were precoated by adding 10 μl/well of mixture of recombinant human M-CSF (10 ng/ml of culture media; Pepro Tech EC Ltd., London, UK) and recombinant human soluble receptor activator of nuclear factor κB (NF-κB) ligand (sRANKL, 50 ng/ml of culture media; Pepro Tech EC Ltd.), which was air dried for 2 hr.
For fluorescent staining, cells were fixed with 2% paraformaldehyde for 15 min at 4C. After washing with PBS and treating with 0.1% Triton X-100 in PBS for 5 min, cells were incubated with Alexa Fluor 488-conjugated phalloidin (diluted 1:100 in PBS; Molecular Probes) for 30 min.
Laser Scanning Confocal Microscopy and Image Processing
Stained calvaria and osteoclast cultures were examined under a laser scanning confocal microscope equipped with an optical laser unit and a scanning unit (Inverted System Microscope IX70; Olympus Optical Co., Tokyo, Japan). Images were obtained with a UPLAPO×20 [numerical aperture (N.A.): 0.70] or a UPLAPO×40 (N.A.: 0.85) objective lens at various magnifications (1 to 10×) obtained using the microscope software. Alexa Fluor 488 and ELF 97 alcohol precipitate, a product generated by the enzymatic cleavage of ELF 97 phosphatase substrate, were excited with the 488-nm line of an air-cooled argon ion laser and detected with a 510/540 band-pass emission filter (FVX-BA510/540). Alexa Fluor 594, Texas Red, rhodamine, Alizarin Red, and TRACP staining were excited with the 568-nm line of an air-cooled krypton ion laser and detected with a 585-nm long-pass barrier filter (FVX-BA585IF) to avoid the carryover between green and red fluorescence. Z-stacks of serial optical sections of XY images taken at the indicated intervals were processed to create a composite image by estimating the regions of best focus using image-processing and analysis software (MetaMorph; Universal Imaging Co., West Chester, PA).
Statistical Analyses
The area and volume of resorption lacunae, the area of regions stained with Alizarin Red S or fluorescent-conjugated phalloidin, and the percent of resorption were averaged from a minimum of three values in replicate experiments from which the mean ± SD (mean ± SEM in area and volume of resorption lacunae) were calculated. Levene's test was used to determine homogeneity of variance, and Bonferroni's procedure was used in the endpoint adjustment of p values for multiplicity, using Statistical Package for Social Science (SPSS) for Windows 11.0.1J (SPSS Japan Inc.; Tokyo, Japan).
Results
TRACP Staining of Non-resorbing and Resorbing Osteoclasts in the Calvaria
We have previously reported that monolayer cultures of prefusion osteoclasts extend many pseudopods and fuse into large multinuclear osteoclasts and that these processes are suppressed in the absence of OPN expression (Suzuki et al. 2002a). To relate these observations to the behavior of osteoclasts in bone in situ, laser scanning confocal microscopy was used to examine neonatal mouse calvaria maintained in culture. Calvaria cultured for various times were stained for TRACP activity after fixation to identify osteoclasts, which varied in size and shape depending on their location. Most of the TRACP-positive cells close to the sagittal suture (Figure 1A) were mononuclear and smaller than those located ∼1.5 mm distant (a piece of parietal bone width ∼5 mm) from the suture (Figure 1B), indicating that the first mononuclear TRACP-positive cells are formed near the soft connective tissue suture and fuse into multinuclear TRACP osteoclasts further away from the suture. The morphological appearance of a typical osteoclast in unstimulated cultures is shown using a series of serial optical sections (Figure 1D). Multinucleated, non-resorbing osteoclasts could be identified extending numerous long pseudopods used for generation of larger, multinucleated osteoclasts (Figure 1C), as observed in monolayer cultures of osteoclasts (Suzuki et al. 2002a). To examine actively resorbing osteoclasts, calvaria were cultured for 48 hr in the presence of 10−8 M PTH to stimulate resorption. Serial optical sections showed strong TRACP staining at the sealing zone (Figure 1E, arrows) coinciding with the edge of resorption lacunae (Figure 1F) and in the basolateral membrane (Figure 1G, sections 8.4-22.4 μm). Notably, a similar morphology was also observed for bone-resorbing osteoclasts in calvaria treated with 10 μg/ml LPS (Suzuki et al. 2003).
Figure 1.
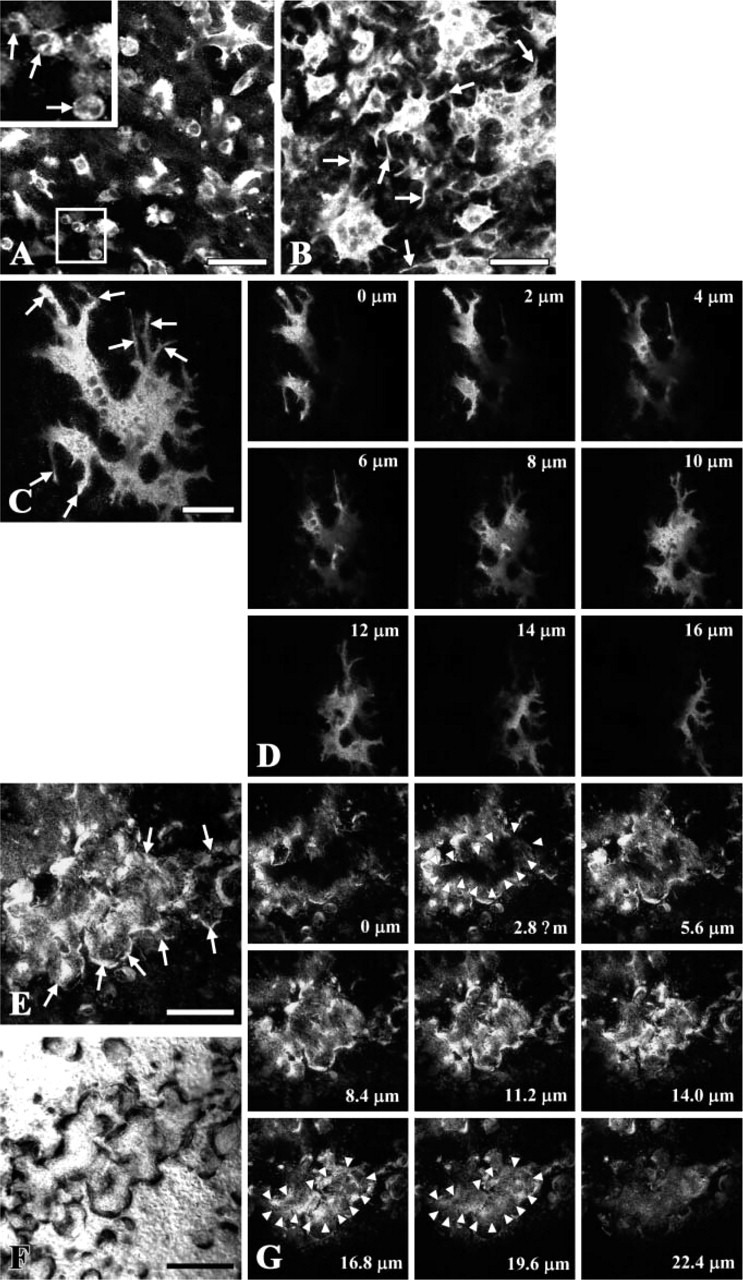
Tartrate-resistant acid phosphatase (TRACP) staining of non-resorbing and resorbing osteoclasts in cultured calvaria. Laser scanning confocal microscopy of neonatal mouse calvaria stained for TRACP activity after culturing for 24 hr (C, D) or 48 hr (A, B, E-G) in the absence (A-D) or presence (E-G) of 10−8 M parathyroid hormone (PTH). The pictures were created by estimating the regions of best focus in the z-stacks made of 11 and 15 images taken at 0.5-μm intervals in A and B, respectively (MetaMorph). Arrows in the picture at higher magnification in A indicate round prefusion osteoclasts and in B indicate long pseudopods extending from the osteoclasts. (C) Picture created by estimating the regions of best focus in a z-stack made of 40 images taken at 0.4-μm intervals in the TRACP-stained calvaria. Arrows indicate long pseudopods. (D) Serial optical sections of a single osteoclast taken at 2-μm intervals from upper (periosteal) surface (0 μm) to the lower (endosteal) surface (16 μm) of the calvaria. (E) Picture created by estimating the regions of best focus in a z-stack made of 37 images. Arrows indicate the sealing zone surrounding resorption areas, also shown using Nomarsky optics (F), associated with resorbing osteoclasts in TRACP-stained calvaria cultured in the presence of 10−8 M PTH. (G) Serial optical sections taken at 2.8-μm intervals from upper surface (0 μm) to lower surface (22.4 μm) of the calvaria. Arrowheads indicate the edge of attachment zone at 2.8 μm. Bars: A, B = 50 μm; C = 30 μm; E, F = 200 μm.
