Abstract
The mechanism of development of the ovarian follicles has been largely unknown. We performed an immunohistochemical (IHC) study to determine the follicular expressions of c-kit, SCF, and inhibin-α at different developmental stages in mouse ovary. Ovaries were obtained from 14 and 16 days post coitum and 2, 7, and 21 days post partum (dpp) mice. IHC for c-kit, SCF, and inhibin-α was carried out. c-Kit and SCF were expressed on oogonia regardless of the developmental stage. Immunoreactive c-kit and SCF antigens were expressed on oocytes of primordial and primary follicles of neonate mouse ovaries. In 21 dpp mouse ovary, the expression of c-kit/SCF in oocytes gradually decreased as the follicles developed. c-Kit/SCF was expressed strongly in oocytes of preantral follicles and weakly in granulosa and thecal cells. Inhibin-α was mainly expressed on granulosa cells of preantral and early antral follicles of the 21 dpp mouse ovaries. These findings suggest that the IHC expression of c-kit/SCF proteins is specific in all developmental stages of ovarian follicles and is decreased after the follicle starts to grow. The expression of inhibin-α is negatively correlated with the expression of c-kit/SCF in the ovarian follicles in mice.
Keywords: mouse ovary, follicle, c-Kit/SCF, inhibin-α
In mammals, ovarian follicular development is the result of a complex and delicate progression of cellular interactions that culminate in a mature follicle capable of ovulating a fertilizable oocyte.
Granulosa cells in developing follicles produce stem cell factor (SCF), which can act on theca cells, stromal cells, and oocytes (Manova et al. 1993; Motro and Bernstein 1993). Differentiated theca cells, undifferentiated stromal cells, and developing oocytes express the receptor c-kit (Horie et al. 1993; Manova et al. 1993; Motro and Bernstein 1993). In rodents, c-kit is highly expressed in oocytes, supporting the role of SCF in granulosa cell-oocyte interactions (Motro and Bernstein 1993). Granulosa cells surround the developing oocyte, providing a critical microenvironment for follicular growth. The SCF and its tyrosine kinase receptor c-kit are encoded at the steel (SI) and white spotting (W) loci of the mouse, respectively (Huang et al. 1990; Zsebo et al. 1990). SCF can directly stimulate proliferation and differentiated functions of theca cells (Parrott and Skinner 1997). The expression patterns of SCF and c-kit in the ovary, as well as the actions of SCF on oocytes and theca cells, suggest that SCF may be important for many stages of follicular development. However, the paracrine signaling factors produced by follicles that regulate follicle development are not well characterized.
Inhibin is a 32-kD glycoprotein composed of two subunits, named α (18 kD) and β (12 kD), linked by disulfide bonds (Burger et al. 1995). Inhibin is a heterodimer composed of a common α-subunit but different β-subunits, denoted βA and βB (Vale et al. 1988). The main role for inhibin, for which it was discovered and named, is suppression of FSH production in the pituitary (Vale et al. 1988). Inhibin regulates growth, proliferation, and differentiation through paracrine and/or autocrine actions in a variety of cell types, including those of the gonads (DePaolo et al. 1991; Findlay 1993; Mather et al. 1992). Inhibins negatively regulate the production and secretion of FSH from the anterior pituitary, regulate intragonadal events, including follicle development and steroidogenesis, and act as tumor suppressors in the gonads and adrenal cortex (Matzuk et al. 1996; Woodruff et al. 1996). However, in relation to c-kit/SCF expression, elucidation of the ovarian expression of inhibin has only rarely been addressed.
Therefore, to determine the changes in expression profiles between c-kit and SCF, which are expressed at the early stage of follicle development, and inhibin-α, which is expressed at the developing follicles, an IHC study was performed in whole mouse ovaries obtained at different stages of development.
Materials and Methods
Animals and Tissue Preparation
Female mice (ICR strain) received mouse pellets and water ad libitum in a 23C-controlled room with a 12-hr/12-hr (light/dark) cycle. Virgin female mice were placed with a stud of the same strain (7 weeks old) and checked the next morning for the presence of a vaginal plug. If mating had occurred, this was designated as day 1 of pregnancy. Pregnant and lactating female mice were housed in a separate room. Mice were sacrificed at 14 or 16 days of pregnancy and at neonatal days 2, 7, and 21. The ovaries were surgically removed under a dissecting microscope and collected from each group.
To test the validations of the present immunohistochemistries, we used tissues obtained from human patients. Lung tissues were obtained from a 57-year-old woman with small-cell lung carcinoma to test c-kit IHC validation. To test the suitability of SCF IHC, lung tissues were obtained from a 13-week-old fetus aborted from a rubella-infected 32-year-old mother. For inhibin-α IHC validation, the noninvasive ovarian tissues obtained from a 27-year-old woman with squamous cell carcinoma were used.
Histological Preparation
All tissues were fixed in 4% paraformaldehyde or 10% neutral buffered formalin for 12 hr, embedded in paraffin, and sectioned at 5 μm for routine hematoxylin-eosin staining and IHC for c-kit, SCF, and inhibin-α was performed. For routine histology, sections were deparaffinized with xylene for 3 min, quickly rehydrated, and stained with hematoxylin and eosin. After clearing with fresh xylene, slides were mounted with Canada balsam (Sigma; St. Louis, MO) for observation. For IHC, sections were prepared on poly-l-lysine (Sigma)-coated slides.
