Abstract
ATP-sensitive K+ (KATP) channel subunits on the subcellular structures of rat cardiomyocytes were studied with antibodies against Kir6.1 and Kir6.2. According to the results of Western blot analysis, Kir6.1 was strongly expressed in mitochondrial and microsome fractions, and faintly expressed in cell membrane fraction, whereas Kir6.2 was mainly expressed in the microsome fraction and weakly in cell membrane and mitochondrial fractions. Immunohistochemistry showed that Kir6.1 and Kir6.2 were expressed in the endocardium, atrial and ventricular myocardium, and in vascular smooth muscles. Immunoelectron microscopy revealed that Kir6.1 immunoreactivity was mainly localized in the mitochondria, whereas Kir6.2 immunoreactivity was mainly localized in the endoplasmic reticulum and a few in the mitochondria. Both Kir6.1 and Kir6.2 are candidates of mitochondrial KATP channel subunits. The data obtained in this study will be useful for analyzing the composition of KATP channels of cardiomyocytes and help to understanding the cardioprotective role of KATP channels during heart ischemia.
Keywords: ATP-sensitive K+ channels, mitochondria, endoplasmic reticulum, rat, cardiomyocytes, immunoelectron microscopy
ATP-sensitive K+ (KATP) channels were originally discovered in the heart (Noma 1983), and have since been found in many tissues, including pancreatic β-cells, skeletal muscle, kidney, and brain (Inagaki et al. 1995). KATP channels close at high intracellular ATP concentrations and open during ischemia (lower intracellular ATP concentrations) (Yokoshiki et al. 1998). They are hetero-octameric and consist of four pore-forming subunit molecules, Kir6.x (Kir6.1 or Kir6.2), and four regulatory subunit molecules, sulfonylurea receptors (SUR): SUR1, SUR2A, or SUR2B (Clement et al. 1997).
Brief exposure of myocardium to ischemia and reperfusion, named ischemia preconditioning, can dramatically reduce lethal injury to the heart followed by prolonged severe ischemia, but the mechanism of such a cardioprotection remains controversial—on the one hand being considered to be attributable to the function of sarcolemmal KATP channels (Suzuki et al. 2002), and on the other to be attributable to mitochondrial KATP channels (Das and Sarkar 2003). Kir6.1 mRNA and protein are specifically upregulated during myocardial infarction (Akao et al. 1997). Mice lacking the Kir6.1 gene experience spontaneous ST elevation and atrioventricular block followed by sudden death (Miki et al. 2002). Kir6.1 may be an important candidate to explain the mechanism of cardioprotection, but its precise localization remains to be determined.
Two conflicting views still exit. By the patch-clamp technique, KATP channels were revealed to be localized in the inner membrane of mitochondria with fused giant mitoplasts (Inoue et al. 1991). Later, Kir6.1 was revealed to be localized in the mitochondria in skeletal muscles (Suzuki et al. 1997), and in neurons and glial cells of rat brain (Zhou et al. 1999), whereas the results of the gene transfer technique indicated that neither Kir6.1 nor Kir6.2 was localized in the mitochondria (Seharaseyon et al. 2000). Recently, Kir6.1 and Kir6.2 were expressed in mitochondria in the isolated mitochondrial fraction of mouse cardiomyocytes (Lacza et al. 2003) and isolated rat ventricular myocytes (Singh et al. 2003). In contrast, there were deniable results that showed no expression of KATP subunits Kir6.1, Kir6.2, SUR2A, and SUR2B in mitochondrial fractions of rat cardiomyocytes by Western blot analysis (Kuniyasu et al. 2003). The specificity of antibodies used and the purity of mitochondrial fractions obtained seemed convincing, but the reason for the discrepancy among the results still remains unknown. Thus it is still necessary to determine the exact cellular and subcellular localization of Kir6.1 or Kir6.2 in cardiomyocytes to identify the molecular identity of KATP channels.
In the present study, we used a new rabbit anti-rat Kir6.1 antibody and a commercial goat anti-human Kir6.2 antibody to analyze the localization of Kir6.1 and Kir6.2 in the mitochondria or endoplasmic reticulum (ER) of rat cardiomyocytes by immunoblot analysis and immunoelectron microscopy. We paid special attention to the characterization of antibody used. Fractions of mitochondria, cell membrane, and microsome of rat heart were carefully purified and distinguished with anti-cytochrome c, anti-Na+/K+ ATPase β1 subunit, and anti-calreticulin antisera. Immunohistochemistry and immunoelectron microscopy were used to detect the localization of KATP subunit proteins on rat heart sections.
Materials and Methods
Generation of Anti-Kir6.1 Antibody
Rabbit anti-Kir6.1 antiserum was raised against a synthetic 14 amino acid peptide, NH2-(C)QFMTPEGNQCPSES-OH, which corresponds to amino acid residues 411-424 of rat Kir6.1 (Gene No. D42145). The polyclonal peptide antiserum production was processed according to Van Bueren et al. (1993), with some modification. In brief, the synthetic peptide representative to rat Kir6.1 was coupled to the carrier protein keyhole limpet hemocyanin via the cysteine residue added to the N-terminal. Two Japanese white rabbits (Japan SLC; Hamamatsu, Japan) weighing 2.5-3.0 kg were injected with ∼200 μg of peptide-keyhole limpet hemocyanin conjugate emulsified with an equal volume of Freund's complete adjuvant (Rockland Immunochemicals; Gilberts-ville, PA) at multiple intradermal sites, followed by three boosters at 2-wk intervals and later by injection with the same dosage of the peptide conjugate emulsified in Freund's incomplete adjuvant (Rockland Immunochemicals) three times. The antiserum was harvested 1 wk after the final injection. The antiserum was purified by immunoaffinity column chromatography before using for Western blot analysis and immunohistochemistry.
