Abstract
Current methods for myelin staining in tissue sections include both histological and immunohistochemical techniques. Fluorescence immunohistochemistry, which uses antibodies against myelin components such as myelin basic protein, is often used because of the convenience for multiple labeling. To facilitate studies on myelin, this paper describes a quick and easy method for direct myelin staining in rodent and human tissues using novel near-infrared myelin (NIM) dyes that are comparable to other well-characterized histochemical reagents. The near-infrared fluorescence spectra of these probes allow fluorescent staining of tissue sections in multiple channels using visible light fluorophores commonly used in immunocytochemistry. These dyes have been used successfully to detect normal myelin structure and myelin loss in a mouse model of demyelination disease.
Keywords: myelin, near-infrared, dye, fluorescence
Reagents that specifically stain myelin have been an important part of the histopathological examination of the nervous system. Colorimetric histological stains for myelin include classic dyes such as Luxol Fast Blue (Klüver and Barrera 1953), Sudan Black (Stilwell 1957), and more recently introduced Black-Gold (Schmued and Slikker 1999). All react with the phospholipids or lipoproteins in myelin. Myelin can also be detected by immunohistochemistry using antibodies that recognize myelin components such as myelin basic protein (MBP) and visualized by enzymatic reaction to form colored deposits or by fluorophore-conjugated secondary antibodies that emit fluorescence at specific wavelengths when excited. Fluorescence immuno-histochemistry has distinct advantages for multiple labeling because different colored fluorophores can be discriminated by using proper filters. This paper describes novel dyes that specifically bind myelin and when excited emit fluorescence in the near-infrared range (700 nm or above). These new dyes, therefore, allow quick and easy fluorescent staining of myelin, alone or in combination with other fluorescent reagents in tissue sections. To rationally design fluorescent dyes for specific binding to white matter, high lipophilicity of the molecule is a major prerequisite for specific binding. In our design, we chose a traditional “push-pull” architecture with donor and acceptor moieties at opposite ends of the molecule interconnected by an easily polarizable π-conjugated bridge. This architecture allows for a simple manipulation of photophysical properties of the dye (including absorption and emission wavelengths) by selecting appropriate donor and acceptor groups. To improve binding to white matter, we incorporated a hydrophobic dithienyl bridge. As an additional advantage, this bridge possesses high electronic polarizability, which allows for an efficient electronic transduction between donor and acceptor, therefore contributing toward decreasing the energy gap between the frontier orbitals and resulting in further bathochromic shifting of adsorption/emission (Kuhn and Schweig 1967; Zollinger 2003). A very important design feature is the utilization of the asymmetric squarylium dye architecture (Nakazumi et al. 2003; Welder et al. 2003). This allowed for a unique possibility of alteration of regions of high hydrophobicity and polar hydrophilic regions along the dye molecule. We show in this report that these dyes can be used successfully to detect normal myelin structure and myelin loss in a mouse model of demyelination disease.
Materials and Methods
NIM Dye Synthesis and Characterization
Synthesis of the near-infrared dyes NIM-1 and NIM-2 is outlined in Figure 1 and can be easily scaled-up to prepare multi-gram quantities. The detailed procedures will be published elsewhere. Preparation of 1, 2, 3, 3-tetremethylindolenium iodide was as described previously (Hirano et al. 2002). 2-Bromo-5, 5'-dithiophene, diisopropyl squarate and other starting materials and reagents were obtained from Sigma-Aldrich (St Louis, MO). The dyes were fully characterized by proton nuclear magnetic resonance (1H NMR) spectroscopy and high-resolution mass spectrometry. The absorption and fluorescence spectra are shown in Figure 2. UV-visible spectra were recorded on a Cary 50 UV-Vis spectrophotometer (Varian Inc.; Palo Alto, CA) using a 1-cm quartz cuvette. Fluorescence studies were carried out with a Spex FluoroLog-3 fluorometer (HORIBA Jobin Yvon Inc.; Edison, NJ) with the following absorbance and emission peaks: NIM-1 (absorbance 650 nm, emission 670 nm); NIM-2 (absorbance 690 nm, emission 820 nm).
Figure 1.