Immunohistochemistry
IHC staining for c-kit, SCF, and inhibin-α was performed on deparaffinized and rehydrated Paraplast sections. The sections were immersed in 0.1% trypsin in CaCl2 solution for 30 min at 37C to remove proteins and then incubated in 2 N HCl for 30 min. The slices were immediately soaked in 0.1% borax solution to stop the reactions and washed with tapwater. Sections were immersed twice for 5 min in 10 mM citrate buffer (pH 6.0) and irradiated three times for 5 min in a standard microwave oven (750 W) to optimize IHC staining. Between the cycles, evaporated buffer was supplemented with hot distilled water. The slices were allowed to cool for 15 min at room temperature (RT) after heating in the microwave. Then the sections were washed with Tris-buffered saline (TBS: 0.05 M Tris-HCl plus 0.15 M NaCl, pH 7.6) and the immunostaining procedure started. Nonspecific staining was blocked twice, first with 3% H2O2 in methanol for 15 min to inhibit endogenous peroxidase activity and second with 5% normal rabbit serum for c-kit and SCF and 5% normal goat serum for inhibin-α for 10 min at RT to block nonspecific sites. Next, the sections were processed for visualization of c-kit, SCF, and inhibin-α using the IHC techniques. After washing with TBS, sections were incubated overnight at 4C in a humidified chamber in the presence of the primary antibodies (all antibodies were used at 1:100 dilution). Antibodies for c-kit and SCF were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody for inhibin-α was purchased from Oxford Bio-Innovation (Oxfordshire, UK). Next, the slices were incubated for 20 min with biotinylated anti-goat IgG (1:200) for c-kit and SCF and with biotinylated goat anti-mouse IgG (1:200) for inhibin-α. Finally, sections were washed again in TBS and incubated for 10 min in a solution of streptavidin-ABC-HRP at a dilution of 1:100. Staining was performed in TBS containing DAB (3,3′-diaminobenzidine tetrahydrochloride, 0.5 mg/ml), 0.01 M imidazole, and 0.3% H2O2 for c-kit and inhibin-α. For SCF, staining was carried out using AEC (3-amino-9-ethylcarbazole). For negative controls, primary antibodies were omitted. Then the sections were rinsed in tapwater and counterstained with Mayer's hematoxylin. The slides were mounted with Canada balsam for c-kit and inhibin-α IHC and with glycerin jelly for SCF. The sections were then examined under a microscope (Zeiss; Jena, Germany).
Identification of Follicles
Primordial, primary, preantral, and antral follicles in the largest cross-sections of the whole ovaries were observed. Primordial follicles were identified by the flattened granulosa cells surrounding the oocyte. Primary follicles were identified by single cuboidal layers of granulosa cells. Preantral and antral follicles were identified by the comparison of the serial sections described elsewhere (Kim and Lee 2000; Lee et al. 2000). If there was a follicular cavity in the serial section, it was identified as an antral follicle.
Evaluation of the IHC Reactions
Ovarian sections obtained from mice of the various ages were observed. The relative intensity of the immunostaining was evaluated by two of the authors in a double-blinded manner as not expressed (-), weakly positive (+), moderate (+ +), or intense (+ + +) for at least three different specimens from each time point. Ambiguous specimens were not considered.
Results
To test the IHC validations of the present experiments, human tissues were used. Each positive and negative IHC control for c-kit, SCF, and inhibin-α is shown in Figure 1 on the basis of hematoxylin and eosin stains (Figures 1A, 1D, and 1G) in serial sections. As expected, the immunoreactive c-kit, SCF, and inhibin-α proteins were localized in small-cell lung carcinoma (Figure 1B), some of the epithelial cells in terminal bronchi (Figure 1E), and in granulosa and theca cells of the antral follicles (Figure 1H), respectively. In the negative controls (Figures 1C, 1F, and 1I), no signals were detected. Therefore, the intensities of staining were completely suppressed.
Figure 1.
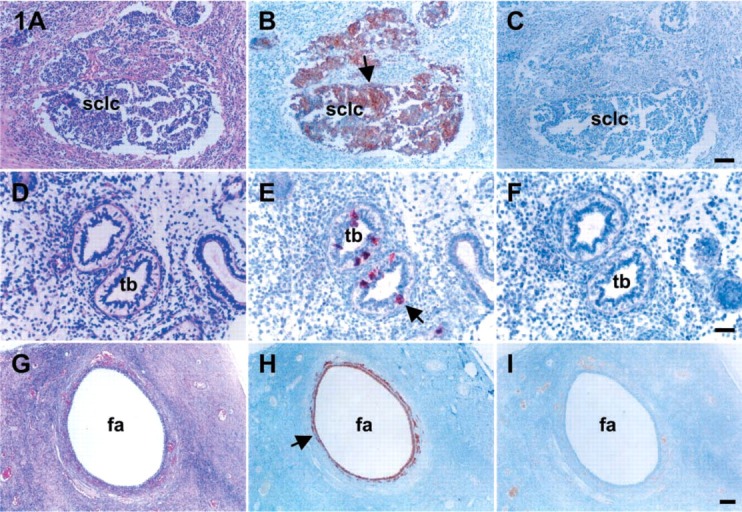
IHC for c-kit, SCF, and inhibin-α in the present experiments was validated using tissues from small-cell lung carcinoma (SCLC) 13-week-old fetal lung, and noninvasive normal ovary. With hematoxylin and eosin (A,D,G), the tissues were localized by IHC for c-kit (B), SCF (E), and inhibin-α (K). The IHC localization of c-kit was identified in SCLC. SCF was localized in the epithelium of terminal bronchi. Immunoreactivity for inhibin-α was localized in the granulosa and theca cells of antral follicles in a 27-year-old human ovary. (C,F,I) Negative controls performed without primary antibodies represent the validities of the present immunohistochemistries. Arrows indicate the immunoreactive localization of c-kit (B), SCF (E), and inhibin-α (H). sclc, small-cell lung carcinoma; tb, terminal bronchi; fa, follicular antrum; SCF, stem cell factor. Bars: A-C = 100 μm; D-F = 50 μm; G-I = 200 μm.
