Abstract
Single-fiber cultures can be used to model satellite cell activation in vivo. Although technical deficiencies previously prevented study of stretch-induced events, here we describe a method developed to study satellite cell gene expression by in situ hybridization (ISH) using protocol modifications for fiber adhesion and fixation. The hypothesis that mechanical stretching activates satellite cells was tested. Fiber cultures were established from normal flexor digitorum brevis muscles and plated on FlexCell dishes with a layer of Vitrogen. After 2 hr of stretch in the presence of BrdU, satellite cells on fibers attached to Vitrogen were activated above control levels. In the absence of activating treatments or mechanical stretch, ISH studies showed 0–6 c-Met+ satellite cells per fiber. Time course experiments demonstrated stable quiescence in the absence of stretch and significant peaks in activation after 30 min and 2 hr of stretch. Frequency distributions for unstretched fiber cultures showed a significantly greater number of quiescent c-Met+ satellite cells than were activated by stretching, suggesting that typical activation stimuli did not trigger cycling in the entire c-Met+ population of satellite cells. These methods have a strong potential to further dissect the nature of stretch-induced activation and gene expression among characterized populations of individual quiescent and activated satellite cells.
Keywords: in situ hybridization, stretch activation, satellite cell, HGF, quiescence, skeletal muscle
Satellite cells are muscle precursor cells that lie between the external lamina and sarcolemma of skeletal muscle fibers. In normal adult muscle these cells are mitotically and metabolically quiescent (Schultz et al. 1978). Once stimulated to leave quiescence, they become activated, a state defined by satellite cell entry into the cell cycle and preparation for DNA synthesis. This initiates the process by which precursors are provided for new muscle formation in growth and repair (Schultz and McCormick 1994). For this reason, satellite cells are essential to normal functions of skeletal muscle. In vivo satellite cells are known to proliferate in response to exercise, stretching, and weight overload, and their proliferation is reduced by loss of weight-bearing in the suspension model of microgravity (Schultz et al. 1994; Norman et al. 2000). However, the mechanism that integrates mechanical stimuli into satellite cell dynamics is not well understood.
According to studies of proliferation, measured using BrdU or [3H]-thymidine incorporation into DNA, hepatocyte growth factor (HGF) and nitric oxide (NO) activate satellite cells from quiescence. HGF is contained in an extract of crushed muscle (CME) and, similar to NO, is demonstrated to activate satellite cells in vivo and in vitro (Bischoff 1986a,1990; Allen et al. 1995; Tatsumi et al. 1998,2001,2002; Anderson 2000; Anderson and Pilipowicz 2002). However, much is still not known about the normal activation pathway or how the population of quiescent satellite cells is distributed on individual normal fibers. In part, this is due to the technical complexity of sampling and studying satellite cells that form such a small constituent of the mass or volume of skeletal muscle tissue. Determining the proportion of satellite cells that is available for activation by a given stimulus would help to interpret the effectiveness of regeneration or growth that follows. C-Met protein, the receptor for HGF, is expressed by both quiescent and activated satellite cells (Cornelison and Wold 1997; Tatsumi et al. 1998; Hawke and Garry 2001), although other gene markers have also been used to study satellite cell populations and responses (e.g., Grounds et al. 1992; Beauchamp et al. 2000; Tamaki et al. 2002).
Isolated cultures of satellite cells have been used for many years to study the dynamics of satellite cell proliferation, gene expression, and myogenic differentiation that ensue after fusion of the precursors. Therefore, isolated cell cultures are useful for simulating some aspects of muscle regeneration in vivo. However, cell cultures are somewhat limited for modeling activation per se, because their isolation disrupts the intercellular relationship between a fiber and quiescent satellite cells. Cell cultures are most useful in exploring activation when they are isolated from older animals because they exhibit a long latent period before activation (DNA synthesis) and can be maintained for control purposes in a quiescent state for almost 48 hr in culture. The initial entry of those satellite-derived cells into cycling activity can then address the process of activation from quiescence (e.g., Tatsumi et al. 2001,2002). Otherwise, dispersed cultures more aptly model tissue responses and new myotube formation after injury (Kästner et al. 2000).
The use of single muscle fibers in culture is an alternative approach to study activation and subsequent events in early regeneration. The method of single-fiber isolation was pioneered by Bekoff and Betz (1976) and firmly established by Bischoff (1986a,b). The method allows the isolation of single intact fibers with satellite cells in their characteristic position, still in a quiescent state beneath the external lamina and not incorporating labeled nucleotides into new DNA under control conditions. This method therefore usefully models many in vivo conditions of the satellite cell microenvironment while maintaining interactions between fibers and satellite cells (Bischoff 1986a,b; Yablonka-Reuveni and Rivera 1997; Allen et al. 1998; Anderson and Pilipowicz 2002). To date, the application of single-fiber cultures to study mechanical signal transduction is not reported.
