Abstract
A single course of antenatal steroids is widely used during preterm labor to promote fetal lung maturation. However, little is known regarding efficacy and safety of multiple courses of antenatal steroids. In animal models and clinical trials, treatment with glucocorticoids can inhibit growth. The present study of single- vs multiple-course steroids in pregnant ewes analyzes the effects of steroids vs placebo on fetal lung histopathology. Single-course groups received dexamethasone (Dex) 6 mg or normal saline every 12 hr for 48 hr at 104-106 days of gestation (term = 150 days). Multiple-course groups received the first course at 76-78 days; this was repeated weekly for 5 weeks. At 108 days, lungs were analyzed using immunohistochemistry for α-smooth muscle actin, a myofibroblast marker and proliferating cell nuclear antigen. Cell injury/death was evaluated using TdT-mediated dUTP digoxigenin nick end labeling (TUNEL) analysis. Although fetal growth was restricted by either single or multiple courses of Dex, alveolar development was accelerated as measured by mean linear intercepts. Alveolar walls were thinner, developing septa were longer, and septal myofibroblasts were increased for both Dex groups compared with controls. Cell proliferation increased following multiple steroid courses, especially in the distal parenchyma, with a corresponding decrease in apoptosis. These observations suggest that Dex promotes alveolarization, whether given in single or multiple courses.
Keywords: glucocorticoids, sheep, immunohistochemistry, morphometry, smooth muscle actin, proliferating cell nuclear antigen, apoptosis
Administration of antenatal corticosteroids (ACS) to women in preterm labor at 24 to 34 weeks gestation decreases the severity of respiratory distress syndrome (RDS) and other complications of prematurity (National Institutes of Health Consensus Development Panel 1994). Liggins and Howie (1972) first introduced this therapy in 1972 when they observed a significant reduction in the incidence of RDS in preterm infants whose mothers were given ACS for fetal lung maturation. Steroid therapy stimulates structural development as well as maturation of surfactant producing type II pneumocytes and improves responses of infants to surfactant therapy after birth (Ballard and Ballard 1995). The benefits of ACS are additive to those derived from surfactant therapy (National Institutes of Health Consensus Development Panel 1994; Ballard and Ballard 1995). Glucocorticoid treatment also reduces protein leak from pulmonary vasculature to airspace and accelerates clearance of lung liquid before delivery (Ikegami et al. 1987). A single course of ACS has been determined to be safe with maximum benefits occurring at 24 hr and lasting up to 7 days (National Institutes of Health Consensus Development Panel 1994). Because this treatment has been reported to lose its effects after 7 days, until recently it has been a common practice to administer multiple courses of ACS when delivery is delayed for a week or more (Crowley 1995). There is some controversy regarding the efficacy and safety of using multiple courses of ACS. Animal studies have shown that repeated courses of ACS have deleterious effects on lung growth and cerebral myelination (Whitelaw and Thoresen 2000). Newborn rats treated with dexamethasone (Dex) in utero have diminished alveolarization (Massaro and Massaro 1992, 2001) and are at an increased risk for developing pulmonary hypertension as adults (le Cras et al. 2000). Evidence from human studies, however, is contradictory and inconclusive (Jobe 2003). Findings from current risk-benefit studies are inadequate to argue for or against the use of multiple-course ACS to promote lung maturation (National Institutes of Health Consensus Development Panel 2001). This National Institutes of Health (NIH) Consensus Conference on the effects of corticosteroids for fetal maturation has recommended prompt use of ACS for women at risk for premature delivery, but that multiple courses of ACS should be used only in clinical trials, and more animal studies evaluating the potential risk and benefits of this therapy are indicated (National Institutes of Health Consensus Development Panel 2001). In the present study, we test the hypothesis that the effects of multiple courses of ACS on fetal lamb lung development differ from those of a single steroid course using clinically recommended doses of steroids and immunohistochemical analyses for several markers of cell function: cell proliferation, differentiation, and programmed cell death. We focused our investigation on the novel parameters of histochemical and cellular changes in the lung parenchyma because this could provide novel and important mechanistic information.
Materials and Methods
Animal Protocol and Methodology
This study was conducted after approval by the Institutional Animal Care and Use Committees of Brown University and Women and Infants’ Hospital of Rhode Island and according to the NIH Guidelines for use of experimental animals.
Surgery was performed under 1-2% halothane anesthesia on 25 time-dated Eastern mixed breed pregnant ewes at 99-101 days of gestation (term = 150 days) as previously described (Stonestreet et al. 2000). Singleton and twin pregnancies were included; however, when a twin gestation was present, only one fetus was catheterized. The thoracic aorta was cannulated via the brachial artery for blood sample withdrawal. Fetuses of ewes assigned to receive the five repeated courses of Dex were catheterized at 98-99 days of gestation after the ewes received four courses of Dex or placebo (sterile normal saline). The final course of Dex or placebo was given after recovery from surgery. Dex was chosen for use in our studies because it is one of the most extensively studied corticosteroids for accelerating fetal lung maturation and has been widely used in experimental studies of lung injury and lung development (Wang et al. 1995; O'Connor et al. 1997; Losada et al. 2000).
Ewes were randomly assigned to one of four treatment groups: (1) single-course Dex, (2) single-course placebo, (3) five repeated courses of Dex, or (4) five repeated courses of placebo. The ewes received a 6-mg intramuscular injection of Dex (Fujisawa; Deerfield, IL) at a concentration = 4 mg/ml, and 1.5 ml was given to each ewe, or placebo (0.9% NaCl) every 12 hr for 48 hr starting at 104-106 days in the single-course groups. The repeated-course groups received Dex or placebo starting at 76, 84, 91, 98, and 105 days of gestation (from the canalicular to the saccular stages). On days 106-108 of gestation, 18 hr after the last dose of Dex or placebo, the ewes were anesthetized and fetuses removed via hysterotomy. Similarly, women in premature labor often deliver <24 hr after a complete course of corticosteroids. Therefore, the Dex dose and treatment regimen that we utilized are similar to those used in pregnant women for fetal maturation and were selected to achieve near-maximal corticosteroid effects while minimizing the risk of premature labor (Willet et al. 1999; Stonestreet et al. 2000), similar to current recommendations of the NIH for clinical use to induce fetal lung maturation in pregnant women with premature labor (National Institutes of Health Consensus Development Panel 1994).