To study whether BPs affect the formation and differentiation of osteoclasts in bone, TRACP-stained calvaria were examined by confocal microscopy after culturing in the presence or absence of clodronate. A wide range of morphological abnormalities was observed in the osteoclasts, ranging from mild (Figure 2A) to severe (Figure 2B) within individual calvaria, even at the same concentration of clodronate (1 μM). The frequency of osteoclast abnormalities, including retraction of pseudopods and vacuolization of cytoplasm, was increased with increasing concentrations of clodronate. Similar effects were observed when risedronate (Figures 2C and 2D) or alendronate (Suzuki et al. 2003) were added to the culture media. Serial optical sections through calvarial bone treated with risedronate showed that the vacuoles are present throughout the osteoclasts (Figure 2D, 0-16 μm). To determine whether these responses were the result of direct effects of the BP on the osteoclasts, preosteoclasts derived from mouse bone marrow cells were incubated for 3 days in the absence or presence of 25 μM clodronate or 2.5 μM risedronate and stained with Alexa Fluor 488-conjugated phalloidin after fixation. Compared with control (Figure 2E), osteoclasts treated with BPs displayed retracted pseudopods (Figures 2F and 2G) and were highly vacuolated (Figure 2G), as observed in the calvarial cultures. In calvaria cultured in the presence of PTH and 25 μM clodronate (Figure 2H) or LPS and 2.5 μM risedronate (Figure 2I), the multinucleated osteoclasts were smaller, displayed severe cytoplasmic vacuolization (Figures 2H and 2I, arrowheads), and the sealing zone was diffuse compared with osteoclasts in calvaria cultured with PTH alone (Figure 1E).
Figure 2.
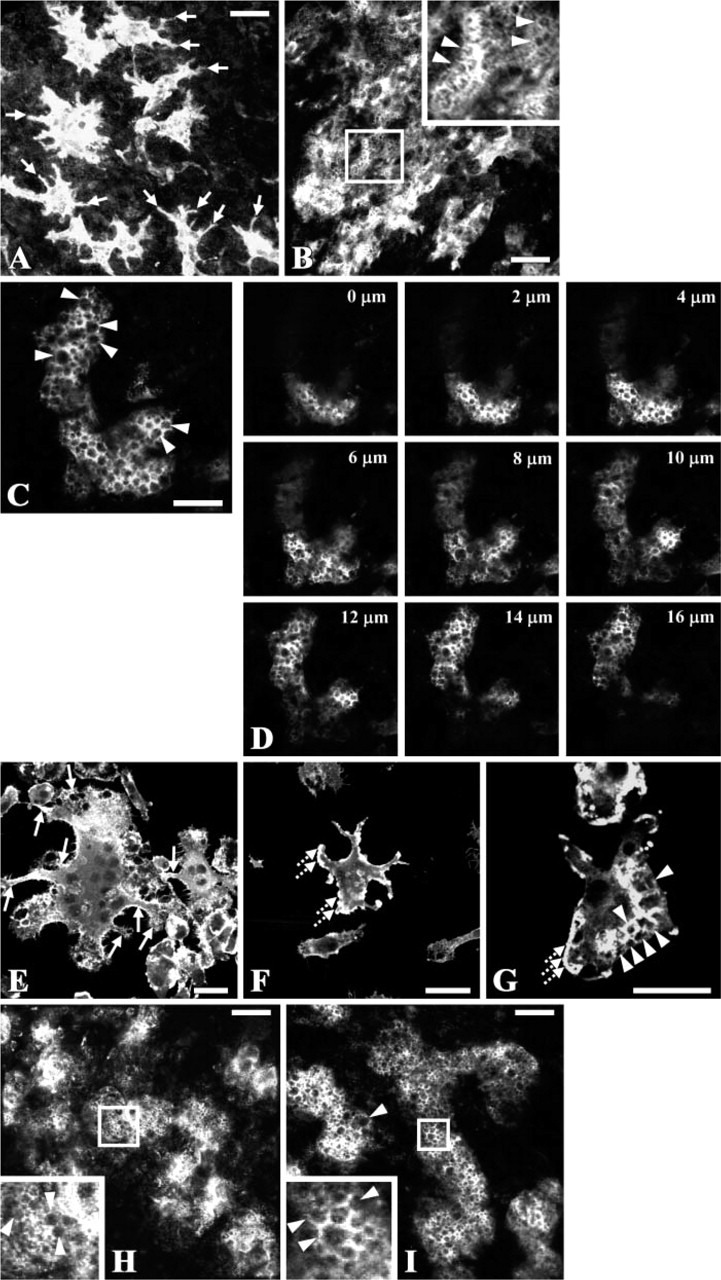
Effects of bisphosphonates (BPs) on the differentiation and activation of osteoclasts. Laser scanning confocal microscopy of neonatal mouse calvaria stained for TRACP activity after culturing for 24 hr (A-D, H) or 48 hr (I) in the presence of BP alone (A-D) or in combination with 10−8 M PTH (H) or 10 μg/ml LPS (I). (A, B) Calvaria were cultured in the presence of 1 μM clodronate alone. The pictures were created by estimating the regions of best focus in the z-stacks made of 44 and 51 images taken at 0.7- and 0.5-μm intervals in A and B, respectively (MetaMorph). Arrows in A indicate cell processes. Arrowheads in the picture at higher magnification in B indicate cytoplasmic vacuolization. (C, D) Calvaria were cultured in the presence of 2.5 μM risedronate alone. (C) Picture created by estimating the regions of best focus in a z-stack made of 43 images taken at 0.5-μm intervals in the TRACP-stained calvaria. Arrowheads indicate cytoplasmic vacuolization. (D) Serial optical sections taken from upper surface (0 μm) to lower surface (16 μm) of the calvarium. (E-G) Preosteoclasts derived from mouse bone marrow cells were incubated for 3 days in the absence (E) or presence (F) of 25 μM clodronate or 2.5 μM risedronate (G) and stained with Alexa Fluor 488-conjugated phalloidin for 30 min. Arrows, dotted arrows, and arrowheads indicate pseudopods extending from the osteoclasts, retracted pseudopods, and cytoplasmic vacuolization, respectively. (H, I) Calvaria were cultured in the presence of 10−8 M PTH and 25 μM clodronate (H) or 10 μg/ml lipopolysaccharides (LPS) and 2.5 μM risedronate (I). The pictures were created by estimating the regions of best focus in the z-stacks made of 26 and 19 images taken at 0.6-μm intervals in H and I, respectively (MetaMorph). Arrowheads indicate the cytoplasmic vacuolization. Bars: A, B, H, I = 50 μm; C, E-G = 30 μm.