The FlexCell culture system delivers a stimulus of cyclical stretching to cells in culture. Using this system, stretch was shown to activate isolated quiescent satellite cells in culture within 2 hr, as judged by significant increases in BrdU incorporation (Tatsumi et al. 2001). Cells of other origins are also known to proliferate and enter tissue-specific processes as a result of stretching (Scott et al. 1993; Li and Xu 2000). Stretch-induced activation has not been examined using the single-fiber model because the conditions required for the experiments have not been developed. Although previous experiments without stretching reported a low level of spontaneous activation by satellite cells (Anderson and Pilipowicz 2002), the frequency distribution, denoting the population response by satellite cells, was not reported. We hypothesized that mechanical stimulation via stretch would activate quiescent satellite cells on single fibers, once appropriate conditions for maintaining the cultures were identified. We also applied the ISH protocol to single-fiber cultures to enable observations of molecular events at the level of individual satellite cells. ISH for c-Met mRNA was used to determine the distribution of quiescent satellite cells on single fibers after modifications to the procedure.
Materials and Methods
Reagents
Vitrogen 100 was supplied by Cohesion Technologies (Palo Alto, CA). Dulbecco's modified Eagle's medium (DMEM), antibiotic/antimycotic, chick embryo extract, fetal bovine serum (FBS), trypsin, and gentamycin were supplied by Invitrogen (Carlsbad, CA). Araldite resin was supplied by Cedarlane (Hornby, ONT, Canada). All other electron microscopy materials were obtained from Electron Microscopy Sciences (Fort Washington, PA). BrdU, Serum Replacement-2 (S-9388; different from the serum replacement used in earlier studies by Yablonka-Reuveni and colleagues, and which is currently not available), HGF, diaminobenzidine, anti-BrdU antibody, HRP-linked anti-mouse antibodies, and diethyl-pyrocarbonate (DEPC) were obtained from Sigma (Oakville, ONT, Canada). The c-Met plasmid template was a gift from Dr. Carola Ponzetto (University of Turin, Italy). FlexCell plates were obtained from FlexCell International (Hillsborough, NC), and Falcon 35-mm plates were obtained from Becton Dickinson (Franklin Lakes, NJ).
Plating Fibers with Vitrogen
Fibers from the flexor digitorum brevis muscle (FDB) of mice were isolated according to published methods, as established by Bischoff (1986a,b) and modified by others (Yablonka-Reuveni and Rivera 1994; Kästner et al. 2000; Anderson and Pilipowicz 2002). Once fibers were isolated, they were plated in six-well FlexCell plates with either a rigid or a flexible surface substrate that was pre-cooled and coated with 80 μl pure Vitrogen 100. The rigid plastic layer under the elastomer substrate provided a control un-stretched condition. ISH experiments had fibers plated on 35-mm culture plates (Falcon). Fibers were allowed to adhere for 20 min at 37C and 5% CO2, after which basal growth medium was added to each well (2 ml/well) as reported (Anderson and Pilipowicz 2002) using DMEM, 20% Serum Replacement-2, 1% FBS, 1% antibiotic/antimycotic, and 0.1% gentamycin. Fibers were allowed to attach overnight at 37C and 5% CO2 before stretching. Fiber preparations that contained tissue debris were discarded and not included in experiments.
Resin Sectioning
Fibers attached to culture plates were fixed for 20 min in acid alcohol (90% absolute ethanol, 5% glacial acetic acid, 5% H2O), rinsed in phosphate buffer (pH 7.4), postfixed in 1% osmium tetroxide, and rinsed in double distilled water. After staining en bloc in 8% uranyl acetate for 60 min, fibers were rinsed in water (1 hr) and dehydrated in ethanols and methanol. After infiltration steps using propylene oxide, fibers were embedded in Araldite resin. The flexible substrate was removed from the hardened resin, which was further embedded in methacrylate to identify and protect the Vitrogen layer. Disks of hardened resin-containing fibers were removed from Petri dishes and fibers were identified and marked under a dissecting microscope. Blocks of a few fibers were cut from the resin, trimmed, and sectioned (2 μm thick). Sections were transferred to slides, stained with toluidine blue, viewed under a light microscope, and photographed with Fujichrome professional slide film (ASA 400). Measurements of the Vitrogen layer thickness were made from nine regions adjacent to each of nine different fibers, and calculated as mean ± SEM.
In Situ Hybridization
Fixations using paraformaldehyde and a variation of acid/alcohol fixations were tested. The former is known to preserve RNA and for this reason is often used for ISH (e.g., Garrett and Anderson 1995). Acid/alcohol fixation is also used for fixation of fibers in Vitrogen (Anderson and Pilipowicz 2002) and was modified as follows for these experiments. Fibers were rinsed with RNase-free 1 × PBS (treated with DEPC) and then fixed in acid/alcohol for 10 min. Fixative was removed and fixed fibers were allowed to air-dry in a laminar flow hood for 10–15 min and rinsed three times with RNase-free 1 × PBS and stored in the same solution at 4C until ready to use. The remaining steps of the in situ protocol were carried out as reported to detect c-Met transcripts in individual satellite cells on fibers (Garrett and Anderson 1995; Anderson et al. 1998). Templates for c-Met receptor were obtained by transforming bacteria with a pBluescript SK+ plasmid containing bases 300–1572 of the Met proto-oncogene cDNA (Park et al. 1987; Dietrich et al. 1999), generously supplied by Dr. C. Ponzetto (Maina et al. 1996). Antisense digoxigenin-labeled riboprobes were synthesized according to Boehringer Mannheim protocols, as reported in detail (Anderson and Vargas, 2003). Labeled probes were run on formaldehyde/Agarose gels, transferred to a nylon membrane, and visualized using anti-digoxigenin antibodies and alkaline phosphatase color detection to confirm the probe size of 1.27 kb. The target mRNA was 9 kb. Hybridized transcripts were localized in satellite cells resident on fibers. In other experiments, a sense riboprobe for c-Met showed no signal, similar to other procedural controls that omitted probe, anti-digoxigenin antibody, or color detection steps.