On the day of harvest, the ewes were anesthetized (ketamine, 50 mg/kg) and hysterotomy performed. The fetuses were removed and weighed, and the lungs were removed. Left lungs were inflated to a distending pressure of 25 mmHg, fixed in 4% paraformaldehyde for 18 to 24 hr, and then routinely processed for paraffin embedding. The lungs were obtained from animals enrolled in a larger series of studies to examine the effects of ACS on blood-brain barrier function and regional water concentrations in ovine fetuses (Stonestreet et al. 2000). Cortisol concentrations were measured in duplicate using Clinical AssaysJ GammaCoatJ Cortisol 125I-radioimmunoassay (DiaSorin; Stillwater, MN). The GammaCoatJ antiserum exhibits 100% cross-reactivity with cortisol. The observed coefficient of variation for inter- and intra-assay precision was 10.1 and 7.9%, respectively.
Histology and Morphometric Analyses
Four-μm-thick sections were used for immunohistochemistry. All lungs were sectioned in a similar manner, with symmetrical slices from the hilum to the pleural surface. The slices within 2 mm of the hilum were designated “proximal” and were excluded from this analysis. The remaining slices were bisected perpendicular to the main axis of the mainstem bronchus: the distal-most section was designated “distal” and the more proximal half was called “medial.” The pleural surface was present in both of the latter groups. The subpleural 5-mm-thick region was used for quantitative morphometry because this is where most new alveoli are forming. As lung maturation varies among different regions of developing lung, all analyses were performed on the distal portion of the left upper lobe within 5 mm of the pleural surface. Hematoxylin and eosin (H and E) staining was carried out on all sections to evaluate overall tissue architecture. Immunostaining for α-smooth muscle actin (SMA) and proliferating cell nuclear antigen (PCNA) were performed using a modification of the avidin-biotin technique (Hsu et al. 1981; Haley et al. 1997). The primary antibodies used were murine IgG2a monoclonal antibody against α-SMA, clone 1A4 (Sigma; St Louis, MO) at 1:500 dilution for SMA, and a murine IgG1 monoclonal antibody against PCNA, clone PC10 (DAKO; Carpinteria, CA) at 1:20 dilution for PCNA. The SMA slides were pretreated with 0.3% Triton X-100 for 5 min, whereas the PCNA slides were preincubated in methanol at −20C for 30 min. A biotinylated secondary antibody, horse anti-mouse IgG (Vector Labs; Burlingame, CA) was used at 1:200 in all immunostaining protocols. The peroxidase substrate was diaminobenzedine (0.05% DAB; Sigma), and 2% aqueous methyl green (Sigma) was used for counterstaining.
Labeling of apoptotic cells was performed using the Apop-Tag In Situ Apoptosis Detection Kit (Intergen; Purchase, NY).
Detailed morphometric analyses were carried out by one observer (ZP) without knowledge of the treatment groups; the slides were subsequently reviewed and the results confirmed by a board-certified staff pathologist (MES). Digitized images were captured from six randomly chosen non-overlapping fields from each of two separate slides per animal (including similar representations of large conducting airways, small airways, and distal lung parenchyma) located below a predetermined clock: the outermost alveolar field at 2 o'clock, two adjacent alveolar fields ∼2-3 mm from the pleural surface at 6 o'clock, and two adjacent alveolar fields ∼4-5 mm from the pleural surface at 10 o'clock using Nikon Labophot Microscope and Optronics camera at ×10 magnification for H and E and SMA immunostaining (Nikon; Melville, NY) and at ×20 for PCNA and apoptosis detection. Image analysis was performed using Scion Image version 1.62 (National Institutes of Health; Bethesda, MD).
Mean linear intercept (MLI) was determined by superimposing a predetermined grid on the image, with set randomly placed lines totaling 1 mm in actual length at ×20 (Figures 1C and 1D). A senior pathology technologist trained in this method (AS) counted the number of times the lines cross an air-tissue interface. The actual MLI was calculated as the inverse of the number of air-tissue interfaces per millimeter × 1000, yielding the average distance from one airtissue interface to the next in units of microns. Alveolar septal length was determined for all free-ended septa in every field by measuring the length of a line drawn from the base to the tip of these septa. Most of the free-ended alveolar septa are believed to be secondary septa in that over 95% arise as simple extensions from a thicker septum at their base and include a single capillary (when we counted 200 septa). Therefore, the vast majority of septa was secondary in all groups. Alveolar wall thickness was measured using lines (60-80 per field) drawn at 90° angles across the narrowest section of alveolar walls (to minimize the number of tangential sections included in the analysis), both free ended and those anchored at both ends. The volume percent of lung tissue immunostaining for SMA was determined using point counting normalized for the total lung parenchymal tissue volume. A linear point-counting grid (22 × 18 lines; 396 intercepts) was superimposed on the digitized images and SMA-positive cells at the intercepts were counted, excluding differentiated smooth muscle cells associated with airways or blood vessels. The formula used for our calculation is as follows: number of intercepts contacting SMA-positive cells/total number of intercepts contacting parenchymal tissue. Morphometric analyses of PCNA- and TdT-mediated dUTP digoxigenin nick end labeling (TUNEL)-positive nuclei were carried out manually: the total number of nuclei was counted separately for airways and distal lung parenchyma; the number of nuclei with PCNA-positive immunostaining was expressed as a percentage of the total number of nuclei in each respective compartment. Apoptotic cells were manually counted because there were only a few TUNEL-positive cells scattered randomly on each slide, and the number of TUNEL-positive cells was expressed by normalizing for the total area of tissue per slide.
Figure 1.