Animals
Male Wistar rats (4-8 wk) were used. The protocols for animal experimentation described were approved by the Animal Research Committee, Akita University. All subsequent animal experiments were conducted in accordance with the Guidelines for Animal Experimentation of the University.
The animals used for immunohistochemistry and immunoelectron microscopy were anesthetized by diethyl ether inhalation and then perfused through left ventricle with 4% paraformaldehyde in 0.1 M PBS, pH 7.4. The hearts were quickly excised and placed in the same fixative for 6 hr at 4C, after which they were transferred to 30% sucrose in PBS. Cryosections 7-8 μm thick were cut, thaw-mounted on MAS-coated glass slides (Matsunami Glass Ind. Ltd.; Kishiwada-city, Japan), and stored at −80C until used.
Subcellular Fractionation of Rat Heart
All operations were carried out at 0-4C. Adult Wistar rats were anesthetized by inhalation of diethyl ether. Their hearts were immediately excised and quickly washed with 0.9% NaCl solution. The hearts were then cut into small pieces and homogenized with 0.25 mM sucrose/50 mM Tris-HCl buffer, pH 7.4, containing proteinase inhibitor cocktail tablets (Roche Diagnostics GMbH; Mannheim, Germany). Subcellular fractions were extracted as described elsewhere (Itoh et al. 2002). Briefly, after centrifugation at 600 × g for 10 min, the precipitate was discarded, and the 600 × g supernatant was centrifuged at 7000 × g for an additional 10 min. The 7000 × g precipitate (P1) was redissolved in the buffer and centrifuged at 5000 × g for 10 min, and the 5000 × g precipitate was used as the mitochondrial fraction. The 7000 g supernatant (S1) was centrifuged at 54,000 × g for 60 min, and the supernatant (S2) was centrifuged at 105,000 × g for an additional 60 min. The 54,000 × g precipitate was used as the cell membrane fraction, the 105,000 × g precipitate as the microsome fraction, and the supernatant as the cytoplasm fraction.
Western Blot Analysis
SDS/PAGE was carried out according to the procedure of Laemmli (Laemmli 1970), using 10% polyacrylamide. Proteins of cellular fractions were electrophoresed (10 μg per lane). After electrophoresis, proteins were electrophoretically transferred to a polyvinylidene difluoride membrane (Bio-Rad; Hercules, CA) with a semi-dry transfer unit (Hoefer TE70 series; Amersham Pharmacia Biotechnology, Little Chalfont, Buckinghamshire, England). After blocking with 5% (w/v) skim milk in PBS and 0.1% Tween-20, the polyvinylidene difluoride membranes were incubated with rabbit anti-rat Kir6.1, goat anti-human Kir6.2 (Sc-11228; Santa Cruz Biotechnology Inc., Santa Cruz, CA), rabbit anti-cytochrome c (Sc-7159; Santa Cruz Biotechnology Inc.), rabbit anti-calreticulin (NB600-101; Novus Biologicals, Littleton, CO), or rabbit anti Na+/K+ ATPase β1 (Sc-16053; Santa Cruz Biotechnology Inc.), diluted in 1:500 to 1:1000, respectively, for 60 min. After rinsing with PBS, they were then exposed to horseradish peroxide-conjugated donkey anti-rabbit IgG (NA9340; Amersham Pharmacia Biotechnology), or donkey anti-goat IgG (AP180P; Chemicon International, Temecula, CA), diluted in 1:3000 to 1:5000 for 30 min. The antigen-antibody complexes were visualized with chemiluminescence detection reagents (Amersham Pharmacia Biotechnology), according to the manufacturer's instructions.
Immunohistochemistry
The heart sections were maintained in PBS containing 0.3% Tween-20 for 45 min or pretreated with 0.4% pepsin in 0.2N HCl for 3 or 5 min at 37C. After preincubation with 5% normal serum (goat or rabbit) for 60 min, the sections were incubated with rabbit anti-rat Kir6.1 at a dilution of 1:200, goat anti-human Kir6.1 (Sc-11224, Santa Cruz Biotechnology) at a dilution of 1:500, or goat anti-human Kir6.2 at a dilution of 1:200 for 12 hr at room temperature. Before incubation, sections were treated with a 0.3% solution of H2O2 in methanol for 10 min to reduce endogenous peroxidase reactions. After thoroughly rinsing with PBS, the sections were exposed to biotinylated goat anti-rabbit IgG or biotinylated rabbit anti-goat IgG (BA-1000 and BA-5000; Vector Laboratories Inc., Burlingame, CA), diluted in 1:200 for 30 min, and then with ABC complex (Vector Laboratories Inc.) according to the manufacturer's instructions. Reaction sites were visualized by the DAB (3, 3'-diaminobenzidine tetrahydrochloride) reaction, and counterstaining was performed with methyl green.