Stretching Single Fibers
After fiber attachment overnight on Vitrogen-coated Flex-Cell plates, 0.02% BrdU was added to medium and fibers were placed immediately in a FlexCell system. Fiber cultures were subjected to mechanical stretching at 4 cycles/min (8 sec on, 7 sec off) with 20 kPa stretch. This applies approximately a 10% stretch (Anderson et al. 1993), which is within the physiological range for muscle contraction. Control plates were also placed in the FlexCell system but did not stretch. In initial experiments, fibers were stretched for 0 or 2 hr. In addition, time course experiments included fibers stretched for 0 hr, 15 min, 0.5-, 1-, 1.5-, 2-, and 3-hr periods. After a given period of cyclical stretch, fibers were maintained at 37C and 5% CO2, still in the presence of BrdU, until 24 hr had elapsed from the initiation of stretching, and then fixed using acid/alcohol. Fibers were immunostained to detect the incorporation of BrdU using anti-BrdU (1:1000) and secondary HRP-linked anti-mouse antibodies (1:300) visualized with diaminobenzidine (25 mg/ml) (Anderson and Pilipowicz 2002). The number of satellite cells that accumulated BrdU per fiber was counted blind, without knowledge of source, by systematically scanning the entire area under each coverslip. Only small numbers of mononuclear cells not adherent to fibers were observed, and they were not counted in this population study because they could not be attributed to a particular fiber of origin. Myonuclei were not positive.
Statistical Analysis
Data were compared by analysis of variance (ANOVA) with repeated measures where appropriate (Statpak; NorthWest Analytical, Portland, OR), with pair-wise comparisons between groups using least significant difference (LSD) tests. Frequency distributions were compared using Chi-square statistics applied to raw data (not percentage data). Significance was determined at the p<0.05 level.
Results
Although mechanical stretching is known to increase activation of quiescent satellite cells in dispersed cultures and in vivo (Tatsumi et al. 2001,2002; Tatsumi et al. personal communication), it is not known whether cultures of isolated muscle fibers can be used to model mechanical activation of satellite cells. Use of fiber cultures would also enable the study of satellite cell gene expression, because these cells are easily accessible to staining procedures. To our knowledge, this is the first report in which satellite cells on single fibers were prepared successfully for ISH studies of c-Met mRNA expression and displayed stretch-induced activation.
In Situ Hybridization Fixation and Staining
Fixation is typically required during immunostaining and ISH protocols, and the latter often employs paraformaldehyde. However, fibers plated on Vitrogen showed a marked and problematic loss of adhesion after brief paraformaldehyde fixation. Here the ISH protocol used to identify c-Met-expressing satellite cells was adapted to incorporate fixation with acid/alcohol, which is used during BrdU immunodetection (Anderson and Pilipowicz 2002). Fixation included the typical ISH wash procedures. This acid/alcohol fixation retained the adhesive properties of Vitrogen without degrading the c-Met mRNA signal supplied by ISH.
ISH procedures produced intense specific staining for c-Met transcripts. The cells were identified in a satellite position outside the sarcolemma, as observed by focusing through the fiber thickness. C-Met-positive cells had a narrow rim of darkly stained cytoplasm located around pale nuclei (Figure 1A). The number of c-Met-positive cells identified in fiber cultures 18 hr after plating and without stretching was 0–6 cells/fiber, with a mean (± SE) of 1.38 ± 0.07. The frequency distribution of c-Met-expressing satellite cells is shown in Figure 1B.
Figure 1.
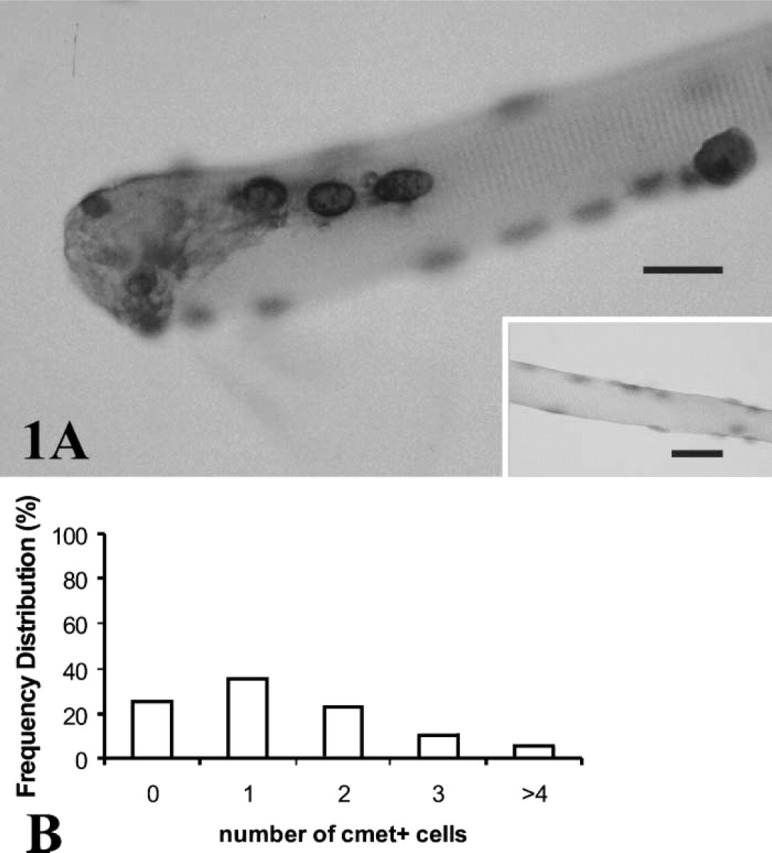
(A) Satellite cells were identified on single fibers using ISH and riboprobes to detect c-Met mRNA. Four cells appear on this fiber, as detected by a dark rim of cytoplasm surrounding paler nuclei, lightly counterstained with hematoxylin. Fiber cytoplasm did not stain for transcripts of c-Met. (Inset) Negative control for the in situ procedure omitting the riboprobe for c-Met shows no signal for mRNA. (B) Frequency distribution [proportion (%) of total fibers counted] of quiescent satellite cells per fiber that were positive for c-Met mRNA in a population of 618 normal mouse FDB fibers, fixed 18 hr after isolation and without stretching. From 0 to 6 cells per fiber expressed c-Met transcripts, with a mean (± SE) of 1.38 ± 0.07 c-Met+ cells/fiber.