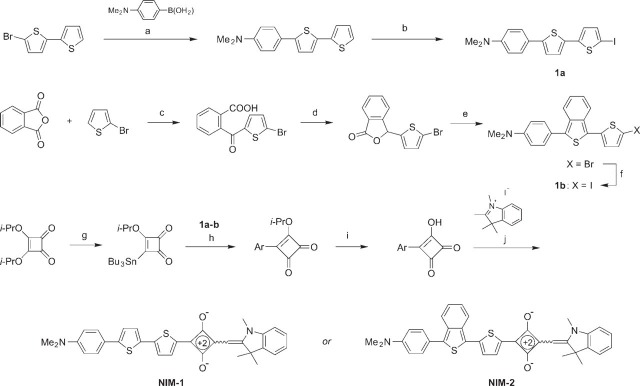
Synthesis of NIM-1 and NIM-2 dyes. Reaction conditions: (a) Pd(PPh3)4 (catalyst), K2CO3, toluene-EtOH-H2O, 85C, 66%; (b) 1) n-BuLi, TMEDA, THF, −70C, 2) I2, −70C to 20C, 75% in two steps; (c) AlCl3, CH2Cl2, 40C, 47%; (d) NaBH4, NaHCO3, H2O, 20C, 75%; (e) 1) 4-Me2N-C6H4-MgBr, THF, 0C to 20C; 2) Lawesson reagent, CH2Cl2, 20C, 58% in two steps; (f) 1) n-BuLi, THF, −70C; 2) I2, −70C to 20C, 75% in two steps; (g) n-Bu3Sn-SiMe3, n-Bu4N+CN- (catalyst), THF, 66%; (h) Pd(PPh3)4 (catalyst), CuI (catalyst), DMF, 60C; (a) 63%; (b) 91%; (i) aqueous HCl, THF, 20C; (a) 83%; (b) 91%; (j) quinoline (catalyst), n-BuOH-C6H6, reflux; (a) 55%; (b) 42%. Abbreviations: TMEDA, N, N, N', N'-tetramethylethylenediamine; THF, tetrahydrofuran; DMF, N, N-dimethylformamide; Cul, copper iodide; Ph, phenyl; Bu, butyl; Me, methyl; Et, ethyl; Pr, propyl; PPh3, triphenylphospine. Ar refers to the bithienyl residue either from 1a (for NIM-1) or from 1b (for NIM-2). The exact stereochemistry of the ethylene double bonds in the structures of the dyes was not determined and is indicated by a squiggly line.
Figure 2.
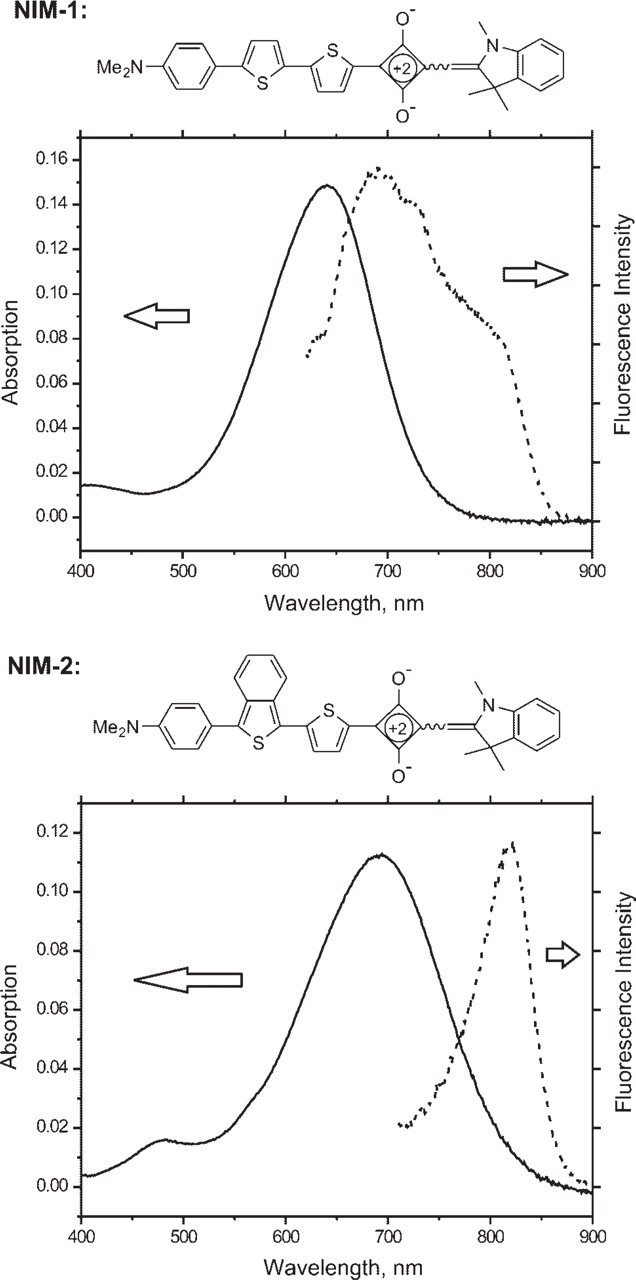
Structure and absorption/emission spectrum of NIM dyes. NIM-1 and NIM-2 were solubilized in DMSO at 2.1 μM (1.1 μg/ml) and 2.1 μM (1.2 μg/ml), respectively. Absorption maxima: NIM-1 641 nm (× = 72,000); NIM-2 692 nm (× = 54,000).