Immunofluorescence Double Staining
The heart sections were maintained in PBS containing 0.3% Tween-20 for 45 min. After preincubation with 5% normal donkey serum in PBS for 1 hr, the sections for double labeling with Kir6.1 and cytochrome c were incubated with goat anti-human Kir6.1 antibody (1:200), and rabbit anti-cytochrome c antibody (1:200) diluted together in PBS, and those for double labeling with Kir6.2 and calreticulin were incubated with goat anti-human Kir6.2 antibody (1:200) and rabbit anti-calreticulin antibody (1:100) diluted together in PBS for 12 hr at room temperature. After rinsing with PBS, the sections were then reacted with Alexa 488-conjugated donkey anti-goat IgG (A11055) and with Alexa 594-conjugated donkey anti-rabbit IgG (A21207; Molecular Probes Inc., Eugene, OR: green for Kir6.1 and Kir6.2, red for cytochrome c and calreticulin) diluted together at 1:1000 in PBS for 30 min. The sections were then cover slipped with PermaFluor aqueous mounting medium (Thermo; Pittsburgh, PA) after counterstaining with 4', 6-diamidino-2-phenylindole, dihydrochloride (DAPI). Fluorescence immunolabeling signals were detected by a laser-scanning microscope (LSM510; Carl Zeiss, Oberkochen, Germany).
Immunoelectron Microscopy
Preembedding Method. Tissue sections showing immunopositive reactions to Kir6.1, and those to Kir6.2 were postfixed in 1% osmium tetroxide (OsO4) for 30 min, dehydrated in a graded ethanol series, and embedded in Quetol 812 (Nisshin EM Co.; Tokyo, Japan). Thin sections were cut and examined with an electron microscope without uranyl acetate and lead citrate staining.
Postembedding Method. After perfusion fixation tissue blocks were washed with PBS, dehydrated in an ethanol series, and embedded in Lowicryl K4M at −20C in an ultraviolet polymerization chamber (Nissin EM) according to the manufacturer's instructions. Thin sections were placed on nickel grids and immunostained by incubating in 1% BSA/PBS for 60 min and then incubated for 12 hr at room temperature with rabbit anti-rat Kir6.1 or goat anti-human Kir6.2 antisera diluted to 1:200 with PBS containing 1% BSA. Normal goat or rabbit serum was used as a negative control. After washing several times with PBS containing 0.1% BSA, the sections exposed to rabbit antisera were incubated with goat anti-rabbit IgG-labeled 5-nm colloidal-gold (G7277; Sigma-Aldrich, Tokyo, Japan) at a dilution of 1:40 with 1% BSA/PBS; those exposed to goat antisera were incubated with rabbit anti-goat IgG-labeled 5-nm colloidal-gold (G5528, Sigma-Aldrich), at a dilution of 1:40 in 1% BSA/PBS for 6 hr at room temperature. After rinsing several times with PBS containing 0.1% BSA, the sections were fixed in 2% glutaraldehyde/PBS for 10 min, rinsed with distilled water, and stained with 2% uranium acetate, and then examined with an electron microscope.
Electron Microscopy of the Mitochondrial Fraction
To confirm the purity of the mitochondrial fraction used in the Western blot, the 5000 × g precipitate obtained by subcellular fractionation was fixed with 2% glutaraldehyde for 2 hr, followed by 1% OsO4 for 2 hr, and then dehydrated with acetone and embedded in Quetol 812. Thin sections were cut and directly examined with an electron microscope after uranyl acetate staining.
Quantitative Evaluation
Unit areas of mitochondria and areas outside the mitochondria were measured with an image-analyzing computer and software (version 1.62, NIH Image; Bethesda, MD) in each of 20 electron micrographs (original magnification × 15,000 or × 20,000) randomly taken in the postembedded sections stained with anti-Kir6.1 antibody or anti-Kir6.2 antibody. The numbers of labeled colloidal gold particles per unit area of mitochondria and area outside the mitochondria, including the cytoplasm, myofilaments, and ER, were calculated. All data were input into an access database by Excel 2000 and analyzed with SPSS software (version 10.0J, SPSS Inc.; Chicago, IL). The data were reported as means ± SE. Differences in the mean particle density between the two groups were analyzed by the unpaired Student's t-test. All statistical tests and p values were two-tailed, and the results were considered significant when the p value was less than 0.05.
Results
Western Blot Analysis
Polyclonal antiserum generated in a rabbit against rat Kir6.1 was affinity-purified to investigate the distribution of Kir6.1 protein in rat cardiomyocytes. The anti-Kir6.1 antibody recognized a prominent ∼43 kDa protein band in the mitochondrial fractions (Figure 1A, Lane 1), microsome fraction (Figure 1A, Lane 3), and a very weak signal was detected in the cell membrane fraction (Figure 1A, Lane 2). The detection signals were completely removed (Figure 1A, Lanes 4-6) by preabsorption with the immunizing peptide antigen. The anti-human Kir6.2 antibody, which crossreacts with rat Kir6.2, recognized a prominent band in the microsome fraction (Figure 1B, Lane 3), and that was weak in the mitochondrial fraction (Figure 1B, Lane 1), and the cell membrane fraction (Figure 1B, Lane 2).
Figure 1.
