Abstract
The enzyme 20α-hydroxysteroid dehydrogenase (20α-HSD) catalyzes the conversion of progesterone into its inactive form, 20α-hydroxyprogesterone. To gain information about the exact sites of 20α-HSD mRNA expression, we performed in situ hybridization using a 35S-labeled cRNA probe in tissues of adult mice of both sexes. 20α-HSD mRNA was expressed in both male and female gonads. In the ovary, high expression was found in luteal cells of corpora lutea, while much lower expression could be detected in granulosa cells of growing follicles. In the testis, a specific hybridization signal was detected only in Leydig cells. In the female reproductive tract, 20α-HSD mRNA was found in the epithelial cells of the uterine cervix. In the adrenal cortex, only the zona reticularis exhibited specific radiolabeling, the expression being very high in the female and very low in the male. In the skin, specific labeling was restricted to sebaceous glands, the hybridization signal being much higher in the female than in the male. In the liver, 20α-HSD mRNA was found in hepatocytes, with a higher degree of expression in the female. In the kidney, specific labeling was observed in the epithelial cells of distal convoluted tubules, the signal being also much more striking in the female than in the male. In non-reproductive tissues, it clearly appears that the expression of 20α-HSD mRNA is higher in the female than in the male, suggesting that 20α-HSD may play an important role in reducing the intracellular concentration of progesterone originating from the circulation at a much higher level in the female.
Keywords: progesterone catabolism, 20α-hydroxysteroid progesterone, 20α-hydroxysteroid dehydrogenase, mRNA expression, in situ hybridization, mouse, autoradiography
The concentration of active steroids is modulated in peripheral tissues by steroid-forming and steroid-inactivating enzymes. The enzyme 20α-hydroxysteroid dehydrogenase (20α-HSD) converts progesterone into its inactive form, 20α-hydroxyprogesterone. Its role is particularly important in the ovary because it induces luteolysis (Strauss et al. 1972; Penning 1997). In progesterone target tissues, 20α-HSD is probably involved in regulating the amount of progesterone that can bind to progesterone receptors. Recently, the cDNAs encoding rat and rabbit ovarian, bovine testicular, and human skin 20α-HSD have been cloned (Lacy et al. 1993; Mao et al. 1994; Miura et al. 1994; Zhang et al. 2000). 20α-HSD from different species belongs to the aldoketo reductase family. In the rat, 20α-HSD mRNA was highly expressed in the ovary, whereas it was almost undetectable in the uterus, kidney, lung and heart (Mao et al. 1994). In human, high expression of 20α-HSD mRNA has been observed in the liver, mammary gland, and brain, whereas low expression has been detected in the prostate, testis, adrenal, uterus, and keratinocytes (HaCaT) (Zhang et al. 2000). In male and female human kidney, 20α-HSD expression has also been detected by RT-PCR (Rumke-Vogl et al. 2002).
Thus far, the precise localization of 20α-HSD at the cellular level has not been reported. We therefore used in situ hybridization (ISH) to localize the 20α-HSD mRNA in several tissues in adult mice of both sexes to gain information about the exact sites of 20α-HSD expression and to determine whether there is a gender-related influence on the expression of the enzyme.
Materials and Methods
Animals
Four adult male (26–30 g) and female (24–27 g) C57BL6 mice were housed under constant temperature (21 ± 1C) and light (lights on from 0600 to 2000 hr) regimen. The animals received Purina Chow (Ralston-Purina; St Louis, MO) and tapwater ad libitum. The experiment was conducted in an animal facility approved by the Canadian Council on Animal Care (CCAC) and by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The study was performed in accordance with the CCAC Guide for Care and Use of Experimental Animals. The animals were all perfused between 0900 and 1000 hr for histological procedures as described below. The females were on diestrous day 1.
Histological Procedures
All the animals were perfused transcardially with 50 ml 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The different tissues, i.e., liver, kidney, jejunum, pancreas, skin, adrenal, pituitary, testis, prostate, ovary, uterus, vagina, and mammary gland were excised and postfixed in the same fixative for 24 hr at 4C. The tissues were placed in 15% sucrose in 0.1 M phosphate buffer before being quickly frozen in isopentane chilled in liquid nitrogen.
In Situ Hybridization
Frozen sections (10 μm thick) were serially cut at −20C and mounted on gelatin- and poly-l-lysine-coated slides. The vector used for the production of cRNA probe was constructed by insertion into a pBSKSII+ vector (Stratagene; La Jolla, CA) of a cDNA fragment of 232 bp of mouse 20α-HSD (GenBank accession number AB059565). The cDNA fragment located at position 36–288 downstream from the ATG start codon was obtained by amplification using the polymerase chain reaction (PCR). ISH with the antisense and sense 35S-labeled cRNA probes was performed as previously described (Givalois et al. 1997). Briefly, the sections were prehybridized at room temperature (RT) in a humid chamber for 2 hr in 450 μl/slide of a prehybridization buffer containing 50% formamide, 5 × SSPE (1 × SSPE being 0.1 M NaCl, 10 mM NaH2PO4, pH 7.4, mM EDTA), 5 × Denhart's buffer, 200 mg/ml denatured salmon testis DNA (Sigma), 200 μg/ml yeast tRNA, 2 μg/ml Poly A (Boeringher-Mannheim; Montreal, PQ, Canada) and 4% dextran sulfate. After prehybridization treatment, 100 μl hybridization mixture (prehybridization buffer containing in addition 10 mM dithiothreitol and 35S-labeled cRNA probe at a concentration of 10 × 106 cpm/ml) was spotted on each slide, sealed under a coverslip, and incubated at 37C overnight (15–20 hr) in a humid chamber.
After hybridization, coverslips were removed and slides were rinsed in 2 × SSC at RT for 30 min. Sections were digested by RNase A (20 μg/ml in 2 × SSC) at 37C for 30 min, rinsed in decreasing concentrations of SSC (2 × SSC and 1 × SSC) for 30 min at RT, washed in 0.5 × SSC for 30 min at 37C, followed by 90 min at RT in 0.5 × SSC, at 60C in 0.1 × SSC, and finally for 30 min at RT in 0.1 × SSC.