Stretch-induced Activation and Proliferation
Initial stretching experiments indicated that a Vitrogen substrate was essential for stable fiber adhesion in FlexCell plates. Fibers were observed embedded at various depths in the Vitrogen layer, as visualized with resin sections, from a position anchored on top of the Vitrogen to one completely surrounded by Vitrogen. The Vitrogen layer itself varied from 40 to 200 μm in thickness when examined in 2-μm-thick resin sections (Figure 2). Although fibers initially adhered in the absence of Vitrogen coating, many fibers rapidly detached from the collagen-coated elastomer layer when the plates were subjected to stretch.
Figure 2.
Light micrograph of an isolated fiber in transverse section partially embedded in the Vitrogen layer. Sections through all layers were made perpendicular to the original culture plate (2 μm in thickness) and stained with toluidine blue to show the fiber (dark) and the paler Vitrogen layer (delimited by arrows) above the region of resin that replaced the flexible substrate (lower gray region). Note the variation in the thickness of the Vitrogen layer. Bar = 20 μm.
Once fiber attachment was stabilized by Vitrogen, the fiber model was used to identify mechanical stretch as an activating stimulus. Satellite cells on stretched fibers were significantly activated after 2 hours (p<0.05; Figure 3), as previously reported for dispersed cultures of satellite cells (Tatsumi et al. 2001,2002). In the absence of stretch (on rigid substrate), very few satellite cells stained positive for BrdU, indicating that satellite cells were quiescent. To confirm that attachment was required for transduction of the mechanical signal, fibers plated directly on collagen-coated FlexCell plates without Vitrogen did not show activation of satellite cells after 2 hr of stretch (Figure 3).
Figure 3.
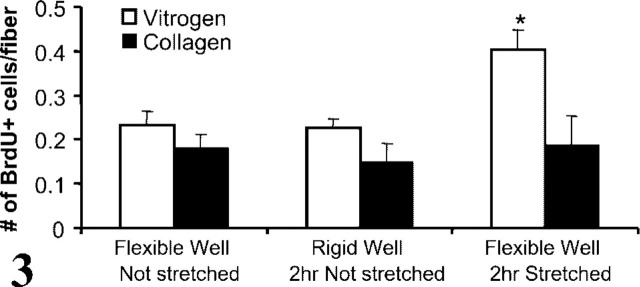
Graph showing the number of activated (BrdU+) satellite cells/fiber plated either in collagen-coated FlexCell dishes (as supplied) or in the same dishes with an additional layer of Vitrogen gel. Fibers were plated on flexible or rigid wells and allowed to adhere overnight (16 hr). BrdU was added and cultures were subject to stretching for 2 hr (plates with rigid-bottomed wells were placed in vacuum mats but not stretched). There was no difference in activation between fibers plated on collagen-coated or Vitrogen-covered dishes in the absence of stretch for either flexible- or rigid-bottomed wells. However, stretching for 2 hr induced a significant increase in activation (BrdU+ cells/fiber) when fibers were plated into Vitrogen-covered dishes !(∗p<0.05). There was no change in activation induced by stretching fibers that were plated onto collagen-coated wells.