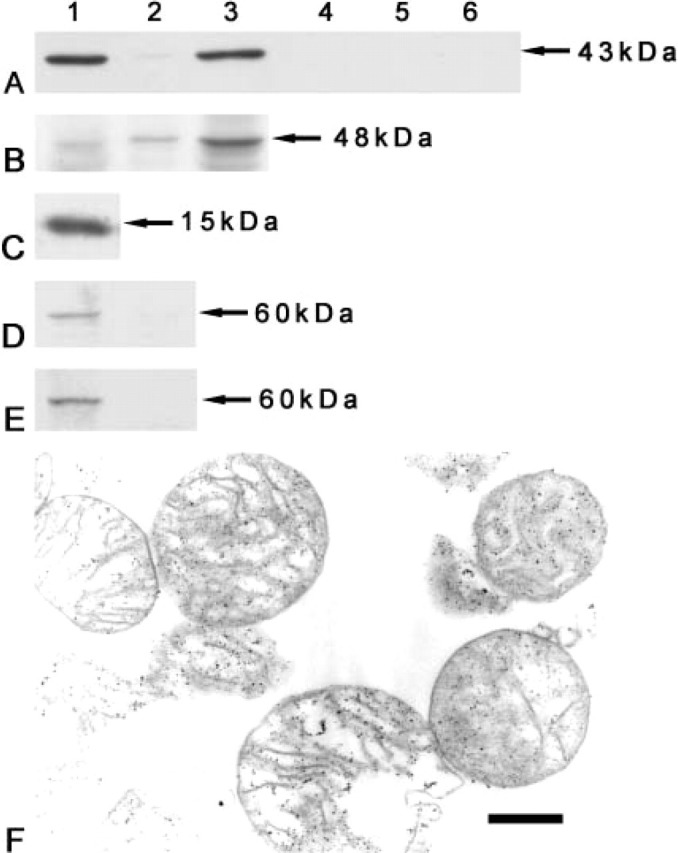
Western blot analysis of Kir6.1 and Kir6.2 in the rat heart and purification of isolated cellular fractions. (A) In the three left lanes, the rabbit anti-rat Kir6.1 antibody recognizes a prominent ∼43 kDa band in the mitochondrial (Lane 1) and microsome (Lane 3) fractions, but very weak in the cell membrane fraction (Lane 2). In the three right lanes, after preincubation with immunizing peptide antigen the detection bands have disappeared (Lanes 4-6). (Lanes 1, 4) Mitochondrial fraction; (Lanes 2, 5) plasma membrane fraction; and (Lanes 3, 6) microsome fraction. (B) A corresponding band (∼48 kDa) is detected by the goat anti-human Kir6.2 antibody in the microsome fraction (Lane 3) of the rat heart, weakly in the cell membrane (Lane 2) and the mitochondrial (Lane 1) fractions. (C) A corresponding band (15 kDa) is strongly detected in the mitochondrial fraction by the rabbit anti-cytochrome c antibody. (D) The anti-calreticulin antibody recognizes a corresponding band (60 kDa) only in the microsome fraction (Lane 1), but not in the mitochondrial fraction (Lane 2). (E) The anti-Na+/K+ ATPase β1 subunit antibody recognizes a corresponding band (60 kDa) in the plasma membrane fraction (Lane 1), but not in the mitochondrial fraction (Lane 2). (F) Most mitochondria were intact shown in the electron micrograph, proving the purity of the mitochondrial fraction. Bar = 500 nm.
Confirmation of the Cellular Fractions
Anti-cytochrome c antibody was used to confirm the purity of the mitochondrial fraction. The mitochondrial fraction showed intense immunoreactivity to anti-cytochrome c antibody in the Western blot analysis (Figure 1C). The microsome fractions was confirmed by anti-calreticulin antibody (an ER-specific antibody), which reacted to microsome fraction (Figure 1D, Lane 1), but not to mitochondrial fraction (Figure 1D, Lane 2). The purity of the cell membrane fraction was confirmed by anti-Na+/K+ ATPase β1 subunit antibody (a membrane-specific antibody), which reacted to plasma membrane fraction (Figure 1E, Lane 1), but not to the mitochondrial fraction (Figure 1E, Lane 2). The purity isolated mitochondrial fraction was also confirmed by electron microscopy. In the electron photograph, most of the visual field was covered with complete or some broken mitochondria (Figure 1F).
Immunohistochemistry of Kir6.1 and Kir6.2
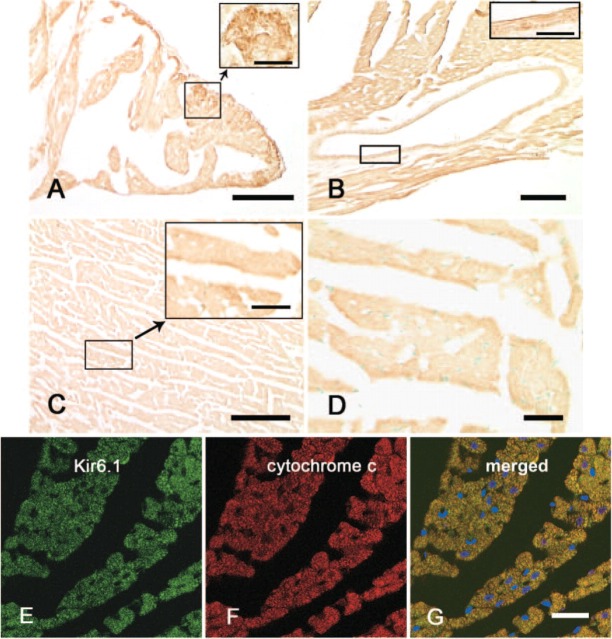
In the atria, intense immunoreactivity with anti-Kir6.1 antibodies was observed in the atrial cardiomyocytes (Figure 2A). In the ventricles, moderate to intense immunoreactivity with Kir6.1 antibodies was detected in both the transverse sections and longitudinal sections of cardiomyocytes (Figure 2B). Kir6.1 protein was also detected in the papillary muscles, endocardium, interatrial septum, and interventricular septum. The immunoreactivity with anti-Kir6.1 antibodies in cardiomyocytes was prominent because of punctate-immunoreactive products in the cytoplasm (Figure 2C). Weak to moderate immunoreactivity with Kir6.1 was also observed in the smooth muscle and endothelium of blood vessels (Figure 2B). The anti-human Kir6.1 antibody, which crossreacts with rat Kir6.1, also exhibited the similar immunoreactivity in rat cardiomyocytes as observed with rabbit anti-rat Kir6.1 antibody (Figure 2D).