The sections were then dehydrated and exposed to
Kodak Biomax MR films for 3–8 days before being coated with liquid photographic emulsion (Kodak-NTB2; diluted 1:1 with water). Slides were exposed for 1–45 days, developed in Dektol developer (Kodak; Rochester, NY) for 2 min, and fixed in rapid fixer (Kodak) for 4 min. Thereafter, tissues were rinsed in running water for 30 min, counterstained with hematoxylin, and rapidly dehydrated through graded concentrations of ethanol, cleared in toluene, and cover-slipped with Permount (Fisher Scientific; Montreal, PQ, Canada).
Results
After 3–8-day exposure of the films, specific labeling was found in the ovary, uterine cervix, mammary gland, testis, skin, adrenal cortex, liver, and kidney. No specific hybridization signal could be detected in the vagina, prostate, pituitary, jejunum, and pancreas. From photographic emulsion-coated sections, information could be obtained about the cell types expressing 20α-HSD mRNA in the different tissues. In the ovary, as shown in X-ray films and emulsion-coated slides after a short exposure time (7 days), labeling was restricted to corpora lutea (Figure 1 and 2). Because the mice were in diestrous day 1, only old corpora lutea were present. Cell counting performed on at least two corpora lutea per ovary (four mice) established that approximately 30% of luteal cells were labeled. At longer exposure times (28 days), weak specific labeling was also observed in granulosa cells of growing follicles at all stages of development (Figure 3).
Figure 1.
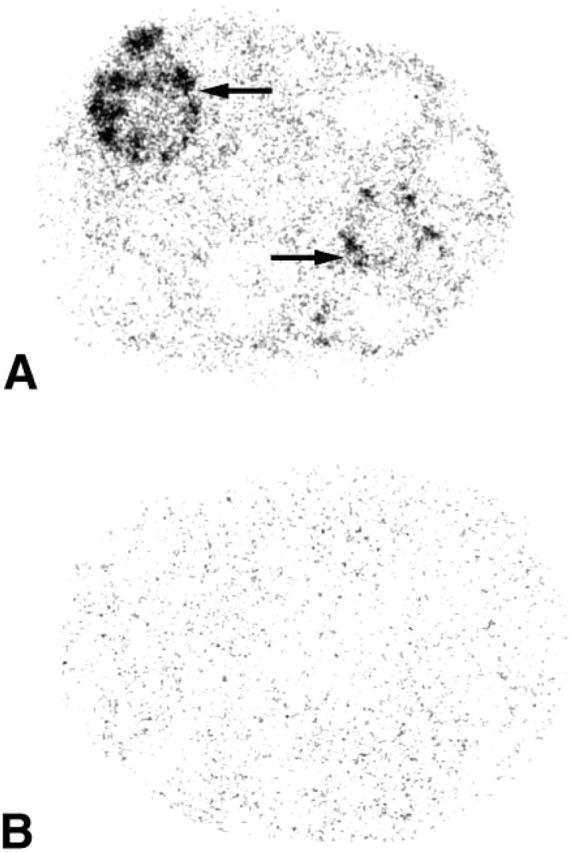
X-ray film autoradiographs illustrating the localization of 20α-HSD mRNA in the ovary. (A) Section hybridized with the antisense riboprobe. Labeling is observed in two corpora lutea (→). (B) Consecutive section hybridized with the sense riboprobe. Only a weak diffuse background can be observed. Exposure time 3 days.
Figure 2.
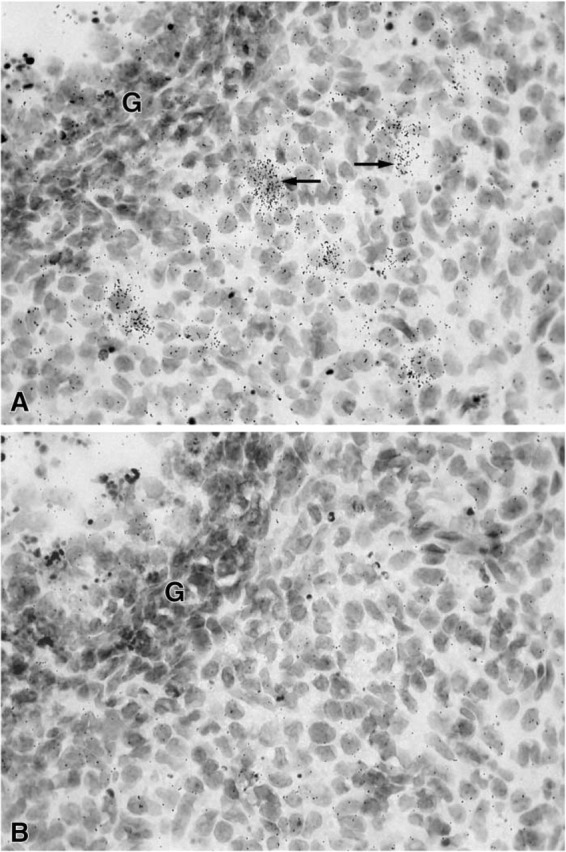
(A) Micrographs showing radiolabeling of luteal cells (→) in a corpus luteum after a short exposure time (7 days). (B) Consecutive section hybridized with the sense probe. Very weak labeling can be observed. G, granulosa cells. Original magnification ×700.
Figure 3.