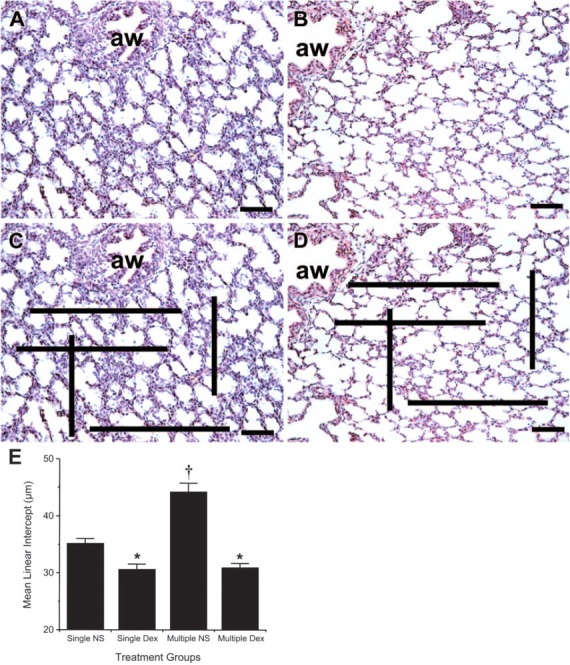
Alveolar morphology and mean linear intercept in lambs given multiple doses of ACS vs placebo. Ewes were treated with Dex or placebo as described in Materials and Methods. At harvest, lamb lungs were instilled intratracheally with 4% paraformaldehyde to a pressure of 25-cm H2O, then fixed overnight before being processed routinely into paraffin blocks. For morphometric analyses, all sections were taken from the distal portion of the left upper lobe. Image analysis was performed on hematoxylin and eosin-stained 4-μm-thick sections using Scion Image version 1.62 to evaluate overall tissue architecture. Bars in lower right-hand corners = 100 μm. (A) Lung from multiple-course placebo-treated lamb (NS = normal saline). (B) Lung from multiple-course Dex-treated lamb. (C, D) To demonstrate the principle of mean linear intercept (MLI), the same sections are shown with the superimposed predetermined line. The number of times this line crosses alveolar septa (air-tissue interfaces) was quantified using computerized image analysis, aw, airway. (C) Lung from multiple-course placebo-treated lamb with grid superimposed. (D) Lung from multiple-course Dex-treated lamb with grid superimposed. Note the larger number of air-tissue interfaces in Dex-treated lambs [∼45 in (D) vs. 31 in (C)]. (E) MLI was determined by an unbiased observer (AS) who had no knowledge of the different experimental groups. For each animal, 12 randomly selected fields of alveolar tissue were captured and used for analysis as detailed in Materials and Methods. MLI is calculated as the inverse of the number of air-tissue interfaces per millimeter or 1000 μm divided by the number of interfaces. (E) Histogram summarizes the data obtained from all of the fetal sheep in the present study. Animals receiving a single course of Dex had significantly lower MLI than single-course NS (∗ p<0.0001). Similarly, animals receiving multiple courses of Dex had significantly lower MLI than those receiving multiple courses of NS (∗ p<0.0001). Unexpectedly, the multiple-course NS group had MLI greater than that of the single-course NS group (°p<0.001).
We evaluated general compartments of epithelial vs mesenchymal cells directly on the slides stained for SMA or PCNA or by the TUNEL method (methyl green counterstain). Epithelial cells in the conducting airways are identified as columnar cells with the typical morphology of Clara cells, ciliated cells, or goblet cells. Epithelial cells in the alveoli (type II cells at this stage of development) are identified as cuboidal cells protruding into and directly lining the airspaces. Both types of epithelial cell are on the ad-lumenal side of the basement membrane, as identified by staining the basement membrane using the periodic acid-Schiff method following diastase digestion to remove glycogen. Mesenchymal cells are defined as interstitial cells clearly separated from the airspaces by other alveolar-lining cells and included endothelial cells lining the capillaries.
Statistical Analysis
All data are reported as mean ± standard error of the mean. Data analysis was performed using Statistical Analysis System software (Cary, NC). Group means were compared using ANOVA. When significant differences defined as p<0.05 were noted among the groups, post hoc analysis was performed using the t-test least significant difference (LSD).
Results
Bodyweight and Cortisol Changes with Dex Treatment
Fetuses exposed to multiple courses of Dex weighed 20% less on average than those in the placebo group (p<0.005, Table 1). There was also a 10% decline (p<0.05) in body weight in fetuses exposed to a single course of Dex compared with placebo. There was no difference in fetal weights between the steroid groups. There was no difference in ewe body weights among any of the groups (ANOVA; F = 1.28, p = 0.3, Table 1). There was no difference in fetal serum cortisol levels between the multiple-course groups [Dex: 7.0 ± 0.3 ng/ml (n=10), placebo: 7.0 ± 0.6 (n=8)], whereas the cortisol level of the single-course Dex group was marginally lower than the single-course placebo control group (Dex: 6.1 ± 0.3 ng/ml (n=6), placebo: 8.3 ± 0.8 ng/ml (n=6), p<0.05). There was no significant difference in fetal serum cortisol levels between the single- and multiple-course placebo groups (p>0.10).
Table 1.
Body weights of ewes and fetuses treated with saline or dexamethasone (Dex)
| Groups | Ewe (n) | Fetus (n) | Ewe weight (kg) | Fetus weight (kg) |
| Single-course saline | 6 | 9 | 71 ± 3 | 1.50 ± 0.03 |
| Single-course Dex | 6 | 9 | 71 ± 3 | 1.30 ± 0.03∗ |
| Multiple-course saline | 7 | 11 | 70 ± 8 | 1.50 ± 0.10 |
| Multiple-course Dex | 6 | 11 | 84 ± 5 | 1.20 ± 0.04∗ |
p<0.005 vs saline control.
Alveolar Parenchymal Morphology
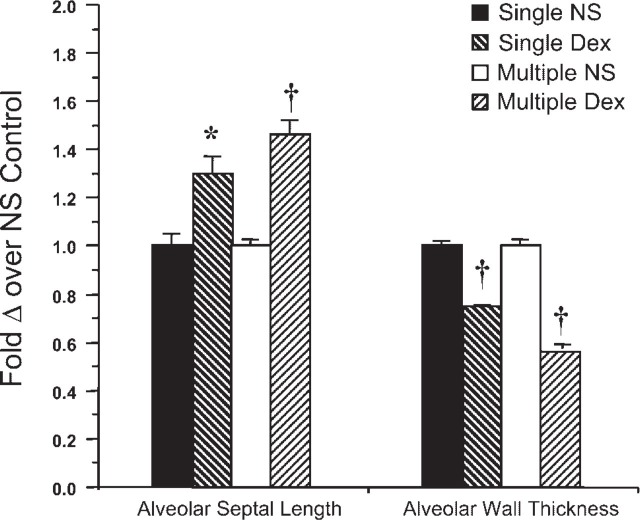
Antenatal Dex, whether given as single or multiple courses, resulted in three significant changes in alveolar architecture: (1) smaller-sized alveoli as determined using MLI to measure the number of air-tissue interfaces per millimeter (Figure 1), (2) the presence of longer free-ended alveolar septa (Figure 2 and Figure 4), and (3) thinning of alveolar walls (Figure 3 and Figure 4). Compared with the corresponding placebo controls (Figures 1A and 1C), we observed smaller alveoli in animals treated with either single- or multi-course Dex (Figures 1B and 1D) on routine histopathological examination of H and E-stained slides. MLI was used to estimate mean alveolar size, which is significantly smaller in sections from Dex-treated animals (Figure 1B). Compared with the corresponding placebo controls (Figure 2A), we also observed longer developing free-ended septa in the Dex-treated groups (Figure 2B). Most of the free-ended alveolar septa appear to be secondary septa (see Materials and Methods). Quantitative analysis demonstrated a 22% increase in mean alveolar free septal length in the single-course Dex group vs the single-course placebo control group (p = 0.011) and a 30% increase in free septal length in the multiple-course Dex group vs multiple-course placebo control group (p<0.001) (Figure 4). Compared with placebo-treated groups (Figure 3A), we also demonstrate decreased alveolar septal thickness in the Dex-treated groups (Figure 3B). Mean alveolar wall thickness was decreased by 25% in the single-course Dex group and by 43% in the multiple-course Dex group compared with the respective control groups (Figure 4). Using post hoc analysis, these differences are statistically highly significant (p<0.001). There are no significant differences in alveolar septal length or alveolar wall thickness between the steroid groups.