Fluorescent microscope images of serial fields covering the entire parietal bone stained with Alizarin Red S showed that LPS increased resorbed areas by ∼8-fold at 24 hr and by ∼15-fold at 48 hr. The LPS-induced resorption at both time points was dose-dependently and significantly decreased by both clodronate and risedronate (Table 1). Despite the increased resorption at 48 hr, both clodronate and risedronate suppressed the LPS-induced resorption to similar levels observed at the 24-hr time point. Notably, risedronate at one-tenth the concentration was more effective than clodronate. Furthermore, a reduction in the size and depth of resorption lacunae formed by osteoclasts in the presence of BPs was evident in the Nomarsky images (Figure 3, Figure 4, and Figure 8) and from the quantitative analysis of the size of individual resorption bays (Table 2).
Table 1.
Inhibitory effects of BPs on bone resorption induced by LPS
| Control | LPS | LPS and CL1 | LPS and CL5 | LPS and CL25 | LPS and RI0.1 | LPS and RI0.5 | LPS and RI2.5 | |
| 24hr | 1.8 ± 0.3c | 14.7 ± 2.6 | 11.8 ± 1.1a | 7.4 ± 1.4b | 5.4 ± 2.1c | 11.3 ± 2.1a | 6.7 ± 2.0b | 4.8 ± 1.4c |
| (3) | (3) | (3) | (3) | (3) | (3) | (3) | (3) | |
| 48 hr | 1.9 ± 0.3c | 29.3 ± 2.3 | 21.4 ± 3.4c | 16.2 ± 4.1c | 5.5 ± 3.1c | 20.8 ± 3.5b | 14.1 ± 1.7c | 4.5 ± 2.5c |
| (3) | (4) | (4) | (4) | (4) | (3) | (3) | (3) |
Not significant.
p<0.05.
p<0.01.
Neonatal murine calvaria were cultured in the absence or presence of 10 μg/ml LPS and/or BP (1, 5, 25 μM clodronate or 0.1, 0.5, 2.5 μM risedronate) for 24 or 48 hr. The % resorption was determined from analyses (MetaMorph) of whole calvaria reconstructed from the X-Y images of mineral staining taken at lower magnification (×5) using conventional fluorescent microscopy. Values are mean ± SD calculated from the number of samples shown in parentheses. Statistical significance of differences observed with bisphosphonates compared to LPS alone was determined by Student's t-test followed by Bonferroni's multiplicity adjustment. BPs, bisphosphonates; LPS, lipopolysaccharides; RI, risedronate.
Figure 3.
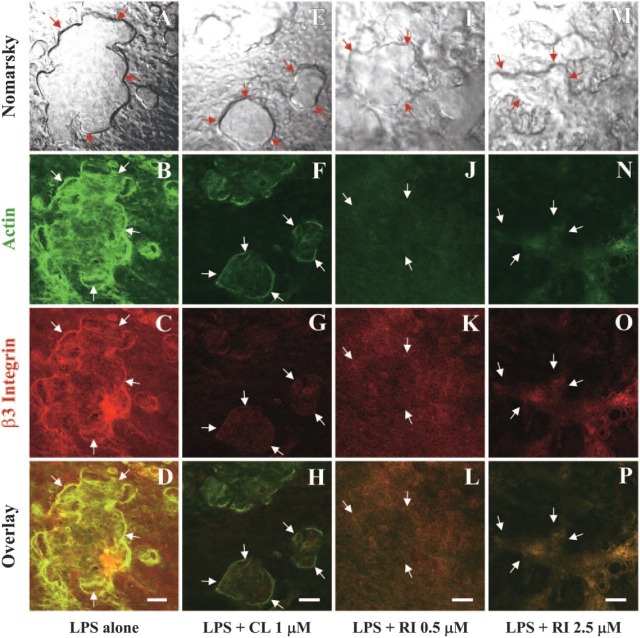
Effects of BPs on the colocalization of actin and β3 integrin in sealing zone of resorbing osteoclasts in calvaria cultured for 48 hr in the presence of LPS. Laser scanning confocal microscopy of neonatal mouse calvaria stained for F-actin (Alexa Fluor 488-conjugated phalloidin) and β3 integrin (hamster anti-mouse β3 integrin antibody followed by Alexa Fluor 594-conjugated anti-hamster IgG) after culturing for 48 hr in the presence of 10 μg/ml LPS alone (A-D) or in combination with 1 μM clodronate (E-H), 0.5 μM risedronate (I-L), or 2.5 μM risedronate (M-P). Arrows indicate the edge of resorption lacunae in the Nomarsky image and corresponding sites in the fluorescent images (actin: B, F, J, N; β3 integrin: C, G, K, O). Bars = 50 μm.
Figure 4.
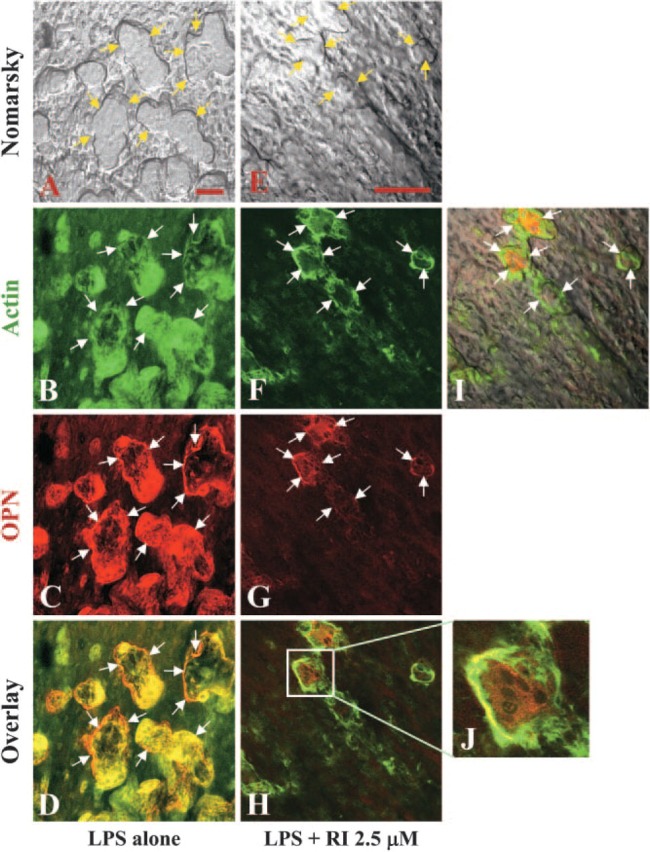
Effects of BP on the colocalization of actin and osteopontin (OPN) in sealing zone of resorbing osteoclasts in calvaria cultured for 48 hr in the presence of LPS. Laser scanning confocal microscopy of neonatal mouse calvaria stained for F-actin (Alexa Fluor 488-conjugated phalloidin) and OPN (LF123 antisera followed by Texas Red conjugated anti-rabbit IgG) after culturing for 48 hr in the presence of 10 μg/ml LPS alone (A-D) or in combination with 2.5 μM risedronate (E-J). Arrows indicate the edge of resorption lacunae in the Nomarsky image and corresponding sites in the fluorescent images (actin: B, F; OPN: C, G). Bars = 50 μm.