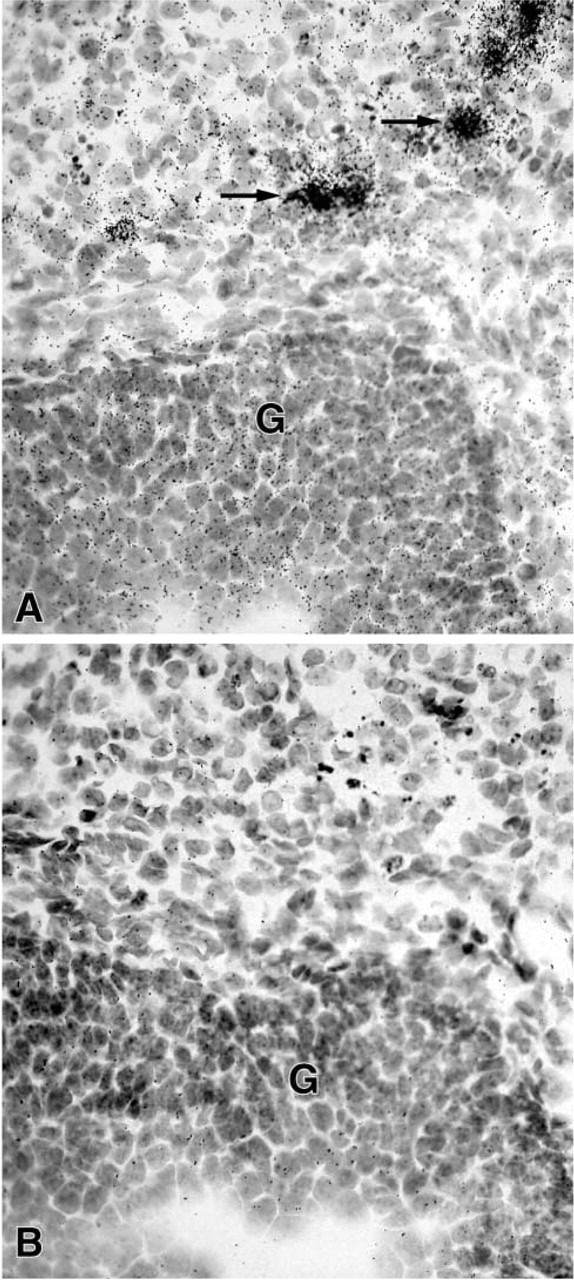
(A) Micrograph in section through the ovary after an exposure time of 28 days. Specific labeling can be seen over granulosa cells (G) of a follicle. Luteal cells are strongly labeled (→). (B) Consecutive section hybridized with the sense probe. Only diffuse labeling can be observed. G, granulosa cells. Original magnification ×470.
In the uterine cervix, the hybridization signal was found in both endocervix and exocervical epithelial cells, the stroma being devoid of any specific labeling (Figure 4). In the female mouse mammary gland, weak specific labeling was restricted to stromal cells surrounding ducts (Figure 5). Typical acini could not be observed. In the male mouse mammary gland, no specific labeling could be detected in any cell type at the longest time interval studied (45 days). In the testis, a specific hybridization signal was found only over Leydig cells, the tubules being devoid of any specific reaction (Figure 6).
Figure 4.
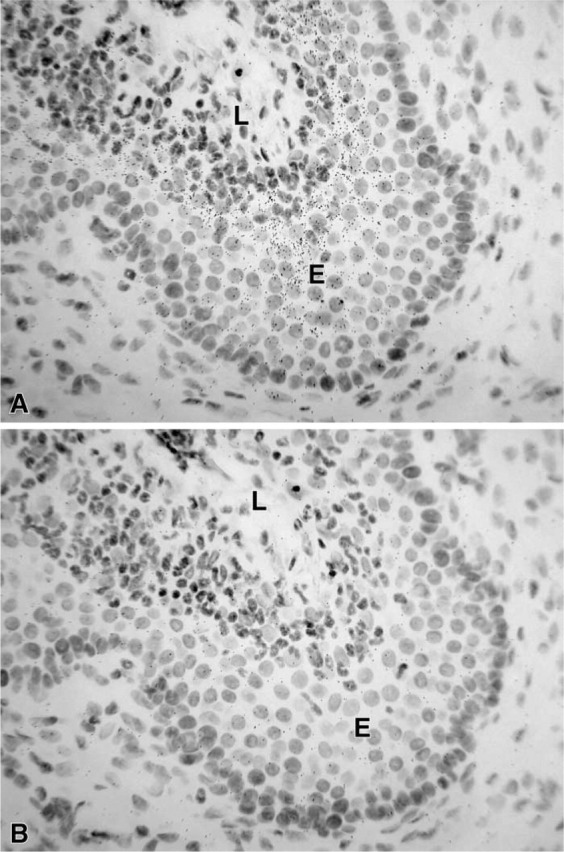
(A) Micrographs illustrating the hybridization signal obtained in the epithelial cells (E) of the uterine cervix. (B) Consecutive section hybridized with the sense probe. No accumulation of silver grains can be detected. L, lumen. Exposure time 21 days. Original magnification ×580.
Figure 5.