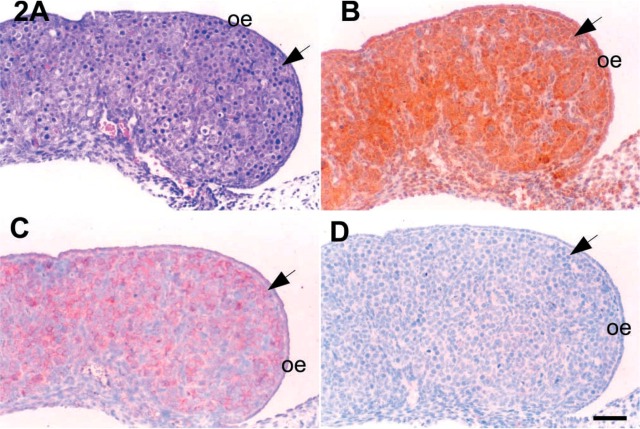
IHC localizations for c-kit, SCF, and inhibin-α in the various cell types of fetal mouse ovaries are summarized in Table 1. The expressions of c-kit, SCF, and inhibin-α were not detected by IHC in the interstitial cells in fetal mouse ovaries at 14 and 16 days post coitum (dpc). However, the antigens for c-kit and SCF were expressed on oogonia regardless of the developmental stage (Figures 2, 3, 4, and 5).
Table 1.
Immunohistochemical localizations of c-kit, SCF, and inhibin-α in various cell types of mouse ovarian follicles at 14 and 16 dpc a
| 14 dpc | 16 dpc | |||||
|---|---|---|---|---|---|---|
| Cell type | c-Kit | SCF | Inhibin-α | c-Kit | SCF | Inhibin-α |
| Germ cell | + | + | − | + | + | − |
| Interstitial cell | / | / | − | / | / | − |
dpc, days post coitum; SCF, stem cell factor; /, not applicable; -, not expressed; +, weakly positive.
Figure 2.
Microphotographs of 14 dpc mouse ovaries. (A) Section stained with hematoxylin and eosin for identification of cellular distribution. IHC localizations for c-kit (B), SCF (C), and inhibin-α (D) were also carried out in the serial sections. Arrows, oogonia. oe, ovarian epithelium; SCF, stem cell factor. Bar = 50 μm.
Figure 3.
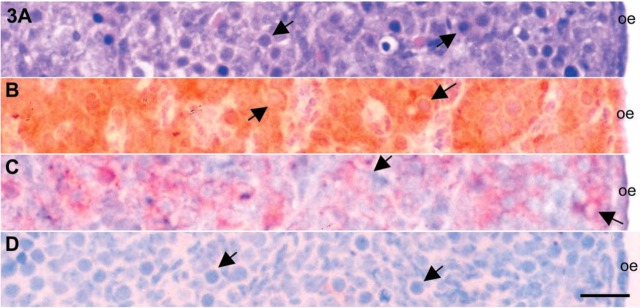
Microphotographs of 14 dpc mouse ovaries. (A) Section stained with hematoxylin and eosin identification of the cell distributions. IHC localizations for c-kit (B), SCF (C), and inhibin-α (D) were also carried out in the serial sections. Arrows, oogonia. oe, ovarian epithelium; SCF, stem cell factor. Bar = 25 μm.
Figure 4.
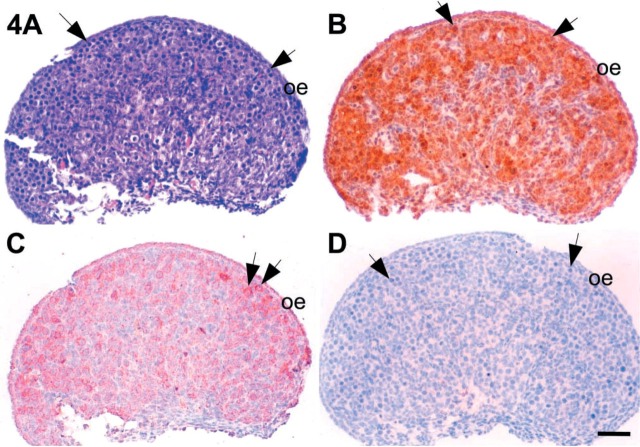
Microphotographs of 16 dpc mouse ovaries. (A) Section stained with hematoxylin and eosin for identification of the cell distributions in the ovary. IHC localizations for c-kit (B), SCF (C), and inhibin-α (D) were also carried out in the serial sections. Arrows, oogonia. SCF, stem cell factor; oe, ovarian epithelium. Bar = 50 μm.
Figure 5.