Figure 2.
Immunohistochemistry showing expression of Kir6.1 protein in rat cardiomyocytes. Immunoreactivity with anti-rat Kir6.1 is seen in the cardiomyocytes of the atrium (A), ventricle (B, C), and smooth muscle of coronary blood vessel (B). Punctate reaction products are seen in the cytoplasm (insets in A, C). A goat anti-human Kir6.1 antibody yielded the same results (D). Immunofluorescence double staining shows the expression of Kir6.1 and cytochrome c in rat cardiomyocytes (E-G). The green fluorescence (Alexa 488) represents the Kir6.1 in the cytoplasm of rat cardiomyocytes (E); the red fluorescence (Alexa 594) represents the cytochrome c in the mitochondria (F). The merged image (G) shows that the Kir6.1 was colocalized with cytochrome c in the mitochondria with yellow. Bars: A-C = 0.1 mm; D = 20 μm; E-G = 20 μm. Inset bars: A, B = 10 μm; C = 20 μm.
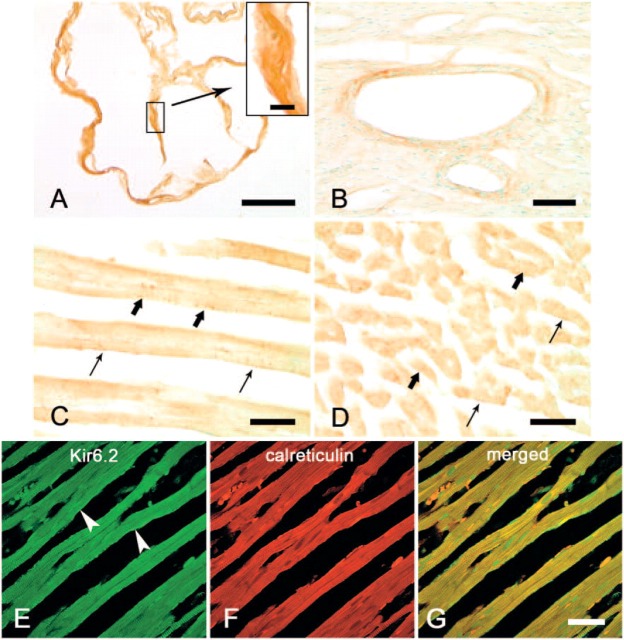
Rat cardiomyocytes in the atria (Figure 3A), ventricles (Figure 3B), papillary muscles, interatrial septum, interventricular septum, and vascular smooth muscle (Figure 3B) showed immunoreactivity with Kir6.2, but the characteristics of Kir6.2 expression were some different from those of Kir6.1 expression. In longitudinal sections, the cell membrane of the cardiomyocytes showed weak immunoreactivity with Kir6.2 (Figure 3C), and some granular or punctate immunoreaction products were also observed in the cytoplasm both in longitudinal and transversal sections (Figures 3C and 3D).
Figure 3.
Immunohistochemistry showing expression of Kir6.2 protein in rat cardiomyocytes. Immunoreactivity with anti-Kir6.2 antibody is seen in cardiomyocytes in the atrium (A). No prominent punctate reaction products were detected in the cytoplasm (inset). Immunoreactivity was also observed in the wall of coronary blood vessels in the rat heart (B). In the longitudinal (C) and transversal (D) section of rat ventricle, weak immunoreactivity to Kir6.2 is seen in the cell membrane (thin arrows); some granular immunoreaction products are shown in the cytoplasm (bold arrows). Immunofluorescence double staining shows the expression of Kir6.2 as green (Alexa 488, E) in the cytoplasm and plasma membrane (arrowheads) and calreticulin as red (Alexa 594, F). The merged image (G) indicates that Kir6.2 is partly colocalized with calreticulin in the endoplasmic reticulum. Bars: A = 0.1 mm; B = 50 μm; C, D = 20 μm; E-G = 20 μm. Inset bar = 10 μm.
Immunofluorescence Double Staining
To determine whether the Kir6.1 is localized in the mitochondria of the rat cardiomyocytes, immunofluorescence double staining was performed. Immunoreactivity of Kir6.1 was detected as green fluorescence (Alexa 488, Figure 2E), and immunoreactivity of cytochrome c as red fluorescence (Alexa 594, Figure 2F). When both images were merged, the yellow fluorescence was detected in the cytoplasm (Figure 2G), indicating that Kir6.1 is overlapped on the mitochondria.