Figure 8.
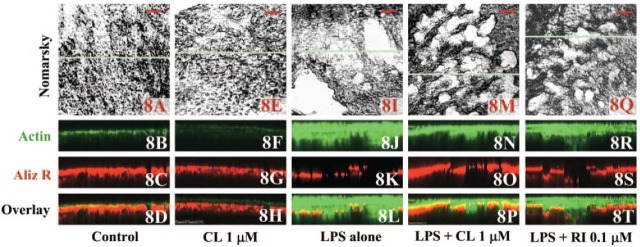
Calvaria cultured for 48 hr under various conditions, i.e., in the presence of vehicle (A-D), 1 μM clodronate alone (E-H), 10 μg/ml LPS alone (I-L) or in combination with 1 μM clodronate (M-P) or 0.1 μM risedronate (Q-T). Calvaria were stained for actin with Alexa Fluor 488-conjugated phalloidin (B, F, J, N, R) and for mineral using Alizarin Red S (C, G, K, O, S). Top panels (A, E, I, M, Q) show the Nomarsky images where white areas indicate the resorption lacuna. XZ (depth)-scans of actin and mineral staining made on the blue horizontal line in the corresponding Nomarsky images. Bars = 100 μm. Depth in the XZ scans is 100 μm.
Table 2.
Effects of BPs on the size of individual resorption bays
| LPS | LPS and CL1 | LPS and CL5 | LPS and CL25 | LPS and RI0.1 | LPS and RI0.5 | LPS and RI2.5 | |
| Mean ± SEM | 20.9 ± 4.5 | 6.6 ± 1.0b | 3.1 ± 0.4b | 2.6 ± 0.4b | 5.8 ± 1.1a | 4.0 ± 0.6a | 1.8 ± 0.2b |
| Area (103 μm2) | |||||||
| Minimum | 0.54 | 0.06 | 0.01 | 0.02 | 0.38 | 0.31 | 0.01 |
| Maximum | 313.2 | 69.2 | 21.2 | 13.9 | 40.7 | 18.3 | 12.4 |
| n | 100 | 95 | 117 | 54 | 38 | 48 | 196 |
| Mean ± SEM | 56.9 ± 13.8 | 7.7 ± 1.5b | 2.6 ± 0.4b | 1.1 ± 0.2b | 11.5 ± 2.7a | 3.6 ± 0.9b | 1.1 ± 0.2b |
| Volume (104 μm3) | |||||||
| Minimum | 0.65 | 0.04 | 0.004 | 0.009 | 0.46 | 0.1 | 0.002 |
| Maximum | 940 | 104 | 24.7 | 6.2 | 97.7 | 39.7 | 19.0 |
| n | 100 | 95 | 117 | 54 | 38 | 48 | 196 |
p<0.05.
p<0.01.
Neonatal murine calvaria were cultured in the presence of 10 μg/ml LPS and/or BP (1, 5, 25 μM clodronate or 0.1, 0.5, 2.5 μM risedronate) for 48 hr. The area and the depth of the resorption bays were measured from X-Y images of Nomarsky and X-Z images of mineral staining, respectively. The volume of the resorption bays was calculated from the area multiplied by corresponding depth. Statistical significance of differences observed with BPs compared to LPS alone was determined by Student's t-test followed by Bonferroni's multiplicity adjustment. BPs, bisphosphonates; LPS, lipopolysaccharides; CL, clodronate; RI, risedronate.
Effects of BPs on the Colocalization of F-actin and β3 Integrin/OPN in Resorbing Osteoclasts
Although αvβ3 integrin and OPN are known to play an important role in the attachment of osteoclasts to bone, we have been unable to show the expression of either protein associated with the actin ring in mature osteoclasts generated from bone marrow or spleen mononuclear cells cultured on glass in the presence of M-CSF and RANKL (unpublished data). To investigate whether the difference in substrata to which the osteoclasts attach influences the expression of β3 integrin and OPN, we examined resorbing osteoclasts in the calvaria after double staining for actin with β3 integrin or OPN, by confocal microscopy. The actin (Figure 3B) and β3 integrin (Figure 3C) show extensive colocalization (Figure 3D, yellow) and are concentrated in the actin ring bordering large resorption lacunae (Figures 3A-3D) in resorbing osteoclasts of calvaria cultured for 48 hr in the presence of 10 μg/ml LPS. However, it is not clear whether the actin and β3 integrin, colocalized inside the sealing zone (Figures 3B-3D), are within the osteoclasts or are present in closely associated osteoblasts. In BP-treated calvaria (Figures 3E-3P), the resorption lacunae were reduced in size and depth (Figures 3E, 3I, 3M) compared with the calvaria cultured with LPS alone (Figure 3A). Furthermore, the presence of actin and β3 integrin along the edge of osteoclasts, which is required for cell attachment to the resorption site, was not readily observed (Figures 3F-3H, 3J-3L, 3N-3P).
Actin (Figure 4B) also colocalized with OPN (Figure 4C), as observed in resorbing osteoclasts stimulated by 10 μg/ml LPS. The colocalization was observed at the periphery of the bone-resorbing osteoclasts (Figure 4D) and coincided with the edges of resorption lacunae (Figure 4A), as seen with the actin and β3 integrin. In cultures treated with 2.5 μM risedronate in combination with LPS (Figures 4E-4J), the resorption lacunae were reduced in size and depth (Figure 4E), and the staining of actin (Figure 4F) and especially the OPN (Figure 4G) were markedly reduced and their peripheral colocalization lost (Figure 4I: merged image of Figures 4E and 4H). The loss of F-actin and OPN, which colocalized in multinucleated osteoclasts, was more clearly evident at higher magnification (Figure 4J).
XZ (Depth) Analyses of Double-stained Calvaria by Using a Laser Scanning Confocal Microscope
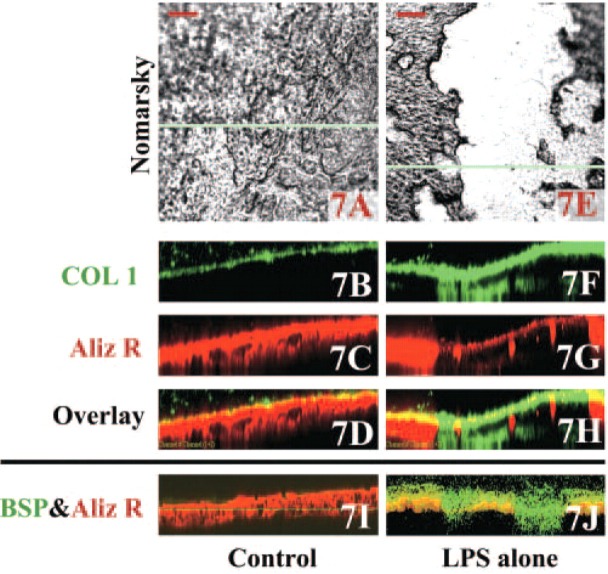
To further demonstrate the spatial organization of actin and β3 integrin, images of osteoclasts double stained for actin and β3 integrin were taken at a focal plane beginning 16.1 μm above (Figures 5A-5D) the level of the sealing zone (Figures 3B-3D) in LPS-treated calvaria. Serial optical sections through to 35.7 μm above the resorption lacunae revealed cells with morphological features of osteoblasts. To determine whether these actin-staining cells were osteoblasts, calvaria were double stained for actin and ALP activity (Figures 6A-6K), for collagen type I and mineral (Figures 7A-7H), or for BSP and mineral (Figures 7I and 7J). The ALP and actin appear to be coexpressed (Figures 6H and 6K, yellow) as expected in osteoblastic cells, which fill the resorbed area and proliferate on top of the resorbed area of bone (Figures 6F-6K). That the osteoblastic cells were newly formed was confirmed by experiments in which bromodeoxyuridine added to the culture media was incorporated into the cell nuclei (data not shown). In actin-stained areas lacking ALP activity at the periphery of the resorption area (indicated as blue and pink lines in Figures 6H and 6K, respectively), the actin appears localized to the sealing zone of bone-resorbing osteoclasts. The osteoblastic cells stained strongly for collagen type I (Figures 7F and 7H), whereas little staining for collagen is observed in control calvaria (Figures 7B and 7D). Similarly, in the LPS-treated cultures the putative osteoblastic cells were concentrated at resorption sites, as indicated by the diminished staining with Alizarin Red, and stained strongly for BSP (Figure 7J), which is a marker of mature osteoblasts (Ganss et al. 1999). In contrast, much weaker staining for BSP was observed in the cells associated with the shallow resorption lacunae in control calvaria (Figure 7I).