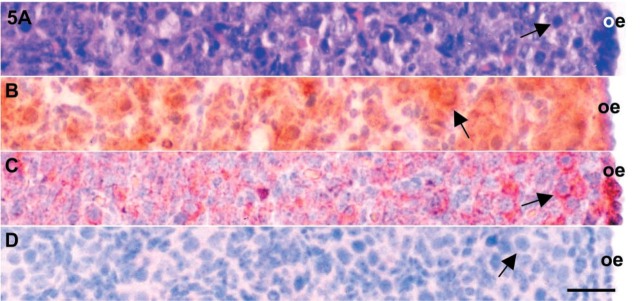
Microphotographs of 16 dpc mouse ovaries. (A) Ovarian slice was stained with hematoxylin and eosin to identify the follicular status. Serial sections were IHC localized for c-kit (B), SCF (C), and inhibin-α (D). Arrows, oogonia. SCF, stem cell factor; oe, ovarian epithelium. Bar = 25 μm.
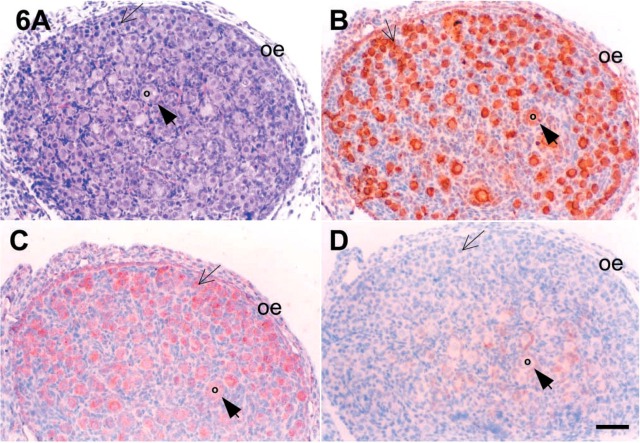
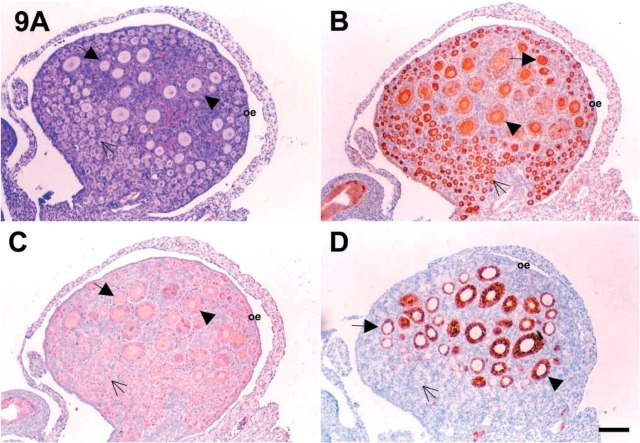
As summarized in Table 2, c-kit and SCF were expressed in oocytes of neonatal mouse ovaries. Intense and moderate immunoreactivity for c-kit and SCF proteins were observed in oocytes of the primordial and primary follicles of two (Figures 6, 7, and 8) and 7 dpp (Figures 9, 10, and 11) mouse ovaries, respectively. As shown in Figure 8, the immunoreactive c-kit and SCF antigens were localized in the ooplasm of oocytes.
Table 2.
Immunoreactivity of c-kit according to follicle status in postnatal mouse ovaries a
| c-Kit | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Oocyte | Granulosa cells | Theca cells | |||||||
| 2 | 7 | 21 | 2 | 7 | 21 | 2 | 7 | 21 | |
| Follicle | dpp | dpp | dpp | dpp | dpp | dpp | dpp | dpp | dpp |
| Primordial | +++ | +++ | +++ | +/− | − | +/− | / | / | / |
| Primary | ++ | ++ | ++ | + | +/− | + | / | / | / |
| Preantral | / | + | + | / | + | + | / | + | + |
| Early antral | / | / | + | / | / | + | / | / | + |
dpp, days post partum; /, not applicable; −, not expressed; +/−, not always expressed; +, weakly positive; ++, moderate; + + +, intense.
Figure 6.
Microphotographs of 2-day-old mouse ovaries. (A) Section stained with hematoxylin and eosin for identification of follicular status. The IHC localizations for c-kit (B), SCF (C), and inhibin-α (D) were also carried out in the serial sections. Thick and thin arrows are primary and primordial follicles, respectively. SCF, stem cell factor; oe, ovarian epithelium. Bar = 100 μm.
Figure 7.
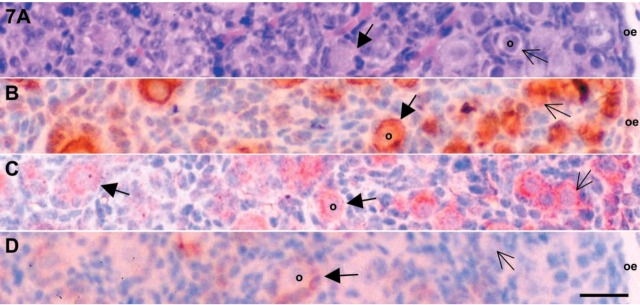
Microphotographs of 2-day-old mouse ovaries. (A) Ovarian slice stained with hematoxylin and eosin to identify follicular status. Serial sections were IHC localized for c-kit (B), SCF (C), and inhibin-α (D). SCF, stem cell factor; o, oocyte; oe, ovarian epithelium. Thick and thin arrows are primary and primordial follicles, respectively. Bar = 50 μm.
Figure 8.