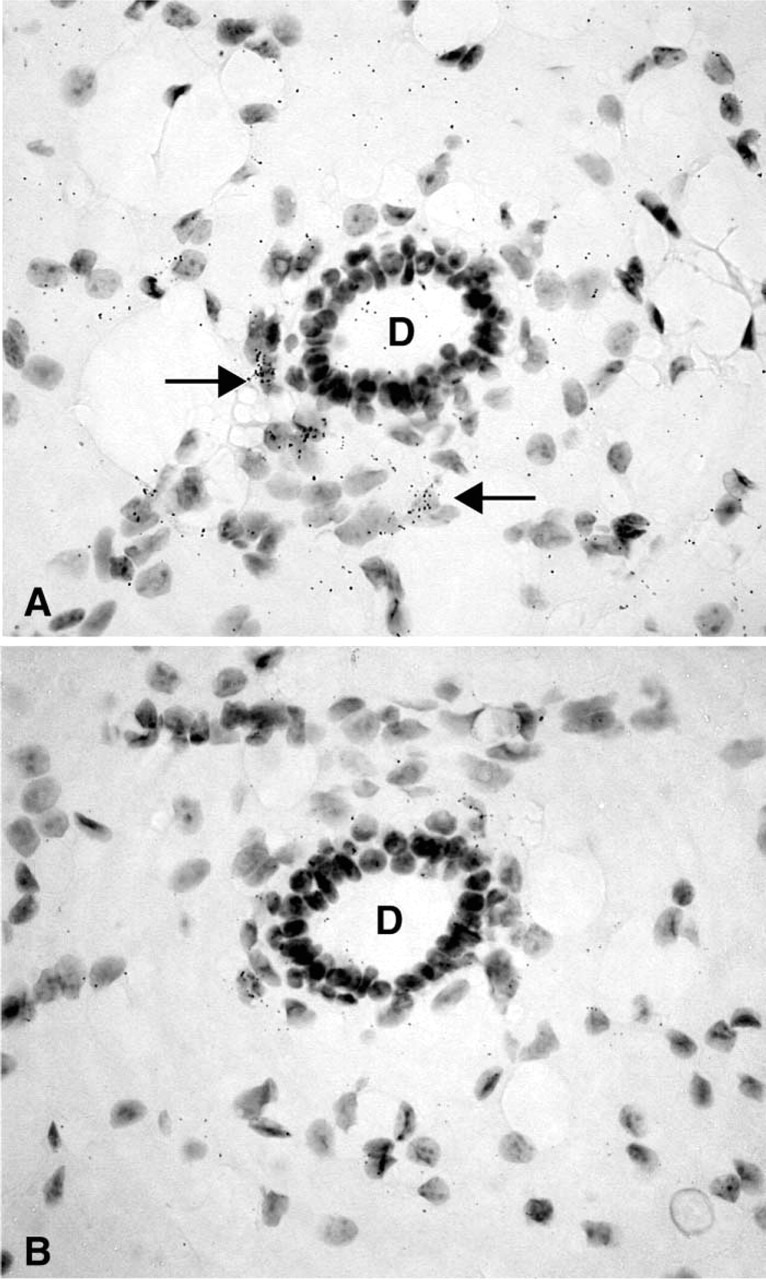
(A) Representative micrograph illustrating the hybridization signal obtained in a mammary gland of a female mouse. Labeling can be observed only over a few stromal cells (→) in proximity to an unlabeled duct (D). (B) Consecutive section hybridized with the sense probe. Only diffuse background can be observed. Exposure time 45 days. Original magnification ×700.
Figure 6.
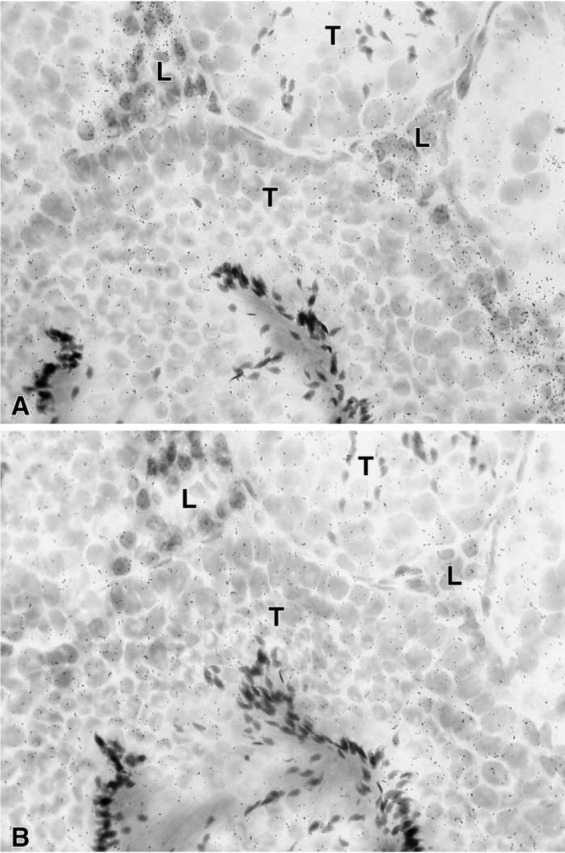
(A) Section through the testis. After hybridization with the anti-sense probe, accumulation of silver grains can be observed only over Leydig cells (L). (B) Consecutive section hybridized with the sense probe. Only diffuse background can be seen. T, seminiferous tubules. Exposure time 21 days. Original magnification ×700.
In female adrenal glands, strong labeling was obtained in the zona reticularis of the adrenal cortex as early as after 1 day of exposure time, whereas the other zones of the cortex and the adrenal medulla were totally negative (Figure 7A). In the male, only weak labeling could be detected in the zona reticularis of the adrenal cortex after the longest exposure time (45 days) (Figure 7B).
Figure 7.
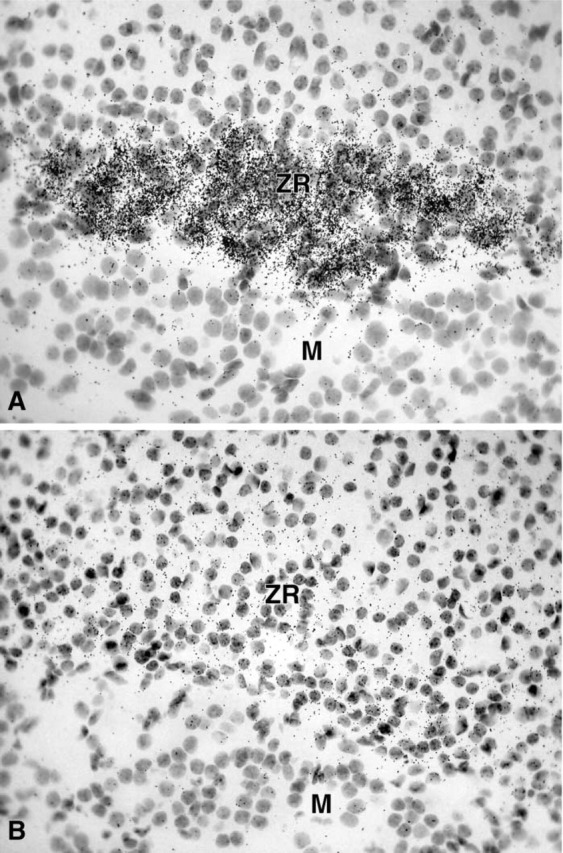
(A) Micrograph showing the strong labeling obtained in the zona reticularis of the adrenal cortex of a female mouse after a 1-day exposure time. (B) In the male, only weak labeling could be obtained in the reticularis zone after 45 days of exposure. ZR, zona reticularis; M, medulla. Original magnification ×570.