Figure 5.
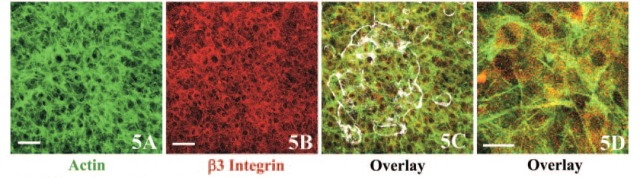
Cell proliferation observed after bone resorption induced by LPS. (A-D) Optical sections of calvaria cultured in the presence of LPS taken at the focal plane 16.1 μm above the level shown in Figure 3B. The sealing zone of osteoclasts observed in LPS-treated calvaria in Figure 3B was superimposed in white (C) on the merged image of actin (A) and β3 integrin (B). (D) Merged image at higher magnification. Bars: A, B = 50 μm; D = 20 μm.
Figure 6.
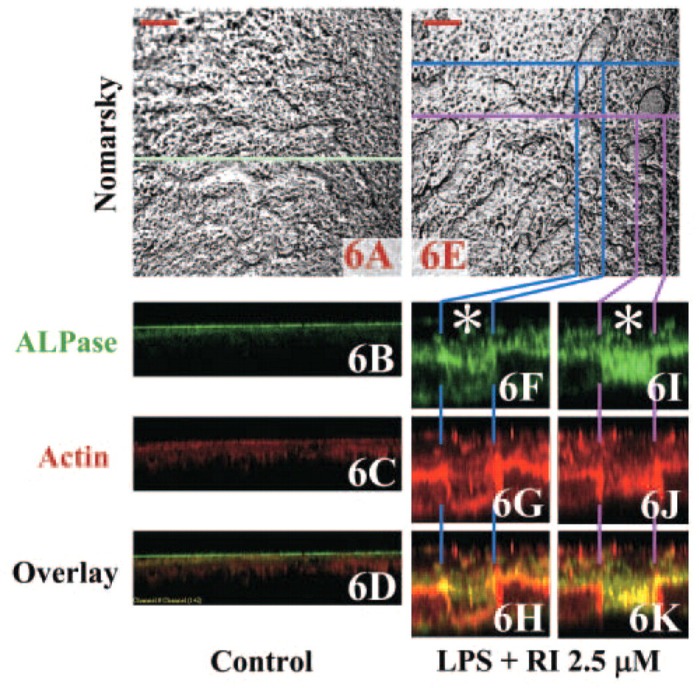
Calvaria cultured for 48 hr with vehicle alone or 10 μg/ml LPS and 2.5 μM risedronate (Nomarsky images; A and E, respectively) were double stained for alkaline phosphatase activity (B, F, I) and actin (C, G, J) using ELF 97 phosphatase substrate and Alexa Fluor 594-conjugated phalloidin, respectively. Depth analyses in the LPS and 2.5-μM risedronate group were made on the blue (F-H) and pink (I-K) lines separately. Asterisks in F and I indicate the resorption lacunae. The horizontal green lines in XZ scans in B and D are due to autofluorescence derived from a glass coverslip. Bars = 100 μm. Depth in the XZ scans is 100 and 70 μm in B-D and F-K, respectively.
Figure 7.
Calvaria cultured for 48 hr with vehicle alone or 10 μg/ml LPS alone (Nomarsky images; A and E, respectively) were double stained with rabbit anti-rat collagen type I antibody (B, F) or rabbit anti-rat bone sialoprotein (BSP) antibody (I, J) followed by Alexa Fluor 488-conjugated anti-rabbit IgG and Alizarin Red S for mineral. XZ (depth)-scans were made on the blue horizontal lines in the Nomarsky images. Bars = 100 μm. Depth in the XZ scans is 100 μm.
From XZ (depth)-scans of the calvaria double stained for actin and mineral after culturing under various conditions (Figure 8), it was evident from the actin staining that cell proliferation had been stimulated in LPS-treated calvaria (Figure 8J). In control (Figure 8B) and clodronate-treated calvaria (Figure 8F), little accumulation of cells was observed, indicating that the proliferation of bone-forming cells occurs in response to accelerated bone resorption induced by LPS. The area of the remaining bone mineral and proliferation of bone-forming cells were measured from the mineral staining in XZ images of calvaria stained with Alizarin Red S and actin staining in XZ images of calvaria stained with Alexa Fluor 488-conjugated phalloidin, respectively, assuming that the ALPase-positive and collagen-producing cells were bone-forming cells. The area of the remaining bone mineral in control and LPS was 12.6 ± 2.6 (mean ± SD; n=40) and 7.0 ± 1.8 μm2 (mean ± SD; n=49), respectively. The areas of proliferation of bone-forming cells were 0.2 ± 0.3 (mean ± SD; n=40) and 12.7 ± 6.0 μm2 (mean ± SD; n=49), respectively. There was a statistically significant correlation between these parameters in the LPS group (r = −0.699, p = 0.002, n = 49). Furthermore, it was evident from the Alizarin Red staining of mineral (Figures 8O and 8S) and the immunostaining of actin (Figures 8N and 8R), that LPS-induced resorption was decreased in the presence of BPs whereas the accumulation of osteoblastic cells was still evident, albeit not as marked as in calvaria treated with LPS only (Figure 8J).
Together these observations suggest that the cells occupying areas where bone resorption has taken place are ALP-positive, collagen type I-, and BSP-producing osteoblastic cells, and that there is an induction of bone formation coupled with LPS-stimulated bone resorption. Notably, similar results were obtained when 10−8 M PTH was used to stimulate bone resorption (Suzuki et al. 2003).