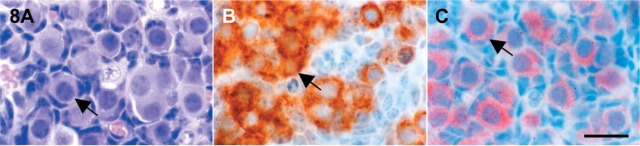
Photomicrographs of 2-day-old mouse ovaries showing the immunoreactive c-kit and SCF localizations. (A) Hematoxylin and eosin stain; (B) c-kit localization; (C) SCF localization. Arrows, primordial follicles. Bar = 20 μm.
Figure 9.
Microphotographs of 7-day-old mouse ovaries. (A) Section stained with hematoxylin and eosin for identification of follicle status. IHC localizations for c-kit (B), SCF (C), and inhibin-α (D) were also carried out in the serial sections. Arrowheads, preantral follicles; thick arrows, primary follicles; thin arrows, primordial follicles. SCF, stem cell factor; oe, ovarian epithelium. Bar = 100 μm.
Figure 10.
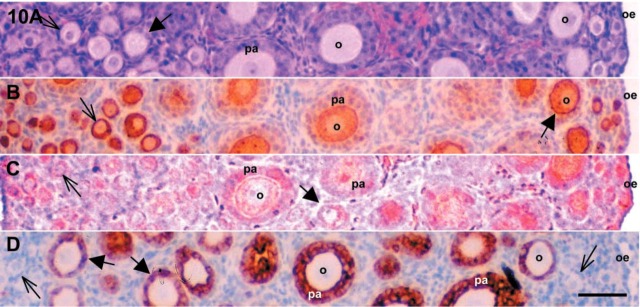
Microphotographs of 7-day-old mouse ovaries. (A) Ovarian slice was stained with hematoxylin and eosin to identify follicular status. Serial sections were IHC localized for c-kit (B), SCF (C), and inhibin-α (D). SCF, stem cell factor; pa, preantral follicle; o, oocyte; oe, ovarian epithelium. Thick and thin arrows are primary and primordial follicles, respectively. Bar = 50 μm.
Figure 11.
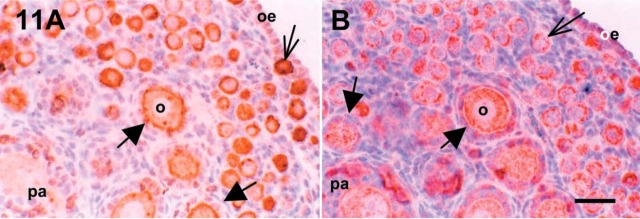
IHC localizations for c-kit (A) and SCF (B) in serial sections of 7 dpp mouse ovary. SCF, stem cell factor; o, oocyte; oe, ovarian epithelium; pa, preantral follicle. Thick and thin arrows are primary and primordial follicles, respectively. Bar = 50 μm.
Immunoreactive inhibin-α protein was strongly expressed in granulosa cells of growing follicles in 7 dpp mouse ovaries. As shown in Figure 9 and 10, the expression of inhibin-α was inversely correlated with that of c-kit and SCF. As shown in Figure 11, the immunoreactivity for c-kit and SCF antigens in 7 dpp mouse ovaries was strong in oocytes of primordial follicles. These proteins were not localized in the granulosa cells of primordial follicles. However, immunoreactivity was detected in a few granulosa cells of primary and preantral follicles (Figure 11A and 11B). Especially, as shown in Figure 11, the expression of c-kit and SCF antigens was localized in the same follicles as shown in the serial sections.
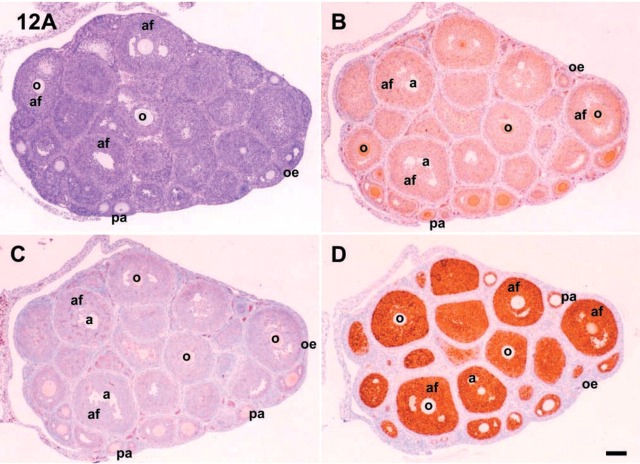
In 21 dpp immature mouse ovaries, the expression of c-kit in oocytes gradually decreased as the follicles developed. In the oocytes of early antral follicles, there was weak immunoreactivity for c-kit. In granulosa cells of 21 dpp mouse ovary, c-kit was expressed regardless of the follicular developmental stage. Some cells in the theca externa of preantral and early antral follicles expressed c-kit (Figure 12 and 13). In 21 dpp mice, SCF was expressed strongly in oocytes of preantral follicles and weakly in granulosa and theca cells. The immunoreactive expression of SCF antigen is summarized in Table 3.
Figure 12.
Microphotographs of 21 dpp mouse ovaries. (A) For observation of follicular status, section was stained with hematoxylin and eosin. IHC localizations for c-kit (B), SCF (C), and inhibin-α (D) were also shown in the serial sections. o, oocyte; a, antrum; af, antral follicle; pa, preantral follicle. Bar = 100 μm.
Figure 13.