In the female mouse skin, a specific hybridization signal was detected in the sebaceous gland cells after 7 days of exposure (Figure 8), but the epidermis, stromal cells, and hair follicles were totally unlabeled. In the male, only a weak signal could be obtained in the sebaceous glands after a long-term exposure (not shown). In the liver, 20α-HSD mRNA appeared to be expressed in both male and female animals, with a higher signal in the female (Figure 9). As observed by light microscopy, specific labeling was detected in all the hepatocytes (Figure 10). In the kidney, specific labeling was observed in a large area located between the medulla and the outer part of the cortex (Figure 11). As observed for the other tissues, the expression of the enzyme mRNA was higher in the female than in the male (Figure 11). At the light microscopic level, we observed that silver grains were overlying the epithelial cells lining the distal convoluted tubules, whereas the glomeruli, proximal convoluted tubules, collecting tubules, and blood vessels were unlabeled (Figure 12). In all the tissues examined, hybridization with the sense probe generated only a light uniform labeling (for examples, see Figures 1, 2, 4, 5, 6, 8, 10, and 12).
Figure 8.
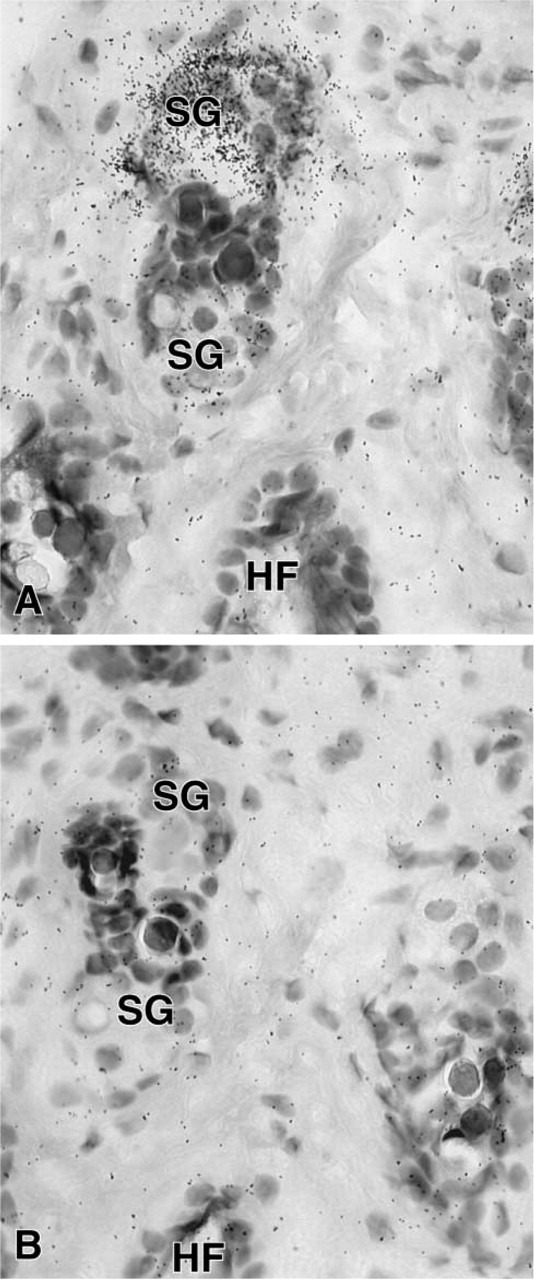
(A) Section through the dorsal skin of a female mouse. After hybridization with the antisense probe, labeling is detected over a sebaceous gland (SG), whereas hair follicle (HF) and stromal cells are unlabeled. (B) In control adjacent section, only diffuse background can be seen. Exposure time 7 days. Original magnification ×670.
Figure 9.
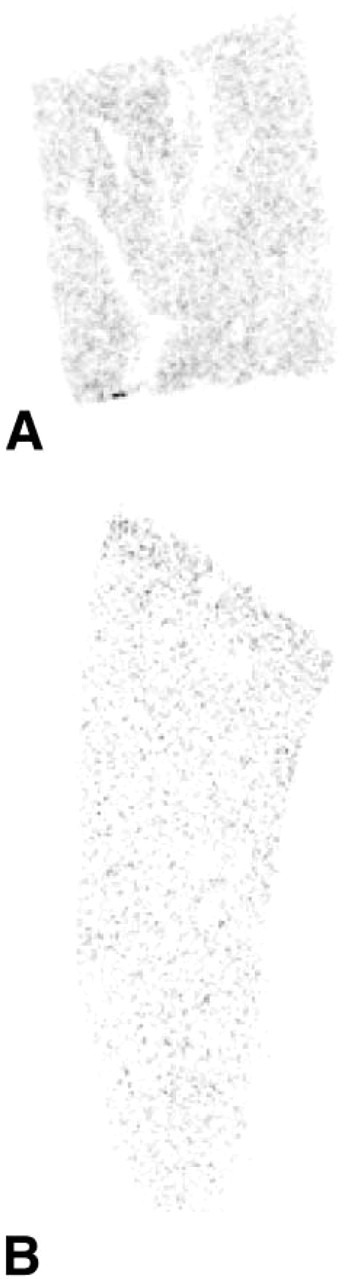
X-ray autoradiograph showing localization of 20α-HSD mRNA in sections through the liver of a female (A) and a male (B) mouse. Labeling is much more striking in female than in male liver. Exposure time 3 days.
Figure 10.