NIM Dye Physical and Chemical Properties
NIM-1 is a golden-green crystalline powder, melting point (mp). 261–262C and NIM-2 is a black crystalline powder, mp. 185–190C (decomposition). In the crystalline state these dyes are stable and show no noticeable signs of decomposition upon storing at room temperature (RT). The dyes are practically insoluble in water but soluble in many organic solvents (methanol, ethanol, chloroform). Both dyes can be solubilized in dimethyl sulfoxide (DMSO) (stock 12.5 mg/ml) and appear dark blue in color. To avoid precipitation, stock solutions should be kept at RT for no more than 2 weeks.
Animals and Cuprizone Treatment
All animal use procedures were in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital. The rodent cuprizone-induced demyelination model produces consistent and massive demyelination in the corpus callosum, which is most pronounced following 6 weeks of cuprizone ingestion (Matsushima and Morell 2001). Cuprizone treatment of mice was carried out exactly as described previously (Matsushima and Morell 2001). Briefly, 8-week-old mice, C57BL/6 strain (Jackson Laboratories; Bar Harbor, ME) were fed ad libitum basal diet (LabDiet 5P75; W.F. Fisher & Son, Richmond, IN) containing 0.2% (w/w) cuprizone (Sigma) that had been milled into the chow for 6 weeks to induce demyelination. Control animals were fed basal diet only, n=4 for each group. Animals were killed by cervical dislocation, and their brains were removed, frozen in 2-methyl butane on dry ice, cut at 10 μm/section in a cryostat (Shandon Cryotome; Thermo Electron Corp., Pittsburgh, PA), and stored at −20C until use.
NIM Dye Staining
NIM-1 and NIM-2 dye-staining solutions, 125 μg/ml in propylene glycol and water (1:1), were freshly prepared because long-term storage (>1 day at RT) results in precipitation. Tissue sections from mouse brain were circled with a Pap pen, and staining solution was applied onto the sections. Sections were stained for 20 min at RT, rinsed briefly in water, and coverslipped with fluorescence mounting medium (Vectashield, Vector Laboratories; Burlingame, CA) for fluorescence microscopy using a Cy5 filter (700-nm long-pass). Images were taken using a Sony digital camera (DSP200; Sony Corp., Tokyo, Japan) and processed using Adobe Photoshop software (Adobe; Mountain View, CA). To examine the photo stability of NIM staining, NIM-1-stained brain sections were exposed to 15 min of continuous laser illumination (Leica TCS; Leica Microsystems AG, Wetzlar, Germany), and images were taken using the same exposure constants at different time points. The staining intensities (average pixel values) in the corpus callosum were quantified using NIH Imaging software (National Institutes of Health; Bethesda, MD).
Luxol Fast Blue Staining
Brain sections were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min, rinsed in water, and allowed to dry. The sections were stained with Luxol Fast Blue according to the standard Klüver-Barrera protocol (Sheehan and Hrapchak 1980). Briefly, sections were incubated in Luxol Fast Blue staining solution (1% in 95% ethanol with 0.5% acetic acid) at 60C overnight. The sections were differentiated briefly in lithium carbonate solution (0.05%), rinsed twice with 70% ethanol, and then dehydrated in an ethanol series, cleared in xylene, and mounted for microscopy.