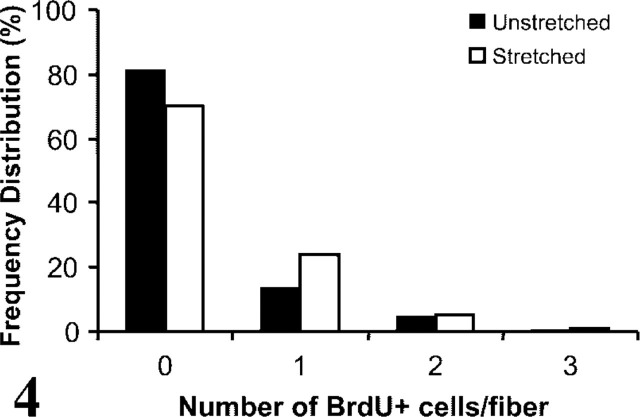
A comparison of the distributions of 2-hr stretched and unstretched fibers (Table 1; Figure 4) showed a significant increase (p = 0.01; χ2 = 9.21; df = 2) in the number of BrdU+ cells/fiber in the stretched compared to unstretched fiber cultures. The frequency distribution of c-Met+ cells per fiber compared to that of BrdU+ satellite cells per fiber on the 2-hr stretched fibers (Table 1; Figure 4) showed a significantly greater number of quiescent satellite cells positive for c-Met (1.38 ± 0.07) than BrdU+ cells (0.4 ± 0.03) after fibers were stretched (Table 1; p < 0.001; χ2 = 147.83; df = 2).
Table 1.
Tabulation of raw data and percentage distribution (parentheses) of the number of c-Met+ (1) and BrdU+ (2 and 3) satellite cells/fiber from three sets of experiments a
| Conditions | 0 | 1 | 2 | 3 | >4 | Total | |
|---|---|---|---|---|---|---|---|
| 1 | c-Met in situ hybridization unstretched | 154 (24.9) | 223 (36.1) | 143 (23.1) | 64 (10.3) | 34 (5.5) | 618 (100) |
| 2 | Control rigid well 2-hr stretched | 236 (81.4) | 39 (13.4) | 14 (4.8) | 1 (0.3) | 0 | 290 (100) |
| Control flexible well 2-hr stretched | 142 (70.3) | 48 (23.8) | 10 (4.95) | 2 (0.99) | 0 | 202 (100) | |
| 3 | Control unstretched | 3799 (65.7) | 1357 (23.5) | 476 (8.2) | 107 (1.8) | 46 (0.8) | 5785 (100) |
| HGF unstretched | 1701 (58.8) | 740 (25.6) | 327 (11.3) | 79 (2.7) | 45 (1.6) | 2892 (100) | |
| CME unstretched | 1330 (43.0) | 959 (31.0) | 513 (16.6) | 195 (6.3) | 96 (3.1) | 3093 (100) | |
Data for 3 are provided for comparison and were derived from Anderson and Pilipowicz (2002).
Figure 4.
Frequency distributions [proportion (%) of total fibers counted] of the number of activated satellite cells on isolated stretched or unstretched fibers. Activation was determined according to immunostaining for the incorporation of BrdU into DNA. The distribution of fibers was shifted to significantly higher numbers of activated satellite cells per fiber by 2 hr of stretching compared to unstretched conditions (p<0.05). Both distributions were significantly lower than the distribution of c-Met+ quiescent satellite cells/fiber shown in Figure 2.
After determining that a period of stretching stimulated satellite cell activation on single fibers, the time course of activation was examined (Figure 5). In all of the six repeated experiments, the satellite cells in unstretched cultures showed a very low level of activation, which was maintained for the duration of the experiment. Again, satellite cells attached to single fibers were activated by stretching (two-way ANOVA, p<0.0001; df=6,1). The increase in activation occurred rapidly, as soon as 30 min after initiation of stretch (p<0.05). Activation peaked at 30 min of stretch, declined, and then rose to a second peak above control levels at 2 hr (p<0.05).
Figure 5.
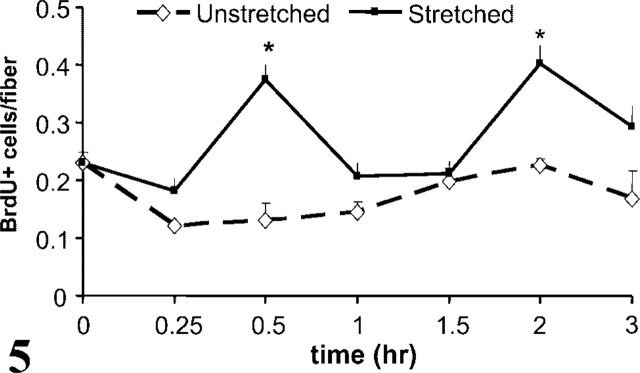
Graph showing time course of the number of activated satellite cells/fiber achieved by stretched and unstretched cultures. There was a significant difference as early as 30 min after the start of stretch and a second peak of activation after 2 hr of stretch (∗p<0.05). Data (mean ± SEM) were collected from four repeated experiments, n = 5–14 wells, with 10–233 fibers each per time point.
Discussion
These experiments established the number and distribution of quiescent c-Met+ satellite cells on a population of normal single fibers. Stretch was also determined to stimulate activation of satellite cells on cultured single fibers, while quiescence was maintained in the absence of stretch. A time course study was conducted to elucidate the nature of stretch-induced activation. The study showed two peaks of activation within 2 hr of stretching. The demonstration of a time-dependent relationship between stretching and activation of satellite cells has direct relevance to the response to exercise and the repair capacity of skeletal muscle.
Vitrogen was essential for adhesion of single fibers to FlexCell plates. In contrast, reports on dispersed cell cultures of skeletal muscle and other cell types that were plated on collagen-coated FlexCell plates did not require Vitrogen (Anderson et al. 1993; Tatsumi et al. 2001,2002). Without a Vitrogen coating, in the present experiments, there was no increase in activation after stretching fibers, probably a result of poor adhesion to the collagen-coated substrate and of shear between long contractile fibers and the underlying substrate. The Vitrogen substrate did not activate satellite cells, as indicated by the low levels of spontaneous activation seen in unstretched cultures. These findings indicate that secure adhesion of fibers is required for the stretch stimulus to be transduced to satellite cells through fibers.