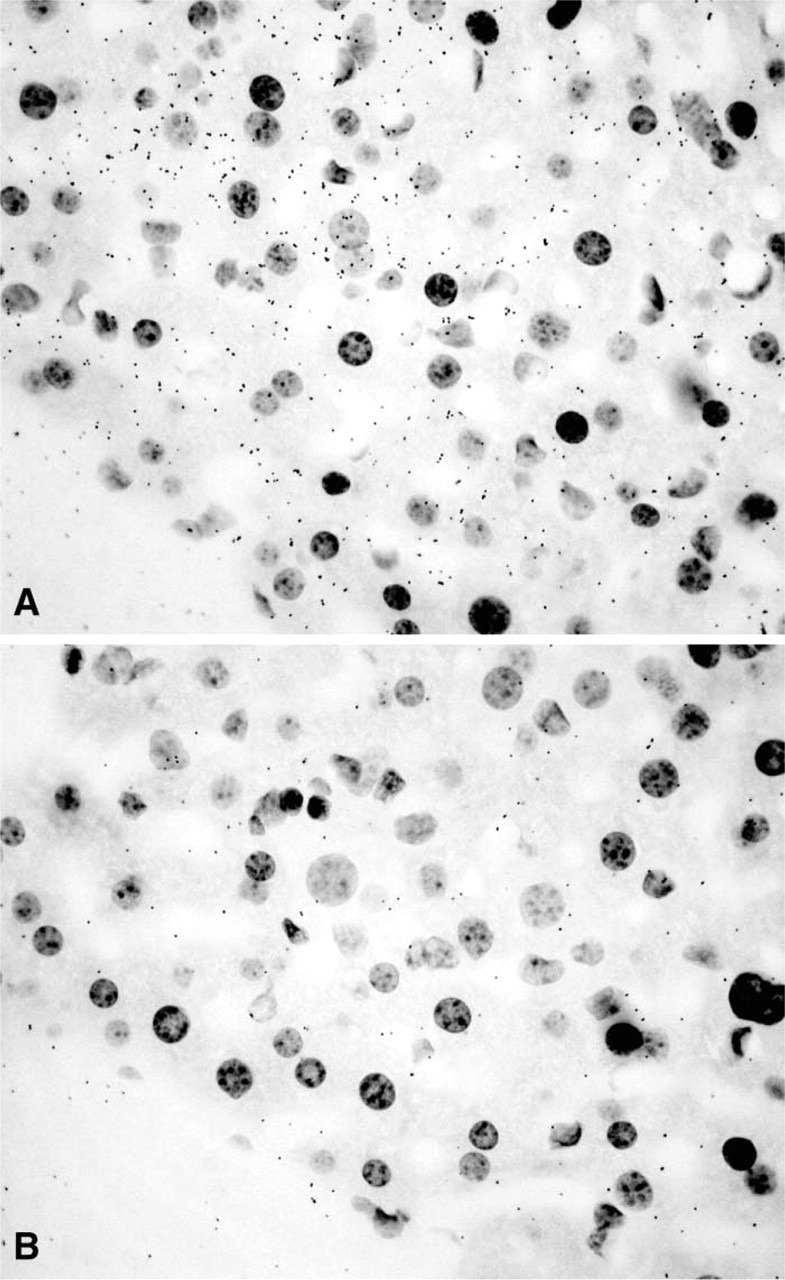
(A) Micrograph of a liver section from a female mouse showing labeling of hepatocytes. (B) Adjacent control section obtained after hybridization with the sense probe. Only diffuse background can be detected. Exposure time 7 days. Original magnification ×730.
Figure 11.
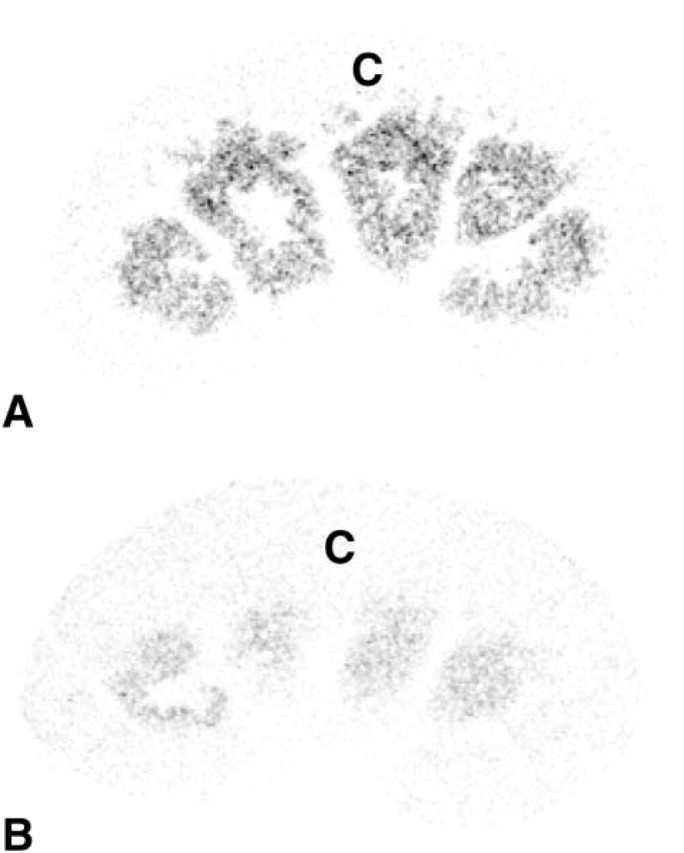
X-ray autoradiograph showing kidney sections from a female (A) and a male (B) mouse. Radiolabeling is present in a large area extending between the cortex (C) and the medulla. The intensity of labeling is higher in the female than in the male. Exposure time 3 days.
Figure 12.
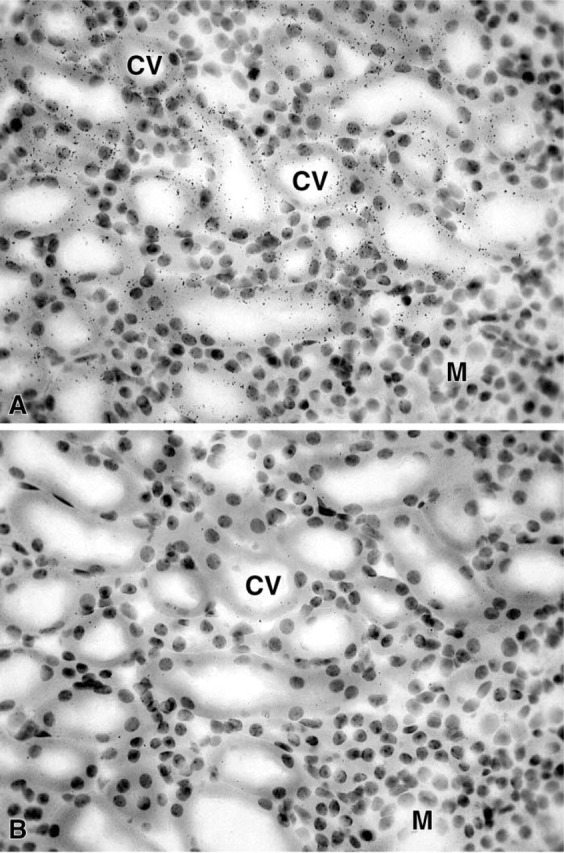
(A) Micrograph illustrating 20α-HSD mRNA expression in a female kidney. Silver grains are seen overlying epithelial cells of distal convoluted tubules (CV). The medulla region (M) is devoid of any labeling. (B) In an adjacent control section, very few silver grains can be seen. Exposure time 7 days. Original magnification ×460.