Immunohistochemistry
Brain sections were fixed in 4% paraformaldehyde for 30 min and rinsed in PBS. After permeabilization for 1 hr at RT with 0.2% Triton X-100 in PBS and 5% normal donkey serum to block nonspecific binding, the sections were incubated with primary antibodies: goat anti-MBP (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-NeuN (1:1000; Chemicon, Temecula, CA), all diluted in PBS with 1% normal donkey serum. After three washes with PBS, the sections were incubated with IgG Cy2-conjugated secondary antibodies at RT for 1 hr in the dark. After three washes with PBS, the sections were incubated with IgG Cy2-conjugated secondary antibodies at RT for 1 hr in the dark. Secondary antibodies were IgG (donkey) Cy2-conjugated anti-goat and IgG (donkey) Cy2-conjugated anti-mouse (Jackson ImmunoResearch Laboratories; West Grove, PA), all at 1:200 in PBS with 1% donkey serum. After PBS washes, the sections were mounted with fluorescence mounting medium containing the nuclear dye DAPI (Vectashield; Vector Laboratories) and coverslipped for microscopy using an Olympus microscope (BX60; Olympus Optical, Tokyo, Japan) equipped with epifluorescent optics.
Results
We synthesized squarylium-based near-infrared fluorescent dyes that preferentially bind myelin and allow multi-channel fluorescent imaging in tissue sections from the rodent nervous system. The synthesis and structure of these two novel dyes, NIM-1 and NIM-2, is shown in Figure 1. Squarylium-based compound dyes typically have relatively low fluorescent quantum efficiencies in solution, which are substantially increased upon binding to substrate (Nakazumi et al. 2003; Welder et al. 2003). The fluorescent spectra of these compounds are shown in Figure 2 and are red-shifted enough to enable simultaneous fluorescent measurements with visible light fluorophores from blue to far red: NIM-1 (absorbance 650 nm, emission 670 nm); NIM-2 (absorbance 690 nm, emission 820 nm).
To confirm the specificity of white matter staining, tissue sections from mouse brain were stained with the NIM-1 dye. We tested optimum staining conditions by varying concentrations of the dye and staining solution components. The dye is insoluble in water, and stock solution was prepared in DMSO. Direct dilution for working staining solution in water or PBS resulted in tiny precipitates. To facilitate even distribution of the dye, different proportions of ethanol, DMSO, or propylene glycol were added. We found that propylene glycol is best when mixed 1:1 with water. Ethanol also gives good results with a slightly higher background, whereas DMSO is the worst. Higher dye concentration gives stronger fluorescence; however, the background also increases due to staining of cell membranes. The optimum staining was achieved at 125 μg/ml in water-propylene glycol mix (1:1). Similar results were found for NIM-2.
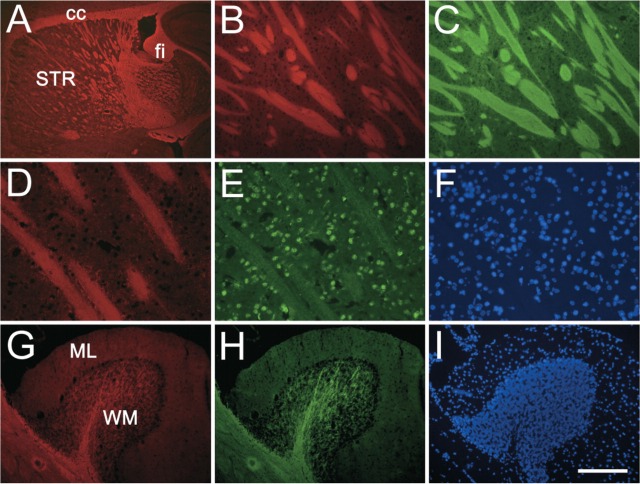
In para-sagittal frozen sections, major myelinated structures such as the corpus callosum, cerebellar medulla, and fiber bundles in the striatum were strongly stained with NIM-1 (Figure 1).
To confirm specificity for myelin, sections were costained with antibody to the myelin component MBP. In the striatum, small myelinated fiber bundles are well stained with the antibody and demonstrated complete colocalization with the NIM-1 staining (Figures 3B and 3C). In the cerebellum, NIM-1 staining is very strong in the medulla region where myelinated Purkinje cell axons are located, colocalizing with MBP staining (Figures 3G and 3H). To demonstrate compatibility with other fluorescence stainings, sections were costained with antibody against neurons (NeuN) and nuclear dye DAPI. The absence of interference between these stainings indicates that the dye can be used in combination with other fluorescent immuno-histochemical reagents.
Figure 3.