The modification of the fixation protocol for ISH was necessary because the typical paraformaldehyde fixation for ISH was problematic and allowed fibers to detach from the Vitrogen substrate (Rivera and Yablonka-Reuveni unpublished observations; Anderson unpublished observations). Previous reports of single-fiber cultures examined by immunostaining used methanol fixation and were able to maintain the adhesive properties of Vitrogen (e.g., Yablonka-Reuveni and Rivera 1994; Yablonka-Reuveni et al. 1999). However, methanol fixation has not been investigated in connection with ISH. Acid/alcohol fixation, typically used for anti-BrdU immunostaining, maintained the adhesive properties of Vitrogen during in situ protocols. Although c-Met is also expressed by non-satellite cells in other tissues, including pericytes (Matsumoto et al 1995; Takayama et al. 1996; Catizone et al. 2001; Skibinski et al. 2001), c-Met expression is reported as a marker of satellite cells as viewed on fibers in sections of skeletal muscle (Tatsumi et al. 1998; Anderson 1998,2000) and, in single-fiber cultures (Anderson and Pilipowicz 2002), any fibroblast that theoretically might remain attached to a fiber would not be positive for c-Met expression.
The number of c-Met+ cells/fiber, here identified using ISH, was taken to represent the number of satellite cells expressing c-Met under quiescent conditions. This assumption was based on the low levels of spontaneous activation under basal conditions, measured by BrdU incorporation, in the parallel experiments presented here. Because c-Met is expressed by satellite cells regardless of the activation state (Cornelison and Wold 1997; Tatsumi et al. 1998), the size of the satellite cell population on normal mouse fibers is now available for detailed comparisons among different muscles, transgenic strains, environments, and treatments.
The average number of quiescent c-Met+ cells per fiber was 1.38 ± 0.07. This number apparently under-represents the population with the potential to become activated according to earlier studies. Bischoff (1986b,1990) reported that 2.1–3 cells per rat FDB were activated after 48 hr in proliferation medium. Yablonka-Reuveni and colleagues (1994) reported that four or five cells per rat fiber were positive for proliferating cell nuclear antigen after 36 hours in basal medium containing 1% horse serum and controlled processed serum replacement [a product named CPSR2 by Sigma, and somewhat different from controlled serum replacement used here and by Anderson and Pilipowicz (2002)].
Four explanations may account for the apparent differences between the satellite cell populations identified to date. First, different methods of isolation and culture probably contribute to populations defined by expression of particular gene products (Lee et al. 2000; Beauchamp et al. 2000; Tamaki et al. 2002). Second, FDB fibers are longer in rats than in mice and would show more satellite cells if the density per unit length was the same. Third, the method using Vitrogen to anchor fibers to dishes may affect penetration of the riboprobe used to define c-Met+ satellite cells. Although a thick layer of Vitrogen substrate could potentially impede penetration of a 1.2-kb riboprobe, causing some satellite cells to be unidentified by this protocol, this explanation is unlikely because satellite cells were observed in all positions relative to the fiber and the Vitrogen layer, and showed intense staining for c-Met mRNA regardless of location. Finally, c-Met expression may be restricted to a subset of satellite cells when probed in cultures under conditions that promote satellite cell quiescence. This possibility is under investigation because it suggests that some activating stimuli may induce significant de novo expression of c-Met in a second population of satellite cells, in addition to activating those cells that already express c-Met mRNA (and presumably c-Met protein) during quiescence.
The number of c-Met+ cells per fiber apparently over-represents the number of satellite cells that were activated by stretch in these experiments or by various stimuli reported previously (Anderson and Pilipowicz 2002). The low numbers of satellite cells positive for either c-Met or BrdU on a single fiber under quiescent conditions demonstrate that the basal conditions maintained quiescence and allowed only a small number of satellite cells to become activated during the approximately 40 hr in culture after plating (Table 1). In the presence of an activating stimulus (HGF, CME, or 2-hr stretch), there was a contingent of c-Met+ cells that were not activated (i.e., not BrdU+). These data imply that activating conditions do not uniformly engage the entire population of satellite cells that is presumably available for activation. We are now exploring the idea that there is a subset of satellite cells that reproducibly remain quiescent (i.e., BrdU-negative) even in the presence of the activators known to date.
Satellite cells on single fibers were shown here to be activated by a stretch stimulus. The activation is likely accompanied by an increase in metabolic activity, as suggested by experiments on dispersed cultures of rabbit muscle cells that showed a doubling (p<0.05) of calcium-phosphatidylserine-dependent protein kinase C activity after 1 hr of stretching (McComb, Greenway, Scott, and Anderson, unpublished results). This observation is consistent with the reported increase in metabolic activity in other cell types subjected to stretch (Scott et al. 1993; Li and Xu 2000; Fedorov et al. 2002).