Figure 2.
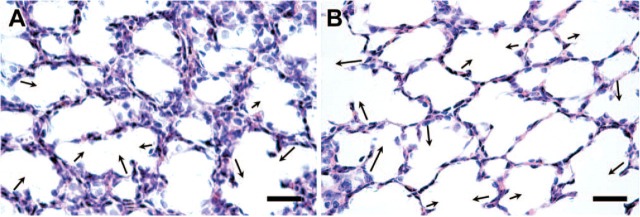
Alveolar free septal length in lambs given multiple doses of ACS vs. placebo preparation of lung tissues is detailed in Figure 1 legend and in Materials and Methods. Alveolar septal length was determined for all free-ended septa (at least 3-μm long) in every field by measuring the distance of a line drawn from the mid-base to the tip of these septa. (A, B) A few representative lines are given as arrows alongside septa, with the arrow directed from the base to the tip of the septa. (A) Lung from multiple-course placebo-treated lamb. (B) Lung from multiple-course Dex-treated lamb. Note the presence of several alveolar septa longer than those observed in (A). Bar = 50 μm.
Figure 4.
Morphometric analyses of alveolar structure. Histogram summarizes the results of morphometric analyses, which are expressed as percentage changes over the mean of the corresponding placebo control. ∗ p<0.02 with respect to single-course placebo control. °p<0.001 with respect to matched placebo control.
Figure 3.
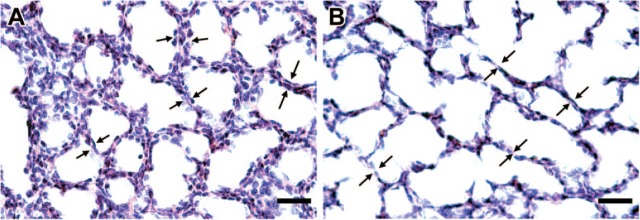
Alveolar septal thickness in lambs given multiple doses of ACS vs. placebo preparation of lung tissues is detailed in Figure 1 legend and in Materials and Methods. Alveolar wall thickness was measured using lines (60-80 per field) drawn at 90° angles across the narrowest segment of each alveolar wall, both free ended and those anchored at both ends. Each panel has four representative pairs of arrows indicating the site of a transseptal line. Bar = 50 μm. (A) Lung from multiple-course placebo-treated lamb. (B) Lung from multiple-course Dex-treated lamb. Note the presence of thinner alveolar septa in (B) as compared with (A).
Immunohistochemical Changes with Dex
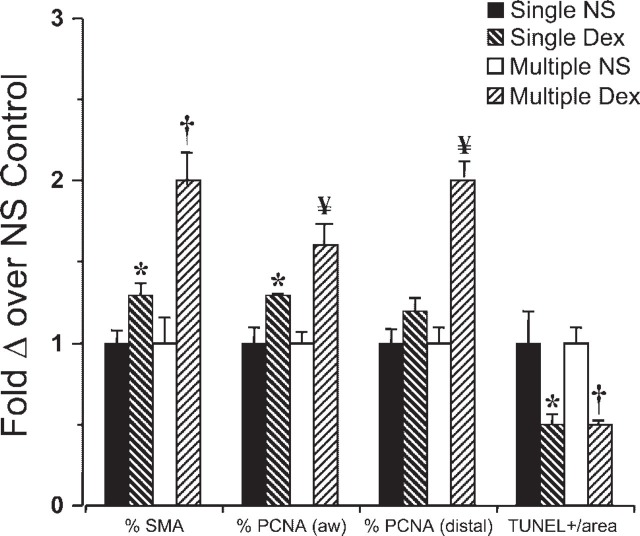
Compared with the control groups (Figure 5A), SMA immunostaining was more prevalent in lung sections from lambs treated with ACS (Figure 5B). The distribution of SMA-positive immunostaining was almost exclusively along the surface of the developing (primitive) alveoli, with rare SMA-immunopositive cells present within the interstitium of the alveolar septa. The difference in SMA immunostaining was of greater magnitude for the multiple-course groups (Figure 7). The mean volume percent SMA staining of the single-course Dex group was 1.3-fold greater than the mean of the matched controls (p = 0.02), whereas the mean of the multiple-course Dex group was 2-fold greater than its corresponding control (p = 0.002) (Figure 7).
Figure 5.
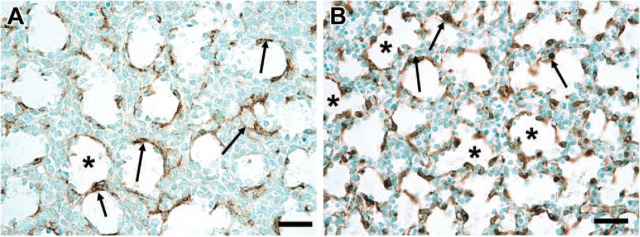
α-Smooth muscle actin (SMA) immunostaining. Preparation of lung tissues is given in Materials and Methods. The volume percent of lung tissue immunostaining for α-SMA was determined using point counting normalized for the total lung parenchymal tissue volume as described in detail in Materials and Methods. Each panel has representative arrows indicating SMA-positive cells. (A) Lung from multiple-course placebo-treated lamb. (B) Lung from multiple-course Dex-treated lamb. Note the presence of more SMA-positive immunostaining in (B) as compared with (A). This SMA positivity is observed frequently lining developing alveoli such as the airspaces indicated by asterisks (∗). Bar = 50 μm.
Figure 7.
Morphometric analyses of functional cell markers. Histogram summarizes the results of morphometric analyses, which are expressed as percentage changes over the mean of the corresponding placebo control. ∗ p<0.03 with respect to single-course placebo (NS) control. °p<0.003 with respect to multiple-course placebo (NS) control. × p<0.0001 with respect to multiple-course placebo (NS) control.