Discussion
To study bone resorption in situ, we used laser scanning confocal microscopy to analyze the effects of PTH and LPS alone or in combination with BPs on osteoclasts in organ cultures of mouse calvaria. To our knowledge, this is the first report examining bone resorption in cultured calvaria using confocal microscopy. Although confocal microscopy has been used to study combined effects of osteoclasts and osteoblasts in vitro (Mulari et al. 2004), isolated cells were monitored sequentially, which does not allow the reciprocal effects of the cells to be assessed. Our approach has many advantages over cell culture systems in which osteoclasts are grown on either plastic/glass plates or on mineralized bone/dentine tissue slices. In particular, osteoclasts in the calvaria retain their three-dimensional relationship with osteoblasts, osteocytes, and the surrounding mineralized bone matrix, and the osteoclasts contact a natural mineralized tissue surface. Also, the effects of agents that influence resorption can be assessed directly, without systemic effects that complicate in vivo studies. Using this approach we have revealed changes in the morphological features of osteoclasts in response to LPS and PTH, which stimulate bone resorption, and to various BPs that suppress bone resorption. In combination with fluorescent labeling, confocal microscopy has revealed the precise spatial localization and codistribution of specific molecules such as actin, TRACP, β3 integrin, and OPN, which have functional importance in osteoclast activities. Collectively, the studies show that BPs inhibit LPS- or PTH-induced osteoclast differentiation, fusion, attachment, actin ring formation, and activation, and that both the β3 integrin and OPN have an important role in cytoskeletal rearrangements associated with cell attachment and resorption in osteoclasts. Moreover, we show that osteoclasts use pseudopods to fuse with each other in the calvaria as well as in monolayer culture, and that BPs inhibit osteoclast formation in the absence of systemic calcium-regulating hormones.
Regulation of osteoclastic activity by BPs is used extensively in the treatment of metabolic bone diseases associated with increased bone resorption. However, the precise mechanism of action on bone metabolism of BPs is still unclear. BPs cause osteoclast retraction, condensation, cellular fragmentation, and induce apoptosis, recognized by morphological changes (Schenk et al. 1973; Flanagan and Chambers 1989; Hughes et al. 1995). BPs also inhibit osteoclast recruitment and differentiation (Lowik et al. 1988; Hughes et al. 1989; Cecchini and Fleisch 1990), the attachment of osteoclasts to the bone surface (Colucci et al. 1998), and ruffled border formation (Sato et al. 1991), which are essential for bone resorption. Notably, these effects may be a consequence of BPs interfering with the remodeling of the actin cytoskeleton (Selander et al. 1994; Murakami et al. 1995) and microtubule formation, which are required for the formation of cell processes and osteoclast fusion (Figure 2). However, BPs also affect osteoblasts, causing the release of a factor that inhibits osteoclast activity or formation (Sahni et al. 1993; Vitte et al. 1996). Although it is unclear which of these processes of bone resorption is most sensitive to inhibition by BPs, it has been suggested that the most likely route by which BPs inhibit bone resorption is through a direct effect on resorbing osteoclasts (Rogers et al. 2000).
Although there have been many investigations on the effect of BPs on osteoclast differentiation and formation, the results remain controversial. In our studies, BP caused vacuolarization (Figures 2B-2D, 2G-2I) and the retraction of pseudopods (Figures 2B-2D, 2F-2I) in some osteoclasts. Although cytoplasmic vacuolarization could reflect toxic effects of BPs, whether it is associated with apoptosis or causes the suppression of bone resorption activity is not clear at present. As observed previously (Gravel et al. 1994; Suzuki et al. 2002a), reduced pseudopod formation is suggestive of an impaired ability of preosteoclasts to fuse, an important step in osteoclastic differentiation. Although BPs may inhibit the resorption-stimulating activity by osteoblasts, it is evident from our studies on TRACP-positive mononuclear cells derived from bone marrow that BPs inhibit M-CSF and RANKL-induced fusion of osteoclast precursors in the absence of osteoblasts (Figures 2F and 2G).
That cell adhesion molecules play an important role in skeletal growth, development, and homeostasis is well established. Cell attachment molecules such as OPN and its αvβ3 integrin receptor are involved in osteoclast differentiation, migration to sites of resorption, fusion of postmitotic osteoclast precursors, cellular polarization, and tight sealing zone formation required for bone resorption (Reinholt et al. 1990; Yamate et al. 1997; Nakamura et al. 1999; Duong et al. 2000; McHugh et al. 2000; Chellaiah and Hruska 2003). The cessation of resorption by detachment of osteoclasts from the bone matrix also involves adhesion molecules (Ek-Rylander et al. 1994; Katayama et al. 1998), which are important for osteoclast survival (Horton et al. 2002). In addition to mediating cell adhesion, cell attachment molecules signal through receptors and affect cytoskeletal remodeling and gene transcription.
OPN, which interacts with the αvβ3 integrin through an RGD sequence (Ross et al. 1993; Grano et al. 1994) and mediates the attachment of osteoclasts to the bone surface (Reinholt et al. 1990; Flores et al. 1992), has a prominent role in bone resorption. OPN is localized to the basolateral surfaces in the clear zone and in ruffled border membranes (Andersson and Johansson 1996; Chellaiah et al. 2003) and is deposited in the resorption pits during bone resorption. Although OPN in the lamina limitans coating the bone surface is produced by osteoblasts following bone formation (McKee and Nanci 1995), osteoclasts also produce OPN (Tezuka et al. 1992; Tong et al. 1994), which is found in resorption lacunae formed during the remodeling of intramembranous and endochondral bone (Maeda et al. 1994) and in developing osteophytes (Dodds et al. 1995). The presence of the OPN is likely important for the chemotaxis and attachment of lining cells that migrate into the resorption lacunae and initiate bone formation (Everts et al. 2002). In addition to mediating attachment, OPN stimulates the resorptive activity of osteoclasts (Horton et al. 1995; Chellaiah and Hruska 2003), reflecting its cytokine properties that also appear to be important in the migration and fusion of osteoclast precursors (Suzuki et al. 2002a).
OPN secreted from the basolateral surfaces of osteoclasts during bone resorption (Chellaiah et al. 2003) acts as an autocrine factor by binding to the αvβ3 integrin (Chellaiah and Hruska 2003). In previous studies we have shown that OPN colocalizes with the β3 integrin exclusively at the cell periphery and in the cell processes of prefusion osteoclasts cultured with M-CSF and RANKL (Suzuki et al. 2002b). However, colocalization of OPN and β3 integrin is not evident in the sealing zone of multinuclear osteoclasts grown on glass, even though an actin ring was formed around the cell periphery (Suzuki et al. 2003). In contrast, in calvarial organ cultures we have confirmed that both OPN and β3 integrin colocalize with actin along the edge of resorbing osteoclasts (Figure 3 and Figure 4). These results indicate that the cytoskeletal reorganization involved in the formation of a sealing zone around resorbing osteoclasts is strictly regulated by the substrata on which the cell locates and emphasizes the importance of studying these cells in their natural environment. Although our results do not directly demonstrate the importance of OPN in cytoskeletal organization, they support previous studies that have demonstrated this relationship (Katayama et al. 1998; Suzuki et al. 2002a).
There have been few reports regarding the effects of BPs on the expression of OPN and αvβ3 integrin in bone cells in spite of their functional importance in bone metabolism. Although BPs have been reported to downregulate OPN mRNA expression by normal osteoblasts in culture (Sodek et al. 1995) and by an osteoblastic cell line (Mackie et al. 2001), which can impact on the recruitment and bone-resorptive activity of osteoclasts, there are no reports on the effect of BPs on OPN expression in osteoclasts. However, BPs have been shown to inhibit the adhesion of human osteoclast-like cells onto coverslips coated with BSP, but not fibronectin, indicative of the interference with receptors such as αvβ3 integrins that specifically recognize bone matrix proteins (Colucci et al. 1998) including OPN. Although it is not known whether BPs have a direct effect on OPN expression by osteoclasts, our studies clearly show that BP treatment of calvaria greatly diminishes the production of OPN, which appears to impact on the colocalization of OPN with actin and the formation of the sealing zone of resorbing osteoclasts (Figure 4).