Two peaks of activation were observed in the time course study of skeletal muscle satellite cells on single fibers. Both peaks occurred within the 2-hr time frame examined in a previous report on dispersed cells in culture, and occurred to the same extent (approximately 1.5–1.8-fold increase) (Tatsumi et al. 2001). The presence of two peaks of activation can be interpreted in two ways. It is possible that the same satellite cells enter proliferation after 30 min and 2 hr of stretch and that each cell requires more than one stimulus to become activated. This implies that activation is not an all-or-none process because satellite cells were not activated 1 hr after stretching, but it does not account for a mechanism that turns off activation processes in the intermediate period. The other possibility is that two (or more) populations of satellite cells enter proliferation after stretch stimuli of different periods (30 min and 2 hr) due to differences in cell-dependent activation characteristics, and that the first requires different stimuli to be maintained. This latter possibility suggests potential for a differential regulation of activation among various satellite cell populations. This would have an impact on anticipating the level of regeneration or growth resulting from different stimuli. However, in either case it is difficult to explain the decrease observed in BrdU incorporation when fibers are stretched for 1 and 1.5 hr. It would be expected that the amount of BrdU observed in cells would be cumulative, because BrdU is present in the medium from the beginning of stretch until fixation 24 hr later. However, if satellite cells on fibers stretched for 1 and 1.5 hr do not exit from G1 or are prevented from making the G1 to S transition, due to an unfavorable environment to initiate DNA synthesis, then BrdU may not accumulate in these cells. However, it is also likely that, once cells are activated by stretch-induced HGF release (Tatsumi et al. 2002), those cells become mobilized and migrate from fibers (Anderson and Pilipowicz 2002), and would not be included in the counts between 1 and 1.5 hr reported here. Analysis of the number of satellite cells that express an earlier marker of activation [i.e. PCNA (Johnson and Allen 1993) or c-fos (Anderson and Vargas 2003)] would address the potential for satellite cells to withdraw from activation under particular circumstances, depending on different thresholds of responsiveness to HGF, stretch, and other activators, including NO.
To our knowledge this is the first report using the single-fiber model for studies of stretch-induced satellite cell activation. Control conditions of these experiments maintained satellite cells in the quiescent state in their normal position and accounted for the potential regulation of activation by subjacent fibers once mechanical stretch was applied. This mimicked in vivo conditions more closely than experiments on stretch-induced activation of dispersed satellite cell cultures. Fiber attachment was required for the study of satellite cells during mechanical stretching, which induced satellite cell activation after 30 min and 2 hr. The entry to cycling was visualized with anti-BrdU immunostaining and was maintained at very low levels in quiescent satellite cells on unstretched fibers. In addition, acid/alcohol fixation was used to enable ISH studies of gene expression in satellite cells attached to the fibers. The size and distribution of the satellite cell population that expressed c-Met were characterized in detail in non-stretched cultures. Population studies of activation using frequency distribution were consistent with the idea of heterogeneity among satellite cells (Rantanen et al. 1995; Yablonka-Reuveni et al. 1999), as prominently suggested by two peaks of stretch-induced activation in the present experiments. With the new information that a mechanical stimulus effects the transition from quiescence to activation and proliferation by resident satellite cells, interactions between stretch and activating treatments can now be explored. This will enable us to fully characterize the signals transduced by fibers and/or inherent to satellite cells that are required for growth and repair.
Acknowledgments
We acknowledge studentships from NSERC (AW: PGS-A) and Children's Hospital Foundation (OP), and grants from the MDA (JEA), NSERC (JES), Paul H.T. Thorlakson Foundation (JEA, JES), NIH (ZY-R: AGI3798, USDA (ZY-R) and U.S.-Israel BARD (ZY-R).
We wish to acknowledge the kind gift of the c-Met cDNA from Dr. Carola Ponzetto (University of Torino, Italy), and the technical assistance of Cinthya Vargas and Paul Perumal. Insightful discussions with Dr Wayne Lautt (Department of Pharmacology and Therapeutics, University of Manitoba) are also gratefully acknowledged.