Discussion
The enzyme 20α-HSD plays an important role in the regulation of the intracellular concentration of progesterone. Cloning of the cDNA encoding mouse 20α-HSD has allowed studies on the histological localization of 20α-HSD mRNA in a series of male and female mouse tissues. In the ovary, strong labeling was observed in about 30% of luteal cells in corpora lutea, while a much weaker signal was obtained in granulosa cells of growing follicles. These findings support the data indicating that, in the rat ovary, 20α-HSD activity was much higher in luteal than in non-luteal cells (Seong et al. 1992). 20α-HSD plays a pivotal role in the regulation of progesterone secretion by corpora lutea. It is generally admitted, however, that there are two types of luteal cells: the luteinizing granulosa cells, which have a large cytoplasm with small lipid droplets, and the theca lutein cells, which are smaller but contain large lipid droplets. It was not possible using frozen sections to establish which luteal cell type(s) was involved in 20α-HSD mRNA expression. On the other hand, it has been shown that, in the rat ovary, all the luteal cells express the enzyme 3β-hydroxysteroid dehydrogenase and can therefore be involved in progesterone secretion (Dupont et al. 1990). In the rat, prolactin strongly inhibits the expression of 20α-HSD, thus contributing to maintenance of progesterone at high levels during pregnancy (Albarracin et al. 1994). The role of 20α-HSD weakly expressed in granulosa cells remains to be clarified.
In the female reproductive tract, 20α-HSD mRNA expression was detected only in the epithelial cells of the uterine cervix. The role of the enzyme might be related to the delivery process. It has been reported that, in mice lacking 5α-reductase type 1, an enzyme that is also involved in progesterone catabolism and is expressed in the cervix, there was a failure of cervical ripening, leading to abnormal delivery (Mahendroo et al. 1999). Such data suggest that 20α-HSD activity does not play a major role in the catabolism of progesterone in the cervix, at least during parturition.
In the mammary gland of the female mouse, weak 20α-HSD expression was detected only in stromal cells surrounding the ducts. Progesterone receptors have been found to be expressed in both epithelial cells of the ducts and stromal cells in the mouse mammary gland (Fenn et al. 1998). It therefore appears that progesterone metabolism is mainly restricted to stromal cells in mammary glands of the cycling mouse.
In the mouse testis, the hybridization signal was observed only in Leydig cells. The testicular localization of the enzyme has already been reported in the bovine and human species (Warren et al. 1993; Zhang et al. 2000). The low expression of 20α-HSD mRNA reported in the human testis (Zhang et al. 2000) can be explained by the fact that the expression of the enzyme is restricted to Leydig cells, which represent only a small proportion of the total number of testicular cells. The role of 20α-HSD might be related to some protective effect from progesterone in testicular tissue.
In the adrenal glands, 20α-HSD mRNA expression was restricted to the cells of the zona reticularis. The hybridization signal was very high in the female and very low in the male. From pig adrenal cytosol, Nakajin et al. (1989) have purified two proteins exhibiting 20α-HSD activities, which they named 20α-HSD I and II. We have recently reported low expression of 20α-HSD mRNA in total adrenal glands in whole human adrenal glands (Zhang et al. 2000). This low expression of 20α-HSD mRNA might be explained by the restricted expression of 20α-HSD mRNA to the zona reticularis, which consists of a few layers of cells and represents a very small proportion of the total number of adrenal cells. The physiological role of 20α-HSD in the adrenal cortex remains to be clarified.
In the skin, 20α-HSD mRNA was strongly expressed in sebaceous glands, but no specific labeling could be detected in the hair follicle and stromal cells or in the epidermis. The presence of progesterone receptors has been reported in sebaceous glands in the human (Wallace and Smaller 1998). The enzyme might therefore regulate the availability of circulating and locally produced progesterone for progesterone receptors and thus control the influence of progesterone on sebaceous gland cell activity. Because it has been shown that progesterone treatment suppresses estrogen receptors in the monkey sex skin (West et al. 1990), one role of progesterone might be to regulate estrogen receptor levels that are expressed in sebaceous glands (Pelletier 2000). The very low expression of 20α-HSD mRNA in the male mouse skin is indicative that 20α-HSD mRNA might be positively regulated by ovarian hormones. In fact, we have recently observed that ovariectomy induced a striking decrease in 20α-HSD mRNA levels in mouse skin (unpublished data).
In the kidney, a specific hybridization signal was found in the epithelial cells in the distal convoluted tubules. As in other tissues, the signal was more striking in the female than in the male. In the human kidney, the expression of 20α-HSD has been reported (Rumke-Vogl et al. 2002) but there was no indication about the cell types involved in the expression of the enzyme. Interestingly, progesterone receptors have been found in epithelial cells of distal convoluted tubules (Rumke-Vogl et al. 2002). From the results obtained in the mouse and human kidney, it appears that the same epithelial cells can express both progesterone receptors and the enzyme that inactivates progesterone. 20α-HSD might be involved in the fine regulation of the action of progesterone, which has been shown to be a potent mineralocorticoid receptor antagonist (Myles and Kuner 1996). In the liver, 20α-HSD mRNA expression was observed in the hepatocytes, the expression being also higher in the female. Because of the large number of hepatocytes, such data indicate that liver might be considered as a major site of progesterone catabolism.