NIM-1 staining in the normal mouse brain. Para-sagittal sections were stained with NIM-1 (red, A, B, D, G) costained with either myelin basic protein (MBP) (green, C, H) or NeuN (green, E), and mounted with the nuclear stain DAPI (blue, F, I). NIM-1 stains distinct white matter structures in the brain (A), such as striatum (STR), corpus callosum (cc), and fimbria (fi). In the striatum (B-F), NIM-1 stains fiber bundles (B), completely overlapping MBP staining (C). The compatibility of NIM-1 staining (D) with other fluorescence staining is further demonstrated by NeuN immunostaining (E) and nuclear stain DAPI (F). In the cerebellum folium, NIM-1 stains the white matter (WM), colocalizes with MBP (H), while leaving molecular layer (ML) unstained. Bars: A = 500 μm; B, C = 100 μm; D-F = 50 μm; G-I = 200 μm.
The dyes were next used to examine myelin content in a mouse cuprizone-induced demyelination model (Matsushima and Morell 2001). Luxol Fast Blue histological staining and MBP immunohistochemical staining were performed on adjacent brain sections to examine the pattern and sensitivity of the NIM-1 dye. Cuprizone feeding for 6 weeks induces consistent and specific demyelination in the corpus callosum of animals (Matsushima and Morell 2001). Luxol Fast Blue revealed light to no staining in the corpus callosum of cuprizone-treated mice compared with controls, indicating severe demyelination (Figures 4A-4B). Sections immunostained with MBP antibody indicated a dramatic loss in myelin compared with controls (Figures 4C-4D). NIM-1 staining also revealed the loss of myelin in cuprizone-fed mice, which was consistent with both Luxol Fast Blue and MBP immunostaining (Figures 4E-4F).
Figure 4.
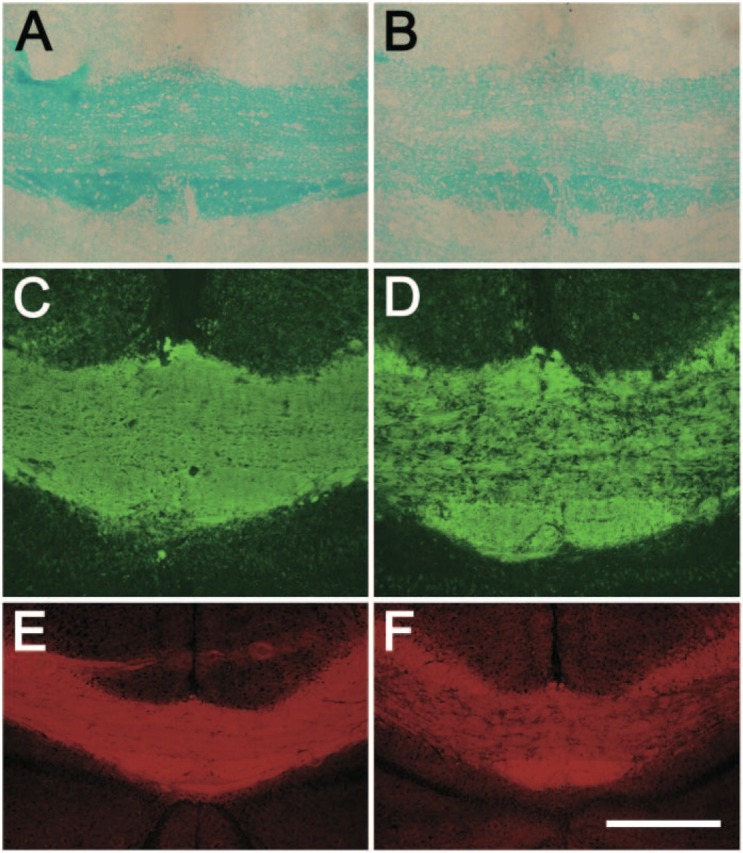
NIM-1 efficiently detects demyelination in a cuprizone-induced animal demyelination model. Brain coronal sections from control mice (A, C, E) and cuprizone-fed mice (B, D, F) were stained with Luxol Fast Blue (A, B), MBP (C, D), and NIM-1 dye (E, F). Shown are middle portions of CC. Bars: A-D = 100 μm; E-F = 200 μm.
Lastly, the photo stability of NIM staining was examined. NIM-1-stained brain sections were exposed to 15 min of continuous laser illumination, and images were taken using the same exposure constants at various time points. The intensity of staining (average pixel values) in the corpus callosum decreased to 80% in 2 min, 70% in 10 min, and 66% after 15 min. The result indicates that NIM-1 is sufficiently photo stable for experimental purposes. NIM-2 was not tested.