Literature Cited
- Allen RE, Sheehan SM, Tayler RG, Kendall TL, Rice GM. (1995) Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 165:307–312 [DOI] [PubMed] [Google Scholar]
- Allen RE, Temm-Grove CJ, Sheehan SM, Rice G. (1998) Skeletal muscle satellite cell cultures. Methods Cell Biol 52:155–176 [DOI] [PubMed] [Google Scholar]
- Anderson JE. (1998) Studies of the dynamics of skeletal muscle regeneration: the mouse came back! Biochem Cell Biol 76:13–26 [PubMed] [Google Scholar]
- Anderson JE. (2000) A role for nitric oxide in muscle repair: nitric oxide-mediated satellite cell activation. Mol Biol Cell 11:1859–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE, Carvalho RS, Yen E, Scott JE. (1993) Measurement of strain in cultured bone and fetal muscle and lung cells. In Vitro Cell Dev Biol 29A:183–186 [DOI] [PubMed] [Google Scholar]
- Anderson JE, Garrett K, Moor A, McIntosh L, Penner K. (1998) Dystrophy and myogenesis in mdx diaphragm muscle. Muscle Nerve 21:1153–1165 [DOI] [PubMed] [Google Scholar]
- Anderson JE, Pilipowicz O. (2002) Activation of muscle satellite cells in single fibers cultures. Nitric Oxide 7:36–41 [DOI] [PubMed] [Google Scholar]
- Anderson JE, Vargas C. (2003) Correlated NOS-Iμ and myf5 expression by satellite cells in mdx mouse muscle regeneration during NOS manipulation and deflazacort treatment. Neuromusc Disord 13:388–396 [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, et al. (2000) Expression of CD34 and myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151:1221–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A, Betz W. (1976) Properties of isolated adult rat muscle fibres maintained in tissue culture. J Physiol 271:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. (1986a) A satellite cell mitogen from crushed adult muscle. Dev Biol 115:140–147 [DOI] [PubMed] [Google Scholar]
- Bischoff R. (1986b) Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol 115:129–139 [DOI] [PubMed] [Google Scholar]
- Bischoff R. (1990) Cell cycle commitment of rat muscle satellite cells. J Cell Biol 111:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catizone A, Ricci G, Galdieri M. (2001) Expression and functional role of hepatocyte growth factor receptor (c-met) during postnatal rat testis development. Endocrinology 142:1828–1834 [DOI] [PubMed] [Google Scholar]
- Cornelison DDW, Wold BJ. (1997) Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 19:270–283 [DOI] [PubMed] [Google Scholar]
- Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, et al. (1999) The role of SF/HGF and c-Met in the development of skeletal muscle. Development 126:1621–1629 [DOI] [PubMed] [Google Scholar]
- Fedorov YV, Jones NC, Olwin BB. (2002) Atypical protein kinase Cs are the Ras effectors that mediate repression of myogenic satellite cell differentiation. Mol Cell Biol 22:1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett KL, Anderson JE. (1995) Colocalization of bFGF and the myogenic regulatory gene myogenin in dystrophic mdx muscle precursors and young myotubes in vivo . Dev Biol 169:596–608 [DOI] [PubMed] [Google Scholar]
- Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW. (1992) Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res 250:563–569 [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551 [DOI] [PubMed] [Google Scholar]
- Johnson SE, Allen RE. (1993) Proliferating cell nuclear antigen (PCNA) is expressed in activated rat skeletal muscle satellite cells. J Cell Physiol 154:39–43 [DOI] [PubMed] [Google Scholar]
- Kästner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. (2000) Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem 48:1079–1096 [DOI] [PubMed] [Google Scholar]
- Lee JY, Qu—Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, et al. (2000) Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol 150:1085–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu Q. (2000) Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal 12:435–445 [DOI] [PubMed] [Google Scholar]
- Maina F, Casgranda F, Audero E, Simeone A, Comoglio PM, Klein R, Ponzetto C. (1996) Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell 87:531–542 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Okazaki H, Nakamura T. (1995) Novel function of prostaglandins as inducers of gene expression of HGF and putative mediators of tissue regeneration. J Biochem 117:458–464 [DOI] [PubMed] [Google Scholar]
- Norman TL, Bradley—Popovich G, Clovis N, Cutlip RG, Bryner RW. (2000) Aerobic exercise as a countermeasure for microgravity-induced bone loss and muscle atrophy in a rat hindlimb suspension model. Aviat Space Environ Med 71:593–608 [PubMed] [Google Scholar]
- Park M, Dean M, Kaul K, Braun MJ, Gonda MA, Vande Woude G. (1987) Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci USA 84:6379–6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen J, Hurme T, Lukka R, Heino J, Kalimo H. (1995) Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest 72:341–347 [PubMed] [Google Scholar]
- Schultz E, Darr KC, Macius A. (1994) Acute effects of hindlimb un-weighting on satellite cells of growing skeletal muscle. J Appl Physiol 76:266–270 [DOI] [PubMed] [Google Scholar]
- Schultz E, Gibson MC, Champion T. (1978) Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool 206:451–456 [DOI] [PubMed] [Google Scholar]
- Schultz E, McCormick KM. (1994) Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol 123:213–257 [DOI] [PubMed] [Google Scholar]
- Scott JE, Yang S-Y, Stanik E, Anderson JE. (1993) Influence of strain on [3H] thymidine incorporation, surfactant-related phospholipid synthesis and cAMP levels from fetal type II alveolar cells. Am J Respir Cell Mol Biol 8:258–265 [DOI] [PubMed] [Google Scholar]
- Skibinski G, Skibinska A, James K. (2001) The role of hepatocyte growth factor and its receptor c-met in interactions between lymphocytes and stromal cells in secondary human lymphoid organs. Immunology 102:506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama H, LaRochelle WJ, Anver M, Bockman DE, Merlino G. (1996) Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci USA 93:5866–5871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, et al. (2002) Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol 157:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret C, Halevy O, Allen RE. (1998) HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol 194:114–128 [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE. (2002) Release of HGF from mechanically stretched skeletal muscle satellite cells and the role of pH and nitric oxide. Mol Biol Cell 13:2909–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. (2001) Mechanical stretch induces activation of skeletal muscle satellite cells in vitro . Exp Cell Res 267:107–114 [DOI] [PubMed] [Google Scholar]
- Yablonka—Reuveni Z, Rivera AJ. (1994) Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol 164:588–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka—Reuveni Z, Rivera AJ. (1997) Proliferative dynamics and the role of FGF2 during myogenesis of rat satellite cells on isolated fibers. Basic Appl Myol 7:189–202 [PMC free article] [PubMed] [Google Scholar]
- Yablonka—Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. (1999) The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol 210:440–455 [DOI] [PMC free article] [PubMed] [Google Scholar]