Compared with placebo controls (Figure 6A), the prevalence of PCNA immunostaining was increased in both steroid-treated groups in the epithelial cells of the conducting airways (Figure 6B). This is quantified as a 1.3-fold increase in the single-course Dex group (p<0.05) and a 1.7-fold increase in the multi-course placebo group (p<0.05) (Figure 7). In the distal lung parenchyma, there was a 2-fold difference in PCNA labeling for the multiple-course Dex group (p<0.0001), whereas no significant difference (1.2-fold increase only) was present in the single-course group (Figure 7). When the two steroid groups were compared, PCNA labeling in the distal parenchyma was greater in the multiple steroid group (p<0.05) (Figure 7).
Figure 6.
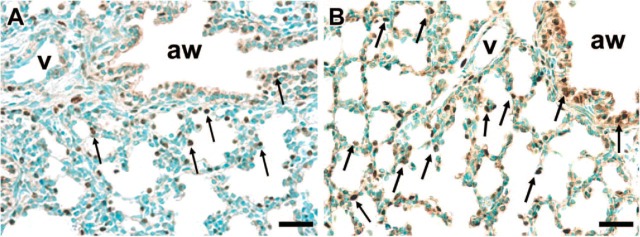
Proliferating cell nuclear antigen (PCNA) immunostaining. Preparation of lung tissues is given in Materials and Methods. Morphometric analyses were carried out by counting the number of PCNA-immunopositive nuclei and expressing this as a percentage of the total number of nuclei in each respective compartment [conducting airways (aw) vs. distal lung parenchyma], as described in Materials and Methods. Arrows in each panel indicate some of the PCNA-positive cells. Bar = 50 μm. (A) Lung from multiple-course placebo-treated lamb. (B) Lung from multiple-course Dex-treated lamb. There is an increase of PCNA-positive cells in both the parenchyma (2-fold, p<0.0001) and conducting airways (1.7-fold, p<0.0004) for multiple Dex-treated animals as compared with multiple placebo controls.
The localization of PCNA-positive cells in developing alveoli occurs in cells localized predominantly along the air-tissue interface (Figure 6), in the same distribution and numbers as the SMA-positive cells (Figure 5). Note that both the SMA- and PCNA-positive cells protrude into the airway lumen in a manner similar to early formation of secondary alveolar septa. This localization is consistent with most of the PCNA-positive cells in the alveoli being myofibroblasts. A few PCNA-positive cells are cuboidal alveolar-lining cells with abundant cytoplasm, consistent with some type II cells being PCNA-positive. Vascular and airway smooth muscle cells are not PCNA positive. Thus, both the proliferating and SMA-positive cells are predominantly (over 70%) mesenchymal in all groups, with no significant differences in distribution among groups.
In addition to increased cell proliferation, there are fewer apoptotic cells observed with both steroid groups, which are decreased by about half compared with their corresponding placebo controls (Figure 7). The apoptotic cells are both interstitial and epithelial in location, with no apparent difference in localization between the Dex- and placebo-treated groups (data not shown).
Discussion
The present study of fetal lamb lung development demonstrates that multiple courses of ACS have cellular and developmental effects similar to those of a single course. These observations are especially meaningful because we used doses of Dex similar to those clinically recommended for mothers in preterm labor. We first determined the effects of Dex on maternal weights and fetal growth, then evaluated morphologic and cellular changes in the developing fetal lungs.
First, we observe growth restriction of fetuses exposed to either single or multiple courses of Dex compared with the corresponding saline controls, consistent with previous animal studies (Ikegami et al. 1987; Jobe et al. 1998a, b). There is no difference in the ewe weights suggesting that the growth restriction is a developmentally specific effect. Jobe et al. (1998a, b) and Ikegami et al. (1987) showed that lambs exposed in utero to either single or repetitive maternal betamethasone had growth restriction from 104 to 121 days of gestation that persisted up to term (150 days). In contrast, single or repetitive courses of betamethasone administered directly to fetuses in utero did not cause growth restriction (Jobe et al. 1998a). In fetal rabbits, increasing courses of betamethasone led to a progressive decrease in birth weight with late treatment, causing more decline compared with early treatments (Pratt et al. 1999a). Monkeys exposed in utero to maternal injections of triamcinolone or Dex manifested growth restriction following both preterm and term deliveries (Johnson et al. 1981; Bunton and Plopper 1984).
Second, serum cortisol levels were suppressed by single-course Dex but not by multiple-course Dex. Tangalakis et al. (1995) found that when Dex was infused for 72 hr to ewes at 80-90 days of gestation, fetal plasma cortisol concentrations were decreased. These authors speculated that at this gestational age, the fetal adrenal gland requires at least 6 hr of adreno-corticotropic hormone exposure to stimulate cortisol secretion. Jobe et al. (1998b) showed that exposure to antenatal betamethasone early in gestation was not associated with a reduction in plasma cortisol concentration when measured in late gestation or full-term fetal sheep. Our findings and those of Tangalakis et al. (1995) and Jobe et al. (1998b) suggest that the fetal plasma cortisol response to exogenous antenatal corticosteroids depends upon the treatment regimen, the time during gestation at which the exogenous steroids are administered, and the timing of cortisol sampling relative to exogenous steroid administration. Our findings suggest that single but not repeated courses of antenatal glucocorticoids in doses similar to those used in the clinical setting to treat women in premature labor may suppress the fetal hypothalamic-pituitary-adrenal axis at mid-gestation. These findings suggest that there may be a resetting of the fetal hypothalamic-pituitary-adrenal axis after repeated glucocorticoid exposure, whereby the negative feedback loop is attenuated (Seckl et al. 1995; Seckl and Miller 1997). Moreover, the lack of change in plasma cortisol concentration after exposure to repeated courses of antenatal Dex might also reflect potential downregulation of central glucocorticoid receptors (Felszeghy et al. 1996).
The major novel observation of our study is that ACS, given either as single or multiple courses, promotes fetal sheep lung morphogenesis both at the cellular level and architecturally. The stage of fetal lung development at the time of harvest is late canalicular/early saccular period, which is characterized by the presence of a transient double capillary network in the alveolar septa (Burri 1974; Burri et al. 1974; Zeltner and Burri 1987).
The Dex-induced acceleration of alveolar development in the present study includes elongation of developing septa and thinning of alveolar walls, either of which would contribute to improved oxygen exchange.