In this study, we also assessed bone formation using the phalloidin-stained actin in combination with ALP, collagen type I and BSP as an indicator of the proliferation and activity of bone-forming cells (Figure 5-Figure 8). Notably, bone formation activity was increased in association with bone resorptive activity induced by LPS and PTH (Suzuki et al. 2003), consistent with the concept that the activities of osteoblasts and osteoclasts are coupled. Although it was not possible to quantify bone resorption by measuring differences in the thickness of the calvaria before and after inducing bone resorption by morphometry, the inclusion of biochemical analyses (Tatrai et al. 1992) in future studies should circumvent these problems.
In conclusion, examination of fluorescent-stained calvaria by laser scanning confocal microscope provides a valuable approach for studying cellular mechanisms of bone remodeling and evaluating the effects of biological agents on bone cells, maintained in their natural environment for a relatively short and controlled experimental time period. Using this approach we have shown the relationship between cell attachment molecules involved in osteoclast activity and how these relationships are influenced by LPS and PTH that stimulate osteoclast activity and BPs that suppress osteoclast activity.
Acknowledgments
This study was funded by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, Culture and Technology (14571776) to K.S. and by a Canadian Institutes of Health Research Grant MOP-36333 to J.S.
We are grateful to Prof. Yasushi Sakai (Division of Physiology, School of Nursing and Rehabilitation Science, Showa University) for providing the confocal microscope facilities and for helpful suggestions for the experiments.
Literature Cited
- Andersson G, Johansson EK. (1996) Adhesion of human myelomonocytic (HL-60) cells induced by 1, 25-dihydroxyvitamin D3 and phorbol myristate acetate is dependent on osteopontin synthesis and the alpha v beta 3 integrin. Connect Tissue Res 35: 163–171 [DOI] [PubMed] [Google Scholar]
- Burdi AR. (1965) Toluidine Blue-Alizarin Red S staining of cartilage and bone in whole-mount skeletons in vitro. Stain Technol 40: 45–48 [DOI] [PubMed] [Google Scholar]
- Cecchini MG, Fleisch H. (1990) Bisphosphonates in vitro specifically inhibit, among the hematopoietic series, the development of the mouse mononuclear phagocyte lineage. J Bone Miner Res 5: 1019–1027 [DOI] [PubMed] [Google Scholar]
- Chellaiah MA, Hruska KA. (2003) The integrin alpha(v)beta(3) and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif Tissue Int 72: 197–205 [DOI] [PubMed] [Google Scholar]
- Chellaiah MA, Kizer N, Biswas R, Alvarez U, Strauss-Schoenberger J, Rifas L, Rittling SR, et al. (2003) Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression. Mol Biol Cell 14: 173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci S, Minielli V, Zambonin G, Cirulli N, Mori G, Serra M, Patella V, et al. (1998) Alendronate reduces adhesion of human osteoclast-like cells to bone and bone protein-coated surfaces. Calcif Tissue Int 63: 230–235 [DOI] [PubMed] [Google Scholar]
- Dodds RA, Connor JR, James IE, Rykaczewski EL, Appelbaum E, Dul E, Gowen M. (1995) Human osteoclasts, not osteoblasts, deposit osteopontin onto resorption surfaces: an in vitro and ex vivo study of remodeling bone. J Bone Miner Res 10: 1666–1680 [DOI] [PubMed] [Google Scholar]
- Duong LT, Lakkakorpi P, Nakamura I, Rodan GA. (2000) Integrins and signaling in osteoclast function. Matrix Biol 19: 97–105 [DOI] [PubMed] [Google Scholar]
- Edelman DB, Keefer EW. (2005) A cultural renaissance: in vitro cell biology embraces three-dimensional context. Exp Neurol 192: 1–6 [DOI] [PubMed] [Google Scholar]
- Ek-Rylander B, Flores M, Wendel M, Heinegard D, Andersson G. (1994) Dephosphorylation of osteopontin and bone sialoprotein by osteoclastic tartrate-resistant acid phosphatase. Modulation of osteoclast adhesion in vitro. J Biol Chem 269: 14853–14856 [PubMed] [Google Scholar]
- Everts V, Delaisse JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, Beertsen W. (2002) The bone lining cell: its role in cleaning Howship's lacunae and initiating bone formation. J Bone Miner Res 17: 77–90 [DOI] [PubMed] [Google Scholar]
- Flanagan AM, Chambers TJ. (1989) Dichloromethylenebisphosphonate (Cl2MBP) inhibits bone resorption through injury to osteoclasts that resorb Cl2MBP-coated bone. Bone Miner 6: 33–43 [DOI] [PubMed] [Google Scholar]
- Flores ME, Norgard M, Heinegard D, Reinholt FP, Andersson G. (1992) RGD-directed attachment of isolated rat osteoclasts to osteopontin, bone sialoprotein, and fibronectin. Exp Cell Res 201: 526–530 [DOI] [PubMed] [Google Scholar]
- Ganss B, Kim RH, Sodek J. (1999) Bone sialoprotein. Crit Rev Oral Biol Med 10: 79–98 [DOI] [PubMed] [Google Scholar]
- Grano M, Zigrino P, Colucci S, Zambonin G, Trusolino L, Serra M, Baldini N, et al. (1994) Adhesion properties and integrin expression of cultured human osteoclast-like cells. Exp Cell Res 212: 209–218 [DOI] [PubMed] [Google Scholar]
- Gravel MR, Zheng ZG, Sims SM, Dixon SJ. (1994) Platelet-activating factor induces pseudopod formation in calcitonin-treated rabbit osteoclasts. J Bone Miner Res 9: 1769–1776 [DOI] [PubMed] [Google Scholar]
- Hollberg K, Hultenby K, Hayman A, Cox T, Andersson G. (2002) Osteoclasts from mice deficient in tartrate-resistant acid phosphatase have altered ruffled borders and disturbed intracellular vesicular transport. Exp Cell Res 279: 227–238 [DOI] [PubMed] [Google Scholar]
- Horton MA, Nesbit SA, Bennett JH, Stenbeck G. (2002) Integrins, and other cell surface attachment molecules of bone cells. In Bilezikian JP, Raisz LG, Rodan GA. eds. Principles of Bone Biology. 2nd ed. San Diego, Academic Press, 265–286 [Google Scholar]
- Horton MA, Nesbit MA, Helfrich MH. (1995) Interaction of osteopontin with osteoclast integrins. Ann NY Acad Sci 760: 190–200 [DOI] [PubMed] [Google Scholar]
- Hughes DE, MacDonald BR, Russell RG, Gowen M. (1989) Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest 83: 1930–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, et al. (1995) Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 10: 1478–1487 [DOI] [PubMed] [Google Scholar]
- Katayama Y, House CM, Udagawa N, Kazama JJ, McFarland RJ, Martin TJ, Findlay DM. (1998) Casein kinase 2 phosphorylation of recombinant rat osteopontin enhances adhesion of osteoclasts but not osteoblasts. J Cell Physiol 176: 179–187 [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, et al. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176 [DOI] [PubMed] [Google Scholar]
- Lakkakorpi P, Tuukkanen J, Hentunen T, Jarvelin K, Vaananen K. (1989) Organization of osteoclast microfilaments during the attachment to bone surface in vitro. J Bone Miner Res 4: 817–825 [DOI] [PubMed] [Google Scholar]