The absence of any hybridization signal in the prostate, uterus, vagina, and pituitary might be explained by a low expression of 20α-HSD that could not be detected by ISH in these tissues. We might also consider the possibility that, in these four tissues, a fine regulation of intracellular progesterone concentrations is not critical for hormonal activity. By RT-PCR, Zhang et al. (2000) have shown low expression of 20α-HSD mRNA in human prostate and uterus, whereas in the present studies we were unable to detect any hybridization signal in the same mouse tissues. These apparent species differences might be explained by a lower sensitivity of ISH compared to that obtained with the highly sensitive RT-PCR technique.
In summary, the present results on the localization of 20α-HSD mRNA in mouse tissues indicate that the enzyme is highly expressed in the male and female gonads and in the uterine cervix. In non-reproductive tissue, the expression of 20α-HSD mRNA is always higher in the female than in the male, thus suggesting that the enzyme plays a predominant role in the inactivation of locally produced or circulating progesterone, which is much higher in the female. Other studies involving ovariectomy and hormone replacement therapy are required to clarify the role of ovarian steroids in the regulation of 20α-HSD expression.
Acknowledgments
The ATLAS program was supported by Genome Canada and Genome Québec.
Literature Cited
- Albarracin CT, Parmer TG, Duan WR, Nelson SE, Gibori G. (1994) Identification of a major prolactin-regulated protein as 20 alpha-hydroxysteroid dehydrogenase: coordinate regulation of its activity, protein content, and messenger ribonucleic acid expression. Endocrinology 89:2453–2460 [DOI] [PubMed] [Google Scholar]
- Dupont E, Zhao HF, Rhéaume E, Simard J, Luu-The V, Labrie F, Pelletier G. (1990) Localization of 3beta-hydroxysteroid dehydrogenase/A5-A4-isomerase in rat gonads and adrenal glands by immunocytochemistry and in situ hybridization. Endocrinology 127:1394–1403 [DOI] [PubMed] [Google Scholar]
- Fenn JL, Roafat AM, Haslann SL. (1998) Mammary gland growth and development from the postnatal period to postmenopause: ovarian steroid receptor ontogeny and regulation in the mouse. J Mam Gland Biol Neopl 3:7–22 [DOI] [PubMed] [Google Scholar]
- Givalois L, Li S, Pelletier G. (1997) Age-related decrease in the hypothalamic CRH mRNA expression is reduced by dehydroepiandrosterone (DHEA) treatment in male and female rats. Mol Brain Res 48:107–114 [DOI] [PubMed] [Google Scholar]
- Lacy WR, Washenick KJ, Cook RG, Dunbar BS. (1993) Molecular cloning and expression of an abundant rabbit ovarian protein with 20 alpha-hydroxysteroid dehydrogenase activity. Mol Endocrinol 7:58–66 [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Porter A, Russell DW, Arnword R. (1999) The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 13:981–992 [DOI] [PubMed] [Google Scholar]
- Mao J, Duan WR, Albarracin CT, Parmer TG, Gibori G. (1994) Isolation and characterization of a rat luteal cDNA encoding 20 alpha-hydroxysteroid dehydrogenase. Biochim Biophys Acta 89:363–364 [DOI] [PubMed] [Google Scholar]
- Miura R, Shiota K, Noda K, Yagi S, Ogawa T, Takahashi M. (1994) Molecular cloning of cDNA for rat ovarian 20 alpha-hydroxysteroid dehydrogenase (HSD1). Biochem J 299:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles K, Kuner JW. (1996) Progesterone binding to mineralocorticoid receptors: in vitro and in vivo studies. Am J Physiol 270:E601–607 [DOI] [PubMed] [Google Scholar]
- Nakajin S, Kawai Y, Ohno S, Shinoda M. (1989) Purification and characterization of pig adrenal 20 alpha-hydroxysteroid dehydrogenase. J Steroid Biochem 33:1181–1189 [DOI] [PubMed] [Google Scholar]
- Pelletier G. (2000) Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol 15:1261–1270 [DOI] [PubMed] [Google Scholar]
- Penning TM. (1997) Molecular endocrinology of hydroxysteroid dehydrogenase. Endocrine Rev 18:281–305 [DOI] [PubMed] [Google Scholar]
- Rumke-Vogl C, Börh V, Hermann SM, Anagnatopoulos I, Oelker W, Quinkler J. (2002) Expression of the progesterone receptor and progesterone metabolizing enzymes in the female and male human kidney. J Endocrinol 175:349–364 [DOI] [PubMed] [Google Scholar]
- Seong HH, Shiota K, Noda K, Ogura A, Asano T, Takahashi M. (1992) Expression of activities of two 20 alpha-hydroxysteroid-dehydrogenase isozymes in rat corpora lutea. J Reprod Fertil 96:573–580 [DOI] [PubMed] [Google Scholar]
- Strauss JF, Foley B, Strambaugh R. (1972) 20-hydroxysteroid dehydrogenase activity in the rabbit ovary. Biol Reprod 6:78–86 [DOI] [PubMed] [Google Scholar]
- Wallace ML, Smaller BR. (1998) Estrogen and progesterone receptors in androgenic alopecia versus alopecia areata. Am J Dermatol 20:160–163 [DOI] [PubMed] [Google Scholar]
- Warren JC, Murdock GL, Ma Y, Goodman SR, Zimmer WE. (1993) Molecular cloning of testicular 20 alpha-hydroxysteroid dehydrogenase: identity with aldose reductase. Biochemistry 32:1401–1406 [DOI] [PubMed] [Google Scholar]
- West NB, Carlisle KS, Brenner RM. (1990) Progesterone treatment suppresses estrogen receptor in the sex skin of Macaca nemstrina. J Steroid Biochem 35:481–485 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dufort I, Rheault P, Luu—The V. (2000) Characterization of a human 20 alpha-hydroxysteroid dehydrogenase. J Mol Endocrinol 25:221–228 [DOI] [PubMed] [Google Scholar]