Immunofluorescence double staining was also performed to determine whether the Kir6.2 is localized in the ER. The expression of Kir6.2 was detected as green fluorescence in the cytoplasm and also detectable on the plasma membrane (Alexa 488, Figure 3E), and the expression of calreticulin, a multifunctional, highly conserved Ca2+-binding protein localized to the ER, detected as red fluorescence (Alexa 594, Figure 3F). When both images were merged, the yellow fluorescence was detected in the cytoplasm (Figure 3G), indicating that Kir6.2 is overlapped on the ER.
Immunoelectron Microscopy for Kir6.1 and Kir6.2
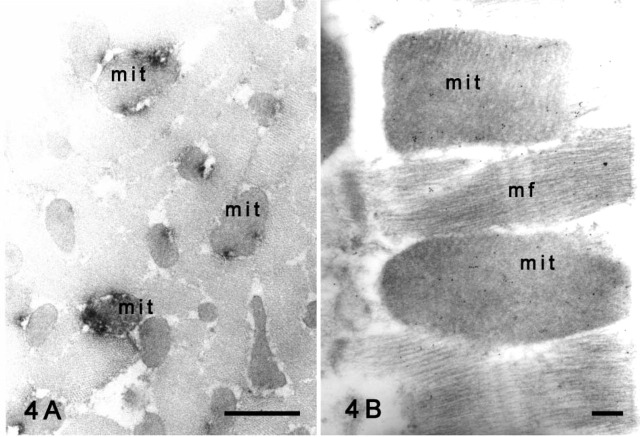
Electron microscopy after the preembedding method revealed the subcellular localization of Kir6.1 in the cardiomyocytes. The punctate immunoreaction products of Kir6.1 observed by light microscopy were detected in the mitochondria of the cardiomyocytes (Figure 4A). No specific labeling was observed on the cell membrane or ER of the rat cardiomyocytes. With the postembedding method, immunogold particles representing Kir6.1 were clearly observed in the mitochondria (Figure 4B), and fewer particles were seen in the myofilaments, nucleus, or cytoplasm of the cardiomyocytes. No specific labeling was observed on the cell membrane.
Figure 4.
Immunoelectron micrographs showing Kir6.1 protein in rat cardiomyocytes. (A) Preembedding method. The immunoreactivity with Kir6.1 is shown in the mitochondria (mit) of the transverse section. (B) Postembedding method. Most gold particles representing Kir6.1 have been deposited in the mitochondria (mit), but some are present in the cytoplasm and myofilaments (mf) in a back-grand level. Bars: A = 1 μm; B = 200 nm.
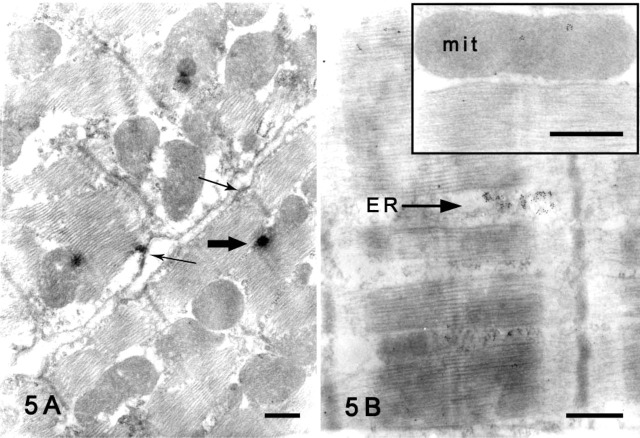
By contrast, Kir6.2 immunoreactivity was observed in the ER and the cell membrane of the rat cardiomyocytes with preembedding method (Figure 5A). Immunogold particles representing Kir6.2 were concentrated in the ER (Figure 5B) with a postembedding method. A few particles were also observed in the mitochondria (Figure 5B, inset).
Figure 5.
Immunoelectron micrographs showing Kir6.2 protein in rat cardiomyocytes. (A) Preembedding method. Immunoreactivity with Kir6.2 was shown in the endoplasmic reticulum (ER, bold arrow) and cell membrane (thin arrows) of cardiomyocytes. (B) Postembedding method. Dense deposition of colloidal gold particles representing Kir6.2 is seen in the ER. Inset micrograph shows that a few gold particles have been deposited in the mitochondria (mit). Bars = 500 nm.
Statistical Analysis
The density of colloidal gold particle labeling was calculated in the mitochondria and outside the mitochondria. With anti-Kir6.1 antibody, particle density in the mitochondria—that is, the number of labeled gold particles per unit area of mitochondria (91.03 ± 9.57/μm2)—was significantly higher than that of outside the mitochondria (25.88 ± 2.81/μm2, p<0.0001, Figure 6A). With anti-Kir6.2 antibody, particle density in the mitochondria (19.33 ± 1.80/μm2) was also significantly higher than outside the mitochondria (12.0 ± 1.25/μm2, p<0.01, Figure 6B).
Figure 6.

Statistical analysis of Kir6.1 and Kir6.2 density in rat cardiomyocytes detected by the postembedding method. (A) The density of colloidal gold particles representing Kir6.1 in the mitochondria (91.03 ± 9.57 per μm2) was significantly higher than outside the mitochondria (28.55 ± 2.81 per μm2, p<0.0001). (B) The density of colloidal gold particles representing Kir6.2 in the mitochondria (19.03 ± 1.80 per μm2) was significantly higher than outside the mitochondria (12.0 ± 1.25 per μm2, p<0.01).