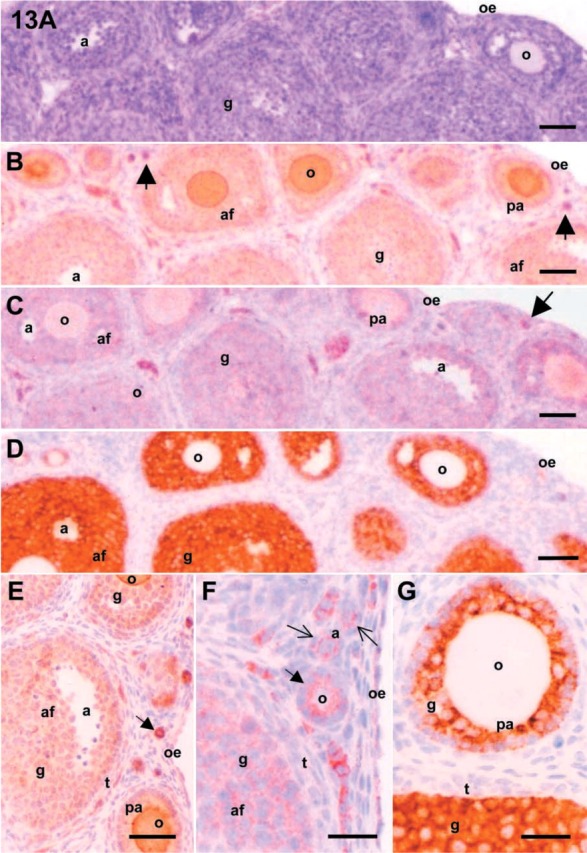
Microphotographs of mouse ovaries. Serial sections from the immature (21 dpp) mice ovaries were stained with hematoxylin and eosin (A) and immunoreactive antigens for c-kit (B), SCF (C), and inhibin-α (D) were localized. The localizations of antigens for c-kit, SCF, and inhibin-α were also shown in panels E-G, respectively. Abbreviation: a, antrum; af, antral follicle; g, granulosa cell; o, oocyte; oe, ovarian epithelium; pa, preantral follicle; t, theca cell. Arrows indicate primordial follicles. Bars: A-D = 50 μm; E = 100 μm; F,G = 25 μm.
Table 3.
Immunoreactivity of SCF according to follicle status in postnatal mouse ovaries a
| SCF | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Oocyte | Granulosa cells | Theca cells | |||||||
| 2 | 7 | 21 | 2 | 7 | 21 | 2 | 7 | 21 | |
| Follicle | dpp | dpp | dpp | dpp | dpp | dpp | dpp | dpp | dpp |
| Primordial | +++ | +++ | +/− | − | − | − | / | / | / |
| Primary | ++ | ++ | + | − | + | − | / | / | / |
| Preantral | / | + | + + | / | + | + | / | − | + |
| Early antral | / | / | + | / | / | + | / | / | + |
dpp, days post partum; SCF, stem cell factor; /, not applicable; −, not expressed; +/−, not always expressed; +, weakly positive; ++, moderate; + + +, intense.
In 21 dpp mouse ovary, as shown in Figure 13A, antigens for c-kit (Figure 13B and 13E) and SCF (Figure 13C and 13F) were expressed strongly in the oocytes and weakly in granulosa cells of primordial, primary, and preantral follicles. These antigens were also expressed in some of the theca cells. Immunoreactive localization of inhibin-α was shown in the granulosa cells of antral follicles. Relatively weak expression was detected in the granulosa cells of preantral and primary follicles. There was no expression in theca cells of antral and preantral follicles and also in primordial follicles (Figure 13D and 13G). The main localizations of inhibin-α expression were in the granulosa cells of primary follicles of 2 dpp and primary and preantral follicles of 7 dpp mouse ovaries. Inhibin-α was mainly expressed in granulosa cells of preantral and early antral follicles of 21 dpp mouse ovaries (Table 4).
Table 4.
Immunoreactivity of inhibin-α according to follicle status in postnatal mouse ovaries a
| Inhibin-α | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Oocyte | Granulosa cells | Theca cells | |||||||
| 2 | 7 | 21 | 2 | 7 | 21 | 2 | 7 | 21 | |
| Follicle | dpp | dpp | dpp | dpp | dpp | dpp | dpp | dpp | dpp |
| Primordial | − | − | − | − | − | − | / | / | / |
| Primary | − | − | − | +/− | + | + | / | / | / |
| Preantral | / | − | − | / | + + | + + | / | − | − |
| Early antral | / | / | − | / | / | + + + | / | / | − |
dpp, days post partum; /, not applicable; −, not expressed; +/−, not always expressed; +, weakly positive; ++, moderate; + + +, intense.
Discussion
In rodents, the pool of primordial follicles formed and established around birth constitutes the complete supply of oocytes available to a female throughout life. Induction of primordial follicles to develop and grow is a fundamental process in ovarian biology and is essential for female reproduction. It was reported that the ovarian follicles expressed c-kit/SCF and inhibin proteins. However, the changes in the expression profiles according to developmental stage was largely unknown. The present study was undertaken to identify the changes in the IHC expression of c-kit, SCF, and inhibin-α in mouse ovary during development.
To confirm the validations of the primary antibodies used in the present experiments, we performed IHC for c-kit, SCF, and inhibin-α using tissues obtained from small-cell lung carcinoma, fetal lung, and adult ovaries, respectively. As shown in the negative controls in Figure 1, there was no immunological staining (Figure 1C, 1F, and 1I). IHC localization for c-kit was identified in small-cell lung carcinoma (SCLC) and has been used as a marker for SCLC (Sekido et al. 1991; Tsuura et al. 1994; Naeem et al. 2002). SCF was expressed in some epithelial cells of fetal lung terminal bronchi. Expression of inhibin-α was identified on the follicular granulosa cells of adult ovary and luteinized theca cells (McCluggage 2001).