- Lowik CW, van der Pluijm G, van der Wee-Pals LJ, van Treslong-De Groot HB, Bijvoet OL. (1988) Migration and phenotypic transformation of osteoclast precursors into mature osteoclasts: the effect of a bisphosphonate. J Bone Miner Res 3: 185–192 [DOI] [PubMed] [Google Scholar]
- Mackie PS, Fisher JL, Zhou H, Choong PF. (2001) Bisphosphonates regulate cell growth and gene expression in the UMR 106–01 clonal rat osteosarcoma cell line. Br J Cancer 84: 951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Kukita T, Akamine A, Kukita A, Iijima T. (1994) Localization of osteopontin in resorption lacunae formed by osteoclast-like cells: a study by a novel monoclonal antibody which recognizes rat osteopontin. Histochemistry 102: 247–254 [DOI] [PubMed] [Google Scholar]
- McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, et al. (2000) Mice lacking β3 integrins are osteosclerotic due to dysfunctional osteoclasts. J Clin Invest 104: 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee MD, Nanci A. (1995) Osteopontin and the bone remodeling sequence. Colloidal-gold immunocytochemistry of an interfacial extracellular matrix protein. Ann NY Acad Sci 760: 177–189 [DOI] [PubMed] [Google Scholar]
- Mostafa YA, Meyer RA, Jr, Latorraca R. (1982) A simple and rapid method for osteoclast identification using a histochemical method for acid phosphatase. Histochem J 14: 409–413 [DOI] [PubMed] [Google Scholar]
- Mulari MT, Qu Q, Harkonen PL, Vaananen HK. (2004) Osteoblast-like cells complete osteoclastic bone resorption and form new mineralized bone matrix in vitro. Calcif Tissue Int 75: 253–261 [DOI] [PubMed] [Google Scholar]
- Murakami H, Takahashi N, Sasaki T, Udagawa N, Tanaka S, Nakamura I, Zhang D, et al. (1995) A possible mechanism of the specific action of bisphosphonates on osteoclasts: tiludronate preferentially affects polarized osteoclasts having ruffled borders. Bone 17: 137–144 [DOI] [PubMed] [Google Scholar]
- Nakamura I, Pilkington MF, Lakkakorpi PT, Lipfert L, Sims SM, Dixon SJ, Rodan GA, et al. (1999) Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J Cell Sci 112: 3985–3993 [DOI] [PubMed] [Google Scholar]
- Reinholt FP, Hultenby K, Oldberg A, Heinegard D. (1990) Osteopontin—a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA 87: 4473–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. (2000) Cellular and molecular mechanisms of action of bisphosphonates. Cancer 88(suppl 12):2961–2978 [DOI] [PubMed] [Google Scholar]
- Ross FP, Chappel J, Alvarez JI, Sander D, Butler WT, Farach-Carson MC, Mintz KA, et al. (1993) Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem 268: 9901–9907 [PubMed] [Google Scholar]
- Sahni M, Guenther HL, Fleisch H, Collin P, Martin TJ. (1993) Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest 91: 2004–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, et al. (1991) Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88: 2095–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk R, Merz WA, Muhlbauer R, Russell RG, Fleisch H. (1973) Effect of ethane-1-hydroxy-1, 1-diphosphonate (EHDP) and dichloromethylene diphosphonate (Cl2 MDP) on the calcification and resorption of cartilage and bone in the tibial epiphysis and metaphysis of rats. Calcif Tissue Res 12: 196–214 [DOI] [PubMed] [Google Scholar]
- Selander K, Lehenkari P, Vaananen HK. (1994) The effects of bisphosphonates on the resorption cycle of isolated osteoclasts. Calcif Tissue Int 55: 368–375 [DOI] [PubMed] [Google Scholar]
- Sodek J, Chen J, Nagata T, Kasugai S, Todescan R, Jr, Li IW, Kim RH. (1995) Regulation of osteopontin expression in osteoblasts. Ann NY Acad Sci 760: 223–241 [DOI] [PubMed] [Google Scholar]
- Stern PH, Krieger NS. (1983) Comparison of fetal rat limb bones and neonatal mouse calvaria: effects of parathyroid hormone and 1, 25-dihydroxy vitamin D3 . Calcif Tissue Int 35: 172–176 [DOI] [PubMed] [Google Scholar]
- Suda K, Udagawa N, Sato N, Takami M, Itoh K, Woo JT, Takahashi N, et al. (2004) Suppression of osteoprotegerin expression by prostaglandin E2 is crucially involved in lipopolysaccharide-induced osteoclast formation. J Immunol 172: 2504–2510 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Takeyama S, Kikuchi T, Sodek J, Yamada S, Shinoda H. (2003) Evidence for the inhibitory effects of bisphosphonates on osteoclast differentiation and activation obtained using laser scanning confocal microscopy. Bone 32(suppl):S162 [Google Scholar]
- Suzuki K, Zhu B, Goldberg HA, Rittling SR, Denhardt DT, McCulloch CAG, Sodek J. (2002b) Intracellular osteopontin in osteoclasts: impaired migration, cell fusion and resorption in osteoclasts from OPN-/- and CD44-/- mice. The Scientific World J 2: 79–81 [Google Scholar]
- Suzuki K, Zhu J, Rittling S, Denhard DT, Goldberg HA, McCulloch CAG, Sodek J. (2002a) Co-localization of intracellular osteopontin with CD44 is associated with migration, cell fusion, and resorption in osteoclasts. J Bone Miner Res 17: 1486–1497 [DOI] [PubMed] [Google Scholar]
- Tatrai A, Foster S, Lakatos P, Shankar G, Stern PH. (1992) Endothelin-1 actions on resorption, collagen and noncollagen protein synthesis, and phosphatidylinositol turnover in bone organ cultures. Endocrinology 131: 603–607 [DOI] [PubMed] [Google Scholar]
- Tezuka K, Sato T, Kamioka H, Nijweide PJ, Tanaka K, Matsuo T, Ohta M, et al. (1992) Identification of osteopontin in isolated rabbit osteoclasts. Biochem Biophys Res Commun 186: 911–917 [DOI] [PubMed] [Google Scholar]
- Themistocleous GS, Katopodis H, Sourla A, Lembessis P, Doillon CJ, Soucacos PN, Koutsilieris M. (2004) Three-dimensional type I collagen cell culture systems for the study of bone pathophysiology. In Vivo 18: 687–696 [PubMed] [Google Scholar]
- Tong HS, Sakai DD, Sims SM, Dixon SJ, Yamin M, Goldring SR, Snead ML, et al. (1994) Murine osteoclasts and spleen cell polykaryons are distinguished by mRNA phenotyping. J Bone Miner Res 9: 577–584 [DOI] [PubMed] [Google Scholar]
- Vaananen HK, Zhao H, Mulari M, Halleen JM. (2000) The cell biology of osteoclast function. J Cell Sci 113: 377–381 [DOI] [PubMed] [Google Scholar]
- Vitte C, Fleisch H, Guenther HL. (1996) Bisphosphonates induce osteoblasts to secrete an inhibitor of osteoclast-mediated resorption. Endocrinology 137: 2324–2333 [DOI] [PubMed] [Google Scholar]
- Yamate T, Mocharla H, Taguchi Y, Igietseme JU, Manolagas SC, Abe E. (1997) Osteopontin expression by osteoclast and osteoblast progenitors in the murine bone marrow: demonstration of its requirement for osteoclastogenesis and its increase after ovariectomy. Endocrinology 138: 3047–3055 [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, et al. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95: 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]