Discussion
Biochemical and immunohistochemical methods revealed the localization of Kir6.1 and Kir6.2 in the subcellular fractions and structures of rat cardiomyocytes. The cellular fractions of mitochondria, cell membrane and microsome were confirmed by their marker antibodies, such as cytochrome c, a marker of mitochondrium; Na+/K+ ATPase β1, a marker of cell membrane; and calreticulin, a marker of ER. Each cellular fraction only reacted to its own marker antibody without to the other antibodies. The mitochondrial fraction was also confirmed by electron microscopy. With these cellular fractions, Western blot analysis was processed.
A polyclonal antibody was generated in a rabbit against the C-terminal domain of rat Kir6.1. The antibody was affinity-purified to investigate the distribution of Kir6.1 protein in the rat heart. The specificity of the antibody was assessed by Western blot analysis. This antibody recognized a prominent band (∼43 kDa) in mitochondrial and microsome fractions, and a faint band in cell membrane fraction. The immunopositive bands were eliminated after preabsorption with immunizing peptide antigen. Kir6.2 was prominently detected in the microsome fraction, weakly in the cell membrane and mitochondrial fractions. Immunohistochemistry showed that Kir6.1 and Kir6.2 were widely expressed in the cardiomyocytes in the atria and the ventricles as well as smooth muscles of coronary artery. Commercial goat anti-human Kir6.1 antibody showed similar distribution of Kir6.1 in the cardiomyocytes as our new antiserum. Double immunofluorescence staining showed that the fluorescence image of Kir6.1 was overlapped with cytochrome c, but not detected on the cell membrane, and the fluorescence image of Kir6.2 was overlapped with calreticulin and on the cell membrane. Preembedding immunoelectron microscopy showed that the punctate Kir6.1 immunoreaction products observed by light microscopy were localized in the mitochondria, and Kir6.2 immunoreaction products were localized in the ER and cell membrane. With the postembedding method, the density of colloidal gold particles representing Kir6.1 that deposited on the mitochondria was significantly higher than outside the mitochondria. The colloidal gold particles representing Kir6.2 were mainly concentrated in the ER and also in the mitochondria. These data strongly indicate that the Kir6.1 was mainly localized in the mitochondria and Kir6.2 was mainly localized in the ER and also in the mitochondria.
The expression of Kir6.1 protein in rat cardiomyocytes and coronary arteries was consistent with the wide distribution of Kir6.1 mRNA in rat heart (Inagaki et al. 1995; Pountney et al. 2001; van Bever et al. 2004) and in the smooth muscle of blood vessels (Yamada et al. 1997; Suzuki et al. 2001; Miki et al. 2002; Li et al. 2003; Miura et al. 2003). The subcellular localization of Kir6.1 in the mitochondria was also consistent with the results in isolated mitochondrial fraction analysis (Lacza et al. 2003) and in isolated ventricular myocytes analysis by colocalized with mitochondrial marker MitoFluor red (Singh et al. 2003). The localization of Kir6.2 in ER and cell membrane in rat cardiomyocytes was similar to the results obtained in our previous immunoelectron microscopy study in neurons and glial cells of rat brain (Zhou et al. 2002). The Kir6.2 immunoreactivity on the ER may represent the site of production before the formation in functional octamers with sulphonylurea receptors on the plasma membrane (Clement et al. 1997; Inagaki et al. 1997; Inagaki and Seino 1998; Zerangue et al. 1999).
Recently, a gene transfer method did not show evidence that mitochondria contain Kir6.1 or Kir6.2 (Seharaseyon et al. 2000). Furthermore, an immunoblot analysis denied that mitochondria contain Kir6.1 or Kir6.2 (Kuniyasu et al. 2003). Thus it was advocated that both Kir6.1 and Kir6.2 were not localized in the mitochondria. On the other hand, Kir6.1 and Kir6.2 were contained in mitochondria by immunoblot analysis and immunoelectron microscopy with mitochondrial fractions (Lacza et al. 2003) and with rat-isolated ventricular myocytes by immunocytochemistry (Singh et al. 2003). The methods that Kuniyasu et al. (2003) used to prepare their antibodies and show the specificities by immunoblot analysis with fusion proteins by gene vector transfer are almost the same as those used by Singh et al. (2003), but they reached different conclusions. The reason for such sharp discrepancies on the subcellular localizations of Kir6.1 and Kir6.2 is still unknown, but interestingly, Singh et al. (2003) and Lacza et al. (2003) pointed out by computer analysis that purified mitochondrial targeting sequence of amino acids colocalizes with Kir6.1 and Kir6.2 proteins.
In the present study, we showed that mitochondria contain Kir6.1 and Kir6.2 by both Western blot analysis and immunohistochemistry. We emphasize that Kir6.1 was mainly localized in the mitochondria and Kir6.2 mainly localized in the ER and also in the mitochondria and plasma membrane. Both Kir6.1 and Kir6.2 may be candidates for mitoKATP channel subunits. Further study is required to determine whether Kir6.1 or Kir6.2 assembles with sulphonylurea receptors on these subcellular structures and forms functional channels, because coexpression with SUR2A or SUR2B on mitochondria still remains to be revealed.
Acknowledgments
This work was supported by a part of Research Grants of Akita University to H.A. and by a part of the Kitasato Graduate School of Medical Sciences (2002-2003) to K.K.
The authors wish to thank Prof. H. Kondo (Tohoku University, School of Medicine) for his kind suggestion for this manuscript. The authors also thank the staff of Bioscience Research-Education Center of Akita University School of Medicine for their useful help and Mr. Nibe for his technical assistance in this study.