Driancourt et al. (2000) reported that mRNA encoding c-kit and SCF has been detected in fetal mouse ovaries between embryonic days 8 and 14, consistent with a role in germ cell migration and proliferation. Previous studies have demonstrated that granulosa cells in developing ovarian follicles express SCF, which may be important for granulosa cell-oocyte interactions (Motro et al. 1991; Manova et al. 1993; Motro and Bernstein 1993; Packer et al. 1994; Yoshida et al. 1997). It was demonstrated that c-kit was transcribed at high levels in oocytes in primordial and growing follicles (Driancourt et al. 2000), and that c-kit and SCF proteins were expressed in the cytoplasm of oogonia of mouse ovaries at 14 and 16 dpc. In the primordial and primary follicles of 2 and 7 dpp postnatal mouse ovaries, immunoreactivity for c-kit and SCF was shown in oocytes. On granulosa cells of the 21 dpp mouse ovary, c-kit was expressed regardless of the follicular developmental stage. Some cells in the theca externa of preantral and early antral follicles exhibited c-kit immunoreactivity. In 21 dpp mice, SCF was expressed strongly in oocytes of preantral follicles but was weak or negative in granulosa and thecal cells. The expression of c-kit on oocytes gradually decreased as the follicles developed in the immature mouse ovary. In the oocytes of early antral follicles, there was no immunoreactivity for c-kit. This implied that the roles of c-kit and SCF were altered during folliculogenesis.
Robinson et al. (2001) reported that c-kit mRNA and protein were expressed in oogonia during the transition from rapid proliferation by mitosis to the formation of primordial follicles in human ovary. Yoshida et al. (1997) reported that a blockade of c-kit function disturbed the onset of primordial follicle development, primary follicle growth, follicular fluid formation in preantral follicles, and penultimate stage ovarian follicle maturation before ovulation. It has been reported that SCF is an important local regulator of ovarian follicular development (Parrott and Skinner 1997) and has a variety of effects on isolated oocytes, including promotion of growth and maintenance of meiotic arrest (Dolci et al. 1991; Godin et al. 1991; Matsui et al. 1991; Packer et al. 1994; Ismail et al. 1996). Functional effects of c-kit/SCF in the ovary may persist into adult life in the activation of primordial follicle growth. A role for c-kit and SCF has also been suggested for the development of preantral follicles from primary follicles (Parrott and Skinner 1999; Yoshida et al. 1997). In the present study, the expression of immunoreactive c-kit/SCF proteins was strong in oocytes and weak in granulosa cells of primordial and primary follicles. However, c-kit/SCF expression in oocyte and granulosa cells of early antral and antral follicles was weak in 21 dpp mouse ovary. The expression was strong in oocytes of primordial and primary follicles at this developmental stage. This means that c-kit/SCF proteins were secreted from oocytes and have roles in follicle growth initiation and selection during folliculogenesis.
In the ovary, the source of inhibin is granulosa cells. Inhibins are members of the transforming growth factor-β superfamily of growth and differentiation factors. Yoshida et al. (1997) reported that ovarian follicle growth is dependent on c-kit during the first 5 days after birth, when the functional FSH receptor is not yet expressed in mouse ovary. In the present study, inhibin-α was expressed on granulosa cells of some primordial, and primary, preantral, and early antral follicles. Inhibin-α was mainly expressed on granulosa cells of preantral and early antral follicles of the immature mouse ovaries. The main localizations of inhibin-α expression were on the granulosa cells of primary and preantral follicles of 7 dpp and primary follicles of 2 dpp mouse ovaries. In the follicles showing strong immunoreactivity for c-kit/SCF, the expression of inhibin-α was weak. It is assumed that inhibin-α was expressed on the growth-initiated primordial, growing primary, preantral, and early antral follicles. However, in the follicles that showed strong inhibin-α immunoreactivity, the expression of c-kit/SCF was relatively weak. This suggests that inhibin-α began to be expressed after c-kit/SCF expression was decreased. It can also be suggested that c-kit/SCF has actions on follicles to be selected for further development. It is assumed that, after follicular acquisition of FSH responsiveness, the expression of inhibin-α gradually increases and that of c-kit/SCF proteins decreases.
The present study shows that the expression of c-kit/SCF is decreased after initiation of follicular growth and that the expression of c-kit/SCF is negatively correlated with the expression of inhibin-α in the ovarian follicles in mice.
Acknowledgments
Supported by the Institute of Women's Medical Science, College of Medicine, Korea University, Seoul, Republic of Korea.