Histochemical analyses demonstrate that both single and multiple courses of Dex promote specific cellular changes associated with lung development, especially the increased prevalence of normal SMA-positive, desmin-negative myofibroblasts in developing alveoli; these myofibroblasts are necessary for alveolar septation (Kapanci et al. 1974; Vaccaro and Brody 1978; Brody and Vaccaro 1979). Myofibroblasts constitute the subpopulation of SMA-positive, vimentin-positive, desmin-negative mesenchymal cells that are rapidly proliferating and also migrating (Skalli et al. 1989; Mitchell et al. 1990) and thus apparently have a direct role in the septation process. For example, the PDGF-A-null mouse has defects in both myofibroblast development and in alveogenesis (Bostrom et al. 1996; Lindahl et al. 1997). This interpretation is supported by our observation of a Dex-associated increase in cell proliferation and decreased apoptosis, as well as increased numbers of alveoli as measured by the MLI technique. In normal adults, SMA-positive cells persist in the distal lung parenchyma where they could regulate airflow.
Similarly, either single- or multiple-course ACS induced cell proliferation in the conducting airways and decreased apoptosis throughout the lung parenchyma. The only major difference we observed between the dosage schedules of Dex was that PCNA-positive cells were increased in developing alveoli with multiple-course, but not single-course ACS. This could explain why the magnitude and/or statistical significance of the observed effects on alveolarization were often greater with multiple- than with single-course ACS as compared with the placebo controls. However, most of these differences between multiple- and single-course groups were not statistically significant.
Our observation of Dex-promoted alveolar development is consistent with studies of lung development in both fetal sheep and mice by other investigators. Glucocorticoids are widely known to be agents for enhancing production and secretion of surfactant by promoting cytodifferentiation of type II pneumocytes (Ballard and Ballard 1995). Recent investigations of preterm lamb lungs by Willet et al. (1999, 2001) support a role for glucocorticoids in structural remodeling during alveolar development of larger mammals including greater alveolar wall thinning after repetitive courses of betamethasone as compared with a single course (Willet et al. 2001). We and others have demonstrated SMA-positive myofibroblasts at the tips of developing secondary septa, where they are believed to be involved in septal growth via migration and production of elastin (Brody and Vaccaro 1979; Vaccaro and Brody 1978). These cells express α-SMA and vimentin but not desmin, consistent with only partial smooth muscle cell differentiation (Skalli et al. 1989). In contrast, myofibroblasts identified in various fibroproliferative pathological lesions frequently express vimentin together with both SMA and desmin (Skalli et al. 1989).
Conversely, newborn mice that are genetically deficient for corticotropin-releasing hormone (CRH) have markedly impaired alveolar structural development (Muglia et al. 1995), which is highly similar to the defective lung development in PDGF-A-deficient mice (Bostrom et al. 1996; Lindahl et al. 1997). The lack of alveolar septation in PDGF-A-null mice is characterized by an arrest of myofibroblast migration. The arrested alveolarization in CRH-null mice is associated with diminished mesenchymal cell proliferation at embryonic day (E) 16.5, then diminished epithelial cell differentiation plus elevated epithelial cell proliferation at E18.5; all of these abnormalities can be completely corrected by treatment with Dex in utero (Muglia et al. 1995). Similar to our observations, glucocorticoids also have been demonstrated to induce cell proliferation in both in vitro and in vivo systems (Emanuel et al. 1999; Gunin and Nikolaev 1999).
Nonetheless, the effect of glucocorticoids on alveolar development remains controversial. Investigations of non-human primates and rodents have demonstrated that repetitive steroids can impair alveolarization. Rhesus macaques exposed to triamcinolone during early gestation had delayed alveolarization (Bunton and Plopper 1984; Blanco et al. 1989). In rats, Dex administration either in utero or during the period of septation resulted in thinning of alveolar walls (Massaro and Massaro 1986, 1992) and markedly diminished septation (Massaro et al. 1985; Blanco and Frank 1993). However, the dose of antenatal Dex used in those rats was over 20-fold higher than the clinical doses used in our fetal sheep studies. A recent study in mice showed that multidose antenatal betamethasone accelerated fetal lung maturation more than a single dose, but this was accompanied by a decrease in lung weight that persisted into adulthood (Stewart et al. 1998). Similarly, lambs treated in utero with repeated maternal betamethasone had reduced body weight but minimal disruption of the hypothalamic-pituitary axis (Sloboda et al. 2000). Furthermore, premature rats receiving ACS were at higher risk for pulmonary fibrosis when they were exposed to hyperoxia postnatally (Chen et al. 1997). Cumulatively, these data suggest that the diverse outcomes of ACS are multifactorial, including differences in species, genetics, timing of ACS, numbers of subjects, and other drugs being administered.
Clinical investigations have yielded contradictory results concerning the effects of ACS on fetal growth. In a retrospective study based on chart reviews, there was a reduction in oxygen use in neonates given multiple courses of ACS, without significant maternal or neonatal complications (Pratt et al. 1999b). In a 3-year retrospective study, French et al. (1999) showed that birth weight and head circumference decreased with increasing number of courses of ACS. However, long-term growth and disability outcomes did not differ between treatment groups, as determined after 3 years of follow-up. Other investigators showed that fetal growth was not disturbed following multiple courses of ACS (Pratt et al. 1999b; Abbassi et al. 2000; Eliminian et al. 2000). Also, these human studies were mostly retrospective and not randomized controlled trials. For instance, in one study the infants receiving multiple courses of ACS were significantly older and heavier than infants in the single-course group (Eliminian et al. 2000). Guinn et al. (2001) carried out the only randomized double-blind placebo-controlled trial published to date, in which there was no apparent benefit of multiple courses and the composite morbidities did not differ between groups receiving single vs multiple courses of ACS.
In conclusion, we observed improved alveolar morphogenesis with either multiple or single courses of ACS. Using a variety of morphological and histochemical parameters to assess cell proliferation and morphogenesis, multiple-course ACS did not offer much advantage over single-course ACS in promoting alveolar development. These results support the concept that clinical application of multiple-course ACS should be approached with caution (Lockshin and Sammaritano 1998) because there is little increased benefit and much more increased risk than with single-course ACS. For instance, glucocorticoids can lead to many systemic effects including pulmonary fibrosis (Chen et al. 1997) and growth restriction of the lung and brain (Johnson et al. 1979, 1981; Salokorpi et al. 1997; Stewart et al. 1998). Considering the cumulative data, it may be unwise to pursue additional randomized human clinical trials to assess long-term pulmonary and neurological function following multiple- vs single-course ACS (Crowley 1995; Whitelaw and Thoresen 2000). In summary, multiple courses of ACS should be avoided unless there is compelling new information from long-term follow-up studies that demonstrate a clear-cut benefit outweighing any potential risks.