Literature Cited
- Akao M, Otani H, Horie M, Takano M, Kuniyasu A, Nakayama H, Kouchi I, et al. (1997) Myocardial ischemia induces differential regulation of KATP channel gene expression in rat hearts. J Clin Invest 100: 3053–3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement JP, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. (1997) Association and stoichiometry of K(ATP) channel subunits. Neuron 18: 827–838 [DOI] [PubMed] [Google Scholar]
- Das B, Sarkar C. (2003) Mitochondrial K ATP channel activation is important in the antiarrhythmic and cardioprotective effects of non-hypotensive doses of nicorandil and cromakalim during ischemia/reperfusion: a study in an intact anesthetized rabbit model. Pharmacol Res 47: 447–461 [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Seino S. (1997) Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett 409: 232–236 [DOI] [PubMed] [Google Scholar]
- Inagaki N, Seino S. (1998) ATP-sensitive potassium channels: structures, functions, and pathophysiology. Jpn J Physiol 48: 397–412 [DOI] [PubMed] [Google Scholar]
- Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, Seino Y, et al. (1995) Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem 270: 5691–5694 [DOI] [PubMed] [Google Scholar]
- Inoue I, Nagase H, Kishi K, Higuti T. (1991) ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 352: 244–247 [DOI] [PubMed] [Google Scholar]
- Itoh H, Komatsuda A, Ohtani H, Wakui H, Imai H, Sawada K, Otaka M, et al. (2002) Mammalian HSP60 is quickly sorted into the mitochondria under conditions of dehydration. Eur J Biochem 269: 5931–5938 [DOI] [PubMed] [Google Scholar]
- Kuniyasu A, Kaneko K, Kawahara K, Nakayama H. (2003) Molecular assembly and subcellular distribution of ATP-sensitive potassium channel proteins in rat hearts. FEBS Lett 552: 259–263 [DOI] [PubMed] [Google Scholar]
- Lacza Z, Snipes JA, Miller AW, Szabo C, Grover G, Busija DW. (2003) Heart mitochondria contain functional ATP-dependent K+ channels. J Mol Cell Cardiol 35: 1339–1347 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Li L, Wu J, Jiang C. (2003) Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. J Membr Biol 196: 61–69 [DOI] [PubMed] [Google Scholar]
- Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, et al. (2002) Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med 8: 466–472 [DOI] [PubMed] [Google Scholar]
- Miura H, Wachtel RE, Loberiza FR, Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. (2003) Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res 92: 151–158 [DOI] [PubMed] [Google Scholar]
- Noma A. (1983) ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148 [DOI] [PubMed] [Google Scholar]
- Pountney DJ, Sun ZQ, Porter LM, Nitabach MN, Nakamura TY, Holmes D, Rosner E, et al. (2001) Is the molecular composition of K(ATP) channels more complex than originally thought? J Mol Cell Cardiol 33: 1541–1546 [DOI] [PubMed] [Google Scholar]
- Seharaseyon J, Ohler A, Sasaki N, Fraser H, Sato T, Johns DC, O'Rourke B, et al. (2000) Molecular composition of mitochondrial ATP-sensitive potassium channels probed by viral Kir gene transfer. J Mol Cell Cardiol 32: 1923–1930 [DOI] [PubMed] [Google Scholar]
- Singh H, Hudman D, Lawrence CL, Rainbow RD, Lodwick D, Norman RI. (2003) Distribution of Kir6.0 and SUR2 ATP-sensitive potassium channel subunits in isolated ventricular myocytes. J Mol Cell Cardiol 35: 445–459 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kotake K, Fujikura K, Inagaki N, Suzuki T, Gonoi T, Seino S, et al. (1997) Kir6.1: a possible subunit of ATP-sensitive K+ channels in mitochondria. Biochem Biophys Res Commun 241: 693–697 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, et al. (2001) Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res 88: 570–577 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, et al. (2002) Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest 109: 509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bever L, Poitry S, Faure C, Norman RI, Roatti A, Baertschi AJ. (2004) Pore loop-mutated rat KIR6.1 and KIR6.2 suppress KATP current in rat cardiomyocytes. Am J Physiol Heart Circ Physiol 287: H850–H859 [DOI] [PubMed] [Google Scholar]
- Van Bueren AM, Moholt-Siebert M, Begley DE, McCall AL. (1993) An immunization method for generation of high affinity antisera against glucose transporters useful in immunohistochemistry. Biochem Biophys Res Commun 197: 1492–1498 [DOI] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. (1997) Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol 499(Pt 3):715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. (1998) ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol 274: C25–37 [DOI] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22: 537–548 [DOI] [PubMed] [Google Scholar]
- Zhou M, Tanaka O, Sekiguchi M, Sakabe K, Anzai M, Izumida I, Inoue T, et al. (1999) Localization of the ATP-sensitive potassium channel subunit (Kir6.1/uK(ATP)-1) in rat brain. Brain Res Mol Brain Res 74: 15–25 [DOI] [PubMed] [Google Scholar]
- Zhou M, Tanaka O, Suzuki M, Sekiguchi M, Takata K, Kawahara K, Abe H. (2002) Localization of pore-forming subunit of the ATP-sensitive K(+)-channel, Kir6.2, in rat brain neurons and glial cells. Brain Res Mol Brain Res 101: 23–32 [DOI] [PubMed] [Google Scholar]