Literature Cited
- Burger HG, Farnworth PG, Findlay JK, Gurusinghe CJ, Healy DL, Mamers P, Mason A, et al. (1995) Aspects of current and future inhibin research. Reprod Fertil Dev 7:997–1002 [DOI] [PubMed] [Google Scholar]
- DePaolo LV, Bicsak TA, Erickson GF, Shimasaki S, Ling N. (1991) Follistatin and activin: a potential intrinsic regulatory system within diverse tissues. Proc Soc Exp Biol Med 198:500–512 [DOI] [PubMed] [Google Scholar]
- Dolci S, Williams DE, Ernst MK, Resnick JL, Brannan CI, Lock LF, Lyman SD, et al. (1991) Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature 352:809–811 [DOI] [PubMed] [Google Scholar]
- Driancourt MA, Reynaud K, Cortvrindt R, Smitz J. (2000) Roles of kit and kit ligand in ovarian function. Rev Reprod 5:143–152 [DOI] [PubMed] [Google Scholar]
- Findlay JK. (1993) An update on the roles of inhibin, activin, and follistatin as local regulators of folliculogenesis. Biol Reprod 48:15–23 [DOI] [PubMed] [Google Scholar]
- Godin I, Deed R, Cooke J, Zsebo K, Dexter M, Wylie CC. (1991) Effects of the steel gene product on mouse primordial germ cells in culture. Nature 352:807–809 [DOI] [PubMed] [Google Scholar]
- Horie K, Fujita J, Takakura K, Kanzaki H, Suginami H, Iwai M, Nakayama H, et al. (1993) The expression of c-kit protein in human adult and fetal tissues. Hum Reprod 8:1955–1962 [DOI] [PubMed] [Google Scholar]
- Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW, Wellner D, et al. (1990) The hematopoietic growth factor KL is encoded by the SI locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell 63:225–233 [DOI] [PubMed] [Google Scholar]
- Ismail RS, Okawara Y, Fryer JN, Vanderhyden BC. (1996) Hormonal regulation of the ligand for c-kit in the rat ovary and its effects on spontaneous oocyte meiotic maturation. Mol Reprod Dev 43:458–469 [DOI] [PubMed] [Google Scholar]
- Kim JK, Lee CJ. (2000) Effect of exogenous melatonin on the ovarian follicles in gamma-irradiated mouse. Mutat Res 449:33–39 [DOI] [PubMed] [Google Scholar]
- Lee CJ, Park HH, Do BR, Yoon YD, Kim JK. (2000) Natural and radiation-induced degeneration of primordial and primary follicles in mouse ovary. Anim Reprod Sci 59:109–117 [DOI] [PubMed] [Google Scholar]
- Manova K, Huang EJ, Angeles M, DeLeon V, Sanchez S, Pronovost SM, Besmer P, et al. (1993) The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of spermatogonia. Dev Biol 157:85–99 [DOI] [PubMed] [Google Scholar]
- Mather JP, Woodruff TK, Krummen LA. (1992) Paracrine regulation of reproductive function by inhibin and activin. Proc Soc Exp Biol Med 201:1–15 [DOI] [PubMed] [Google Scholar]
- Matsui Y, Toksoz D, Nishikawa S, Nishikawa S, Williams D, Zsebo K, Hogan BL. (1991) Effect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature 353:750–752 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Shou W, Coerver KA, Lau AL, Behringer RR, Finegold MJ. (1996) Transgenic models to study the roles of inhibins and activins in reproduction, oncogenesis, and development. Rec Prog Horm Res 51:123–157 [PubMed] [Google Scholar]
- McCluggage WG. (2001) Value of inhibin staining in gynecological pathology. Int J Gynecol Pathol 20:79–85 [DOI] [PubMed] [Google Scholar]
- Motro B, Bernstein A. (1993) Dynamic changes in ovarian c-kit and Steel expression during the estrous reproductive cycle. Dev Dyn 197:69–79 [DOI] [PubMed] [Google Scholar]
- Motro B, van der Kooy D, Rossant J, Reith A, Bernstein A. (1991) Contiguous patterns of c-kit and steel expression: analysis of mutations at the W and SI loci. Development 113:1207–1221 [DOI] [PubMed] [Google Scholar]
- Naeem M, Dahiya M, Clark JI, Creech SD, Alkan S. (2002) Analysis of c-kit protein expression in small-cell lung carcinoma and its implication for prognosis. Hum Pathol 33:1182–1187 [DOI] [PubMed] [Google Scholar]
- Packer AI, Hsu YC, Besmer P, Bachvarova RF. (1994) The ligand of the c-kit receptor promotes oocyte growth. Dev Biol 161:194–205 [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. (1997) Direct actions of kit-ligand on theca cell growth and differentiation during follicle development. Endocrinology 138:3819–3827 [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. (1999) Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology 140:4262–4271 [DOI] [PubMed] [Google Scholar]
- Robinson LL, Gaskell TL, Saunders PT, Anderson RA. (2001) Germ cell specific expression of c-kit in the human fetal gonad. Mol Hum Reprod 7:845–852 [DOI] [PubMed] [Google Scholar]
- Sekido Y, Obata Y, Ueda R, Hida T, Suyama M, Shimokata K, Ariyoshi Y, et al. (1991) Preferential expression of c-kit protoon-cogene transcripts in small cell lung cancer. Cancer Res 51:2416–2419 [PubMed] [Google Scholar]
- Tsuura Y, Hiraki H, Watanabe K, Igarashi S, Shimamura K, Fukuda T, Suzuki T, et al. (1994) Preferential localization of c-kit product in tissue mast cells, basal cells of skin, epithelial cells of breast, small cell lung carcinoma and seminoma/dysgerminoma in human: immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Virchows Arch 424:135–141 [DOI] [PubMed] [Google Scholar]
- Vale W, Rivier C, Hsueh A, Campen C, Meunier H, Bicsak T, Vaughan J, et al. (1988) Chemical and biological characterization of the inhibin family of protein hormones. Recent Prog Horm Res 44:1–34 [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J. (1996) Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology 137:5463–5467 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI. (1997) Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev Biol 184:122–137 [DOI] [PubMed] [Google Scholar]
- Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, et al. (1990) Stem cell factor is encoded at the SI locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 63:213–224 [DOI] [PubMed] [Google Scholar]