Discussion
Various methods have been developed for myelin staining in neuropathological examinations of the brain using either colorimetric histochemical stains such as Luxol Fast Blue, Sudan Black, and Black-Gold, or immunostains using antibodies that recognize specific myelin components. Fluorescence immunohistochemistry has distinct advantages for multiple labeling because different colored fluorophores can be discriminated by using proper filters. We describe in this report two novel squarylium-based lipophilic fluorescent dyes, NIM-1 and NIM-2, that preferentially bind to myelin. We demonstrated the specificity of these dyes in normal mouse tissue as well as in a mouse demyelination model, where dye staining was consistent with both Luxol Fast Blue staining and MBP immunostaining. We developed the NIM dyes not to replace conventional methods such as Luxol Fast Blue histochemical staining and MBP immunostaining but rather to add a new means to the arsenal of myelin detection. The advantage of NIM dyes is that they can be excited and fluoresce in the near-infrared range. Although fluorescent dyes for myelin have been reported (Schmued et al. 1982; Pereyra and Roots 1988; Schmahl et al. 1999), their emission spectra are all within visible light. The unique emission wavelength of NIM dyes allows them to be used in combination with visible light spectra immunohistochemical (e.g., FITC- or Cy2-conjugated secondary antibodies) and histochemical (e.g., DAPI) reagents to allow multi-channel detection of fluorescent signals. Finally, when using high sensitivity emission-detection technology, the near-infrared emission of these dyes should be less limited by thickness of tissue, thus possibly allowing for in vivo myelin detection and quantification.
Acknowledgments
The work was supported by the National Institutes of Health Grants NS-35996 to S.A.R., EB-00768 to B.J.B., AG-08487 to B.T.H.; the U.S. Army through the Institute for Soldier Nanotechnologies under contract DAAD-19-02-D-0002 to T.M.S.; and a Pioneer Award from the Alzheimer's Association to B.T.H.
Literature Cited
- Hirano M, Osakada K, Nohira H, Miyashita A. (2002) Crystal and solution structures of photochromic spirobenzothiopyran. First full characterization of the meta-stable colored species. J Org Chem 67: 533–540 [DOI] [PubMed] [Google Scholar]
- Klüver H, Barrera E. (1953) A method for the combined staining of cells and fibers in the central nervous system. J Neuropathol Exp Neurol 12: 400–403 [DOI] [PubMed] [Google Scholar]
- Kuhn H, Schweig A. (1967) Theoretical treatment of solvent effects on the electronic spectra of polar organic dye molecules. Chem Phys Lett 1: 255–258 [Google Scholar]
- Matsushima GK, Morell P. (2001) The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol 11: 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazumi H, Colyer C, Kaihara K, Yagi S, Hyodo Y. (2003) Red luminescent squarylium dyes for noncovalent HAS labeling. Chem Lett 32: 804–805 [Google Scholar]
- Pereyra PM, Roots BI. (1988) The use of tetracycline hydrochloride as a rapid fluorescent stain for myelin membranes in vertebrates and invertebrates. Brain Res 458: 377–382 [DOI] [PubMed] [Google Scholar]
- Schmahl W, Hermel H, Matiasek K, Mohwald H. (1999) Selective staining by the fluorochrome 5, 5-diphenyl-9-ethyl-oxacarbocyanine. II. Application to paraffin embedded nervous tissue. Biotech Histochem 74: 229–235 [DOI] [PubMed] [Google Scholar]
- Schmued L, Slikker W., Jr (1999) Black-gold: a simple, high-resolution histochemical label for normal and pathological myelin in brain tissue sections. Brain Res 837: 289–297 [DOI] [PubMed] [Google Scholar]
- Schmued LC, Swanson LW, Sawchenko PE. (1982) Some fluorescent counterstains for neuroanatomical studies. J Histochem Cytochem 30: 123–128 [DOI] [PubMed] [Google Scholar]
- Sheehan D, Hrapchak B. (1980) Theory and Practice of Histotechnology. 2nd ed. Columbus, OH, Battelle Press; [Google Scholar]
- Stilwell DL. (1957) A sudan black B myelin stain for peripheral nerves. Stain Technol 32: 19–23 [DOI] [PubMed] [Google Scholar]
- Welder F, Paul B, Nakazumi H, Yagi S, Colyer CL. (2003) Symmetric and asymmetric squarylium dyes as noncovalent protein labels: a study by fluorimetry and capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci 793: 93–105 [DOI] [PubMed] [Google Scholar]
- Zollinger H. (2003) Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments. 3rd revised ed. Zürich, Verlag Helvetica Chimica Acta, Weinheim, Wiley-VCH [Google Scholar]