Acknowledgments
This work was supported by the National Institutes of Health Grants HL-52638 (MES), HD-34618 (BSS), and the Children's Hospital, Boston Neonatology Training Grant (ZJP and AC).
Literature Cited
- Abbassi S, Hirsch D, Davis J, Tolosa J, Stouffer N, Debbs R, Gerdes J. (2000) Effects of single versus multiple courses of antenatal corticosteroids on maternal and neonatal outcome. Am J Obstet Gynecol 182: 1243–1249 [DOI] [PubMed] [Google Scholar]
- Ballard PL, Ballard RA. (1995) Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol 173: 254–262 [DOI] [PubMed] [Google Scholar]
- Blanco LN, Frank L. (1993) The formation of alveoli in rat lung during the third and fourth postnatal weeks: effect of hyperoxia, dexamethasone, and deferoxamine. Pediatr Res 34: 334–340 [DOI] [PubMed] [Google Scholar]
- Blanco LN, Massaro GD, Massaro D. (1989) Alveolar dimensions and number: developmental and hormonal regulation. Am J Physiol 257: L240–247 [DOI] [PubMed] [Google Scholar]
- Bostrom H, Willetts K, Pekny M, Leveen P, Lindahl P, Hedstrand H, Pekna M, et al. (1996) PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85: 863–873 [DOI] [PubMed] [Google Scholar]
- Brody JS, Vaccaro C. (1979) Postnatal formation of alveoli: interstitial events and physiologic consequences. Fed Proc 38: 215–223 [PubMed] [Google Scholar]
- Bunton TE, Plopper CG. (1984) Triamcinolone-induced structural alterations in the development of lungs of the fetal rhesus macaque. Am J Obstet Gynecol 148: 203–215 [DOI] [PubMed] [Google Scholar]
- Burri PH. (1974) The postnatal growth of the rat lung. III. Morphology. Anat Rec 180: 77–98 [DOI] [PubMed] [Google Scholar]
- Burri PH, Dbaly J, Weibel ER. (1974) The postnatal growth of the rat lung. I. Morphometry. Anat Rec 178: 711–730 [DOI] [PubMed] [Google Scholar]
- Chen Y, Martinez MA, Frank L. (1997) Prenatal dexamethasone administration to premature rats exposed to prolonged hyperoxia: a new rat model of pulmonary fibrosis (bronchopulmonary dysplasia). J Pediatr 130: 409–416 [DOI] [PubMed] [Google Scholar]
- Crowley PA. (1995) Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol 173: 322–335 [DOI] [PubMed] [Google Scholar]
- Eliminian A, Verma U, Visintainer P, Tejani N. (2000) Effectiveness of multidose antenatal steroids. Obstet Gynecol 95: 34–36 [DOI] [PubMed] [Google Scholar]
- Emanuel RL, Torday JS, Mu Q, Asokananthan N, Sikorski KA, Sunday ME. (1999) Bombesin-like peptides and receptors in normal fetal baboon lung: roles in lung growth and maturation. Am J Physiol 277: L1003–L1017 [DOI] [PubMed] [Google Scholar]
- Felszeghy K, Gaspar E, Nyakas C. (1996) Long-term selective downregulation of brain glucocorticoid receptors after neonatal dexamethasone treatment in rats. J Neuroendocrinol 8: 493–499 [DOI] [PubMed] [Google Scholar]
- French NP, Hagan R, Evans S, Godfrey M, Newnham JP. (1999) Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol 180: 114–121 [DOI] [PubMed] [Google Scholar]
- Gunin AG, Nikolaev DV. (1999) Effect of acute and chronic glucocorticoid treatments on epithelial cell proliferation in the esophagus and small intestine of rats. J Gastroenterol 34: 661–667 [DOI] [PubMed] [Google Scholar]
- Guinn DA, Atkinson MW, Sullivan L, Lee M, MacGregor S, Parilla BV, Davies J, et al. (2001) Single vs weekly courses of antenatal corticosteroids for women at risk of preterm delivery: a randomized controlled trial. JAMA 286: 1581–1587 [DOI] [PubMed] [Google Scholar]
- Haley KJ, Drazen JM, Osathanondh R, Sunday ME. (1997) Comparison of the ontogeny of protein gene product 9.5, chromogranin A and proliferating cell nuclear antigen in developing human lung. Microsc Res Tech 37: 62–68 [DOI] [PubMed] [Google Scholar]
- Hsu S, Raine L, Fanger H. (1981) A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol 75: 734–738 [DOI] [PubMed] [Google Scholar]
- Ikegami M, Berry D, Elkady T, Pettenazzo A, Seidner S, Jobe A. (1987) Corticosteroids and surfactant change lung function and protein leaks in the lungs of ventilated premature rabbits. J Clin Invest 79: 1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe AH. (2003) Animal models of antenatal corticosteroids: clinical implications. Clin Obstet Gynecol 46: 174–189 [DOI] [PubMed] [Google Scholar]
- Jobe AH, Newnham J, Willet K, Sly P, Ikegami M. (1998a) Fetal versus maternal and gestational age effects of repetitive antenatal glucocorticoids. Pediatrics 102: 1116–1125 [DOI] [PubMed] [Google Scholar]
- Jobe AH, Wada N, Berry LM, Ikegami M, Ervin G. (1998b) Single and repetitive maternal glucocorticoid exposures reduce fetal lung growth in sheep. Am J Obstet Gynecol 178: 880–885 [DOI] [PubMed] [Google Scholar]
- Johnson JWC, Mitzner W, London WT, Palmer AE, Scott R. (1979) Betamethasone and the rhesus fetus: multisystemic effects. Am J Obstet Gynecol 133: 677–684 [DOI] [PubMed] [Google Scholar]
- Johnson JWC, Mitzner W, Beck JC, London WT, Sly DL, Lee PA, Khouzami VA, et al. (1981) Long-term effects of betamethasone on fetal development. Am J Obstet Gynecol 141: 1053–1064 [DOI] [PubMed] [Google Scholar]
- Kapanci Y, Assimacopoulos A, Irle C, Zwahlen A, Gabbiani G. (1974) Contractile interstitial cells in pulmonary alveolar septa: a possible regulator of ventilator-perfusion ratio? Ultrastructural, immunofluorescence, and in vitro studies. J Cell Biol 60: 375–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Cras TD, Markham NE, Morris KG, Ahrens CR, McMurtry IF, Abman SH. (2000) Neonatal dexamethasone treatment increases the risk for pulmonary hypertension in adult rats. Am J Physiol 278: L822–829 [DOI] [PubMed] [Google Scholar]
- Liggins GC, Howie RN. (1972) A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 50: 515–525 [PubMed] [Google Scholar]
- Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. (1997) Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124: 3943–3953 [DOI] [PubMed] [Google Scholar]
- Lockshin MD, Sammaritano LR. (1998) Corticosteroids during pregnancy. Scand J Rheumatol 107(suppl):136–138 [DOI] [PubMed] [Google Scholar]
- Losada A, Tovar JA, Xia HM, Diez-Pardo JA, Santisteban P. (2000) Down-regulation of thyroid transcription factor-1 gene expression in fetal lung hypoplasia is restored by glucocorticoids. Endocrinology 141: 2166–2173 [DOI] [PubMed] [Google Scholar]
- Massaro D, Massaro GD. (1986) Dexamethasone accelerates postnatal alveolar wall thinning and alters wall composition. Am J Physiol 251: R218–224 [DOI] [PubMed] [Google Scholar]
- Massaro D, Massaro GD. (2001) Pulmonary alveolus formation: critical period, retinoid regulation and plasticity. Novartis Found Symp 234: 229–236 [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D. (1992) Formation of alveoli in rats: postnatal effect of prenatal dexamethasone. [published errata appear in Am J Physiol 1992 Sep; 263(3 Pt 1) and Am J Physiol 1993 Feb; 264(2 Pt 1)]. Am J Physiol 263: L37–41 [DOI] [PubMed] [Google Scholar]
- Massaro JD, Teich N, Maxwell S, Massaro GD, Whitney P. (1985) Postnatal development of alveoli: regulation and evidence for a critical period in rats. J Clin Invest 76: 1297–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JJ, Reynolds SE, Leslie KO, Low RB, Woodcock-Mitchell J. (1990) Smooth muscle markers in developing rat lung. Am J Respir Cell Mol Biol 3: 515–523 [DOI] [PubMed] [Google Scholar]
- Muglia L, Jacobson L, Dikkes P, Majzoub J. (1995) Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature 373: 427–432 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Panel (1994) Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 273: 413–418 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Panel (2001) Antenatal corticosteroids revisited: repeat courses—National Institutes of Health Consensus Development Conference Statement, August 17-18, 2000. Obstet Gynecol 98: 144–150 [DOI] [PubMed] [Google Scholar]
- O'Connor V, Heuss C, De Bello WM, Dresbach T, Charlton MP, Hunt JH, Pellegrini LL, et al. (1997) Disruption of syntaxin-mediated protein interactions blocks neurotransmitter secretion. Proc Natl Acad Sci USA 94: 12186–12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L, Magness RR, Phernetton T, Hendricks SK, Abbott DH, Bird AM. (1999a) Repeated use of betamethasone in rabbits: effects of treatment variation on adrenal suppression, pulmonary maturation and pregnancy outcome. Am J Obstet Gynecol 180: 995–1005 [DOI] [PubMed] [Google Scholar]
- Pratt L, Waschbusch L, Ladd W, Gangnon R, Hendricks SK. (1999b) Multiple vs. single betamethasone therapy. Neonatal and maternal effects. J Reprod Med 44: 257–264 [PubMed] [Google Scholar]
- Salokorpi T, Sajaniemi N, Hallback H, Kari A, Rita H, von Wendt L. (1997) Randomized study of the effect of antenatal dexamethasone on growth and development of premature children at the corrected age of 2 years. Acta Paediatr 86: 294–298 [DOI] [PubMed] [Google Scholar]
- Seckl JR, Benediktsson R, Lindsay RS, Brown RW. (1995) Placental 11 beta-hydroxysteroid dehydrogenase and the programming of hypertension. J Steroid Biochem Mol Biol 55: 447–455 [DOI] [PubMed] [Google Scholar]
- Seckl JR, Miller WL. (1997) How safe is long-term prenatal glucocorticoid treatment? JAMA 277: 1077–1079 [PubMed] [Google Scholar]
- Skalli O, Schurch W, Seemayer T, Lagace R, Montandon D, Pittet B, Gabbiani G. (1989) Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest 60: 275–285 [PubMed] [Google Scholar]
- Sloboda DM, Newnham JP, Challis JR. (2000) Effects of repeated maternal betamethasone administration on growth and hypothalamic-pituitary-adrenal function of the ovine fetus at term. J Endocrinol 165: 79–91 [DOI] [PubMed] [Google Scholar]
- Stewart JD, Sienko AE, Gonzalez CL, Christensen HD, Rayburn WF. (1998) Placebo-controlled comparison between a single dose and a multidose of betamethasome in accelerating lung maturation of mice offspring. Am J Obstet Gynecol 179: 1241–1247 [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Sadowski GB, McKnight AJ, Patlak C, Petersson KH. (2000) Exogenous and endogenous corticosteroids modulate blood-brain barrier development in the ovine fetus. Am J Physiol 279: R468–477 [DOI] [PubMed] [Google Scholar]
- Tangalakis K, Moritz K, Shandley L, Wintour EM. (1995) Effect of maternal glucocorticoid treatment on ovine fetal fluids at 0.6 gestation. Reprod Fertil Dev 7: 1595–1598 [DOI] [PubMed] [Google Scholar]
- Vaccaro C, Brody JS. (1978) Ultrastructure of developing alveoli. I. The role of the interstitial fibroblast. Anat Rec 192: 467–479 [DOI] [PubMed] [Google Scholar]
- Wang J, Kuliszewski M, Yee W, Sedlackova L, Xu J, Tseu I, Post M. (1995) Cloning and expression of glucocorticoid-induced genes in fetal rat lung fibroblasts: transforming growth factor-β3. J Biol Chem 270: 2722–2728 [DOI] [PubMed] [Google Scholar]
- Whitelaw A, Thoresen M. (2000) Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed 83: 154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willet KE, Jobe AH, Ikegami M, Kovar J, Sly P. (2001) Lung morphometry after repetitive antenatal treatment in preterm sheep. Am J Respir Crit Care Med 163: 1437–1443 [DOI] [PubMed] [Google Scholar]
- Willet KE, McMenamin P, Pinkerton KE, Ikegami M, Jobe AH, Gurrin L, Sly PD. (1999) Lung morphometry and collagen and elastin content: changes during normal development and prenatal hormone exposure in sheep. Pediatr Res 45: 615–625 [DOI] [PubMed] [Google Scholar]
- Zeltner TB, Burri PH. (1987) The postnatal development and growth of the human lung. II. Morphology. Respir Physiol 67: 269–282 [DOI] [PubMed] [Google